Abstract
Alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor regulation has been shown to be critically involved in synaptic plasticity underlying learning and memory. This regulation occurs through trafficking of the receptor and modulation of the receptor's channel properties, both of which depend on protein phosphorylation. Using homologous recombination (knock-in) techniques we targeted two phosphorylation sites on the AMPA-GluR1 receptor: the Ser831 site, phosphorylated by calcium calmodulin-dependent protein kinase II/protein kinase C, and the Ser845 site, phosphorylated by protein kinase A. Mice with mutations that prevented phosphorylation at one or both of these sites were tested on a single-outcome Pavlovian-instrumental transfer task often used to assess the acquisition of incentive motivation by cues for food reinforcement. Mice were separately trained to associate a Pavlovian cue with food and to perform an instrumental lever-press response to earn that same reward. During a transfer test, the cue was presented while the mice were lever-pressing under extinction conditions. Whereas wild-type control mice showed substantial enhancement of lever-pressing when the cue was presented (i.e. showed Pavlovian-instrumental transfer), mice with mutations at both of these phosphorylation sites showed no evidence of such transfer. By contrast, mice with either serine site mutated alone showed normal transfer. These results suggest critical roles for GluR1 phosphorylation pathways in a form of incentive learning that can play an important part in regulating normal motivated behavior as well as maladaptive behaviors such as addiction and eating disorders.
Keywords: AMPA receptors, GluR1 phosphorylation, incentive motivation, mouse, Pavlovian-instrumental transfer, reward
Introduction
In Pavlovian conditioning, the establishment of a predictive relationship between a relatively neutral conditioned stimulus (CS) and a motivationally significant unconditioned stimulus can endow that CS with motivational or emotional powers. For example, the conditioning of incentive motivation to a CS paired with food delivery enables that CS to reinforce later Pavlovian or instrumental learning and to modulate the performance of other learned (e.g. lever-pressing for food, e.g. Holland & Gallagher, 2003) or unlearned (e.g. feeding, Holland et al., 2002) responses. Many authors have described how such incentive learning can play important roles in the control of aspects of motivated behavior relevant to issues of public health, such as substance abuse and weight control. For example, it has been widely noted that drug-related cues can both enhance craving and drug-seeking behavior and serve as potent conditioned reinforcers for those behaviors, especially in drug-deprived addicts (Everitt et al., 2000).
The Pavlovian-instrumental transfer (PIT) procedure has become a major tool for the investigation of neural mechanisms of incentive learning. In this procedure, animals are separately trained to associate a Pavlovian CS with a reinforcer, such as food, and to perform an instrumental response (such as lever-pressing) to earn that reinforcer. In a test of PIT, the CS is presented for the first time while the animal is performing the instrumental response. Enhanced performance of the instrumental response during CS presentation is taken to indicate that the CS has acquired, as a result of Pavlovian learning, the ability to influence ongoing instrumental behavior. In this simple case of PIT, which uses a single reinforcer for both Pavlovian and instrumental learning, a great deal of research has implicated brain circuitry including the central nucleus of the amygdala (CeA), ventral striatum and ventral tegmental area (e.g. Hall et al., 2001; Holland & Gallagher, 2003; Murschall & Hauber, 2006; El-Amamy & Holland, 2007).
Here we examined the sensitivity of PIT to genetic alterations of two separate pathways for the phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor subunit GluR1. AMPA receptors mediate the majority of fast excitatory neurotransmission in the central nervous system. These ionotropic glutamate receptors, in particular the AMPA GluR1 subunit, are implicated in well-established models of synaptic plasticity thought to underlie learning and memory, such as hippocampal long-term potentiation (LTP) and long-term depression (LTD) (reviewed by Song & Huganir, 2002). Regulation of the GluR1 subunit occurs through direct protein phosphorylation of the receptor at several sites on its intracellular carboxy-terminal domain (Roche et al., 1996; Boehm et al., 2006). The function of two of these sites, in particular, has been examined, i.e. the Ser831 site, which is phosphorylated by calcium calmodulin-dependent protein kinase II (CaMKII) and protein kinase C, and the Ser845 site, which is phosphorylated by protein kinase A (Roche et al., 1996; Barria et al., 1997). Phosphorylation of these sites regulates both synaptic incorporation of the receptor and its channel properties (Barria et al., 1997; Ehlers, 2000; Qin et al., 2005).
Using mice with mutations that rendered both of these phosphor-ylation sites ineffective by replacing serine with alanine, Lee et al. (2003) showed that phosphorylation of the AMPA receptor GluR1 subunit is critical for hippocampal LTP and LTD, as well as for long-term retention on a spatial memory task. These findings suggest an important role for phosphorylation of the GluR1 subunit in the synaptic plasticity that underlies hippocampus-dependent learning and memory. Of course, AMPA receptors are widely distributed in many brain regions and their phosphorylation could also be expected to be important for synaptic plasticity in those regions (e.g. Seol et al., 2007; Svenningsson et al., 2007). In line with this idea, recent findings suggest that forms of learning and memory that are mediated, in part, by circuitries within the amygdala are dependent on normal AMPA receptor functioning within that region (e.g. Rumpel et al., 2005). Therefore, in the experiments reported here, we examined the role of AMPA phosphorylation at Ser831 and Ser845 sites in PIT. Notably, this phenomenon, which reflects prior emotional learning about reward-paired events, requires processing in the amygdala and associated brain systems but is not typically thought to depend on hippocampus.
Materials and methods
Subjects
Double Ser831 + Ser845 and single Ser831 and Ser845 GluR1 mutant mice were generated using polymerase chain reaction mutagenesis (Lee et al., 2003). Briefly, a short genomic DNA fragment including exon 17 of mouse GluR1 was subcloned into pBR322 (an Escherichia coli cloning vector) and alanine substitutions were introduced into Ser831 and Ser845 sites by polymerase chain reaction mutagenesis. Mutated fragments were introduced into the targeting vector in addition to the neomycin resistance gene cassette with lox-P sequences at both sides. Correct homologous recombinant embryonic stem cells were screened by polymerase chain reaction and confirmed by Southern blot analysis. After germline transmission of the mutated GluR1 gene containing 831 alanine and 845 alanine mutations, neomycin resistance gene cassette was eliminated by breeding to Cre transgenic mice (Nagy et al., 1998). The Cre transgene was bred out from the line and heterozygous mice without the Cre gene were used for breeding to produce wild-type (WT) and mutant littermates for these experiments. Mutations of 831 and 845 phosphorylation sites were verified by western blot analysis of brain samples using phosphorylation site-specific antibodies to detect GluR1 (Lee et al., 2003; Crombag et al., 2008b).
All mice were housed four per cage in a light-cycle and climate-controlled facility (12/12 h light/dark) and testing was conducted during the light phase. Starting no less than 3 days prior to training, mice were food-restricted to about 85−90% of their free-feeding weight. This food restriction was maintained throughout the experiment. There were seven mutants and seven WTs in the double-mutation condition, 11 mutants and 12 WTs in the Ser831 condition, and eight mutants and WTs in the Ser845 condition.
All methods and procedures were in accordance with the standards set forth by the National Institutes of Health and the Animal Care and Use Committee of the Johns Hopkins University.
Apparatus
Training and testing for PIT was conducted in standard [21.6 (L) × 17.8 (W) × 12.7 (H) cm] mouse conditioning chambers (Med Associates Inc., St Albans, VT, USA) located inside sound-attenuating enclosures. A fan provided constant ventilation and low-level background noise (∼ 75 dB) to each chamber and an incandescent house light provided low-level (∼ 200 lx) white illumination. Each chamber was equipped with a motorized dipper mechanism (Med Associates Inc.) that delivered a sweetened liquid reward solution (30% condensed milk for the Ser845 and 10% sucrose for the double and Ser831 conditions) in 0.01-mL volumes into a recessed liquid receptacle. We used different rewards for the different genotype conditions because of difficulties in reliably obtaining and using the condensed milk. Infrared photocells recorded entries into the receptacle. A speaker was located on the wall opposite the liquid receptacle and was used to present auditory stimuli. For the instrumental training and PIT test sessions, retractable levers (Med Associates Inc.) were placed at a distance of 5 cm on either side of the liquid reward receptacle. Finally, activity monitors (Coulbourn Instruments, Allentown, PA, USA) were situated on top of each chamber.
Procedures
The procedures for assessing single-outcome PIT were previously described in Crombag et al. (2008a). Training comprised of Pavlovian cue-reinforcer training and instrumental response-reinforcer training. Throughout the experiment, mice received one session/day.
Mice first received two 40-min sessions designed to train them to approach the liquid receptacle and consume the liquid reward that served as the unconditioned stimulus. Next, mice were given daily Pavlovian training sessions intended to establish an association between an auditory stimulus (CS+) and presentation of the rewarding unconditioned stimulus. A second auditory stimulus was presented that was not paired with reward delivery. This CS− served as a control stimulus to assess possible non-associative behavioral effects of auditory stimulus presentations. The CSs were either a broadband white noise stimulus or a 2000-Hz pure tone, both with amplitudes set 5 dB above background (∼75 dB). The identity of the auditory stimulus (white noise or tone) that served as the CS+ or CS− was counterbalanced within each genotype condition. CS+ and CS− presentations were always in random order and for fixed periods of 2 min. During each 2-min CS+ period, liquid reward was delivered at random times with an average delivery interval of 30 s (range 15−45 s). Thus, on average, four unconditioned stimuli were presented during each CS+ period. The intertrial interval (ITI) between cue presentations was varied throughout the session and averaged 2 min (range 60−180 s). In the first two to three Pavlovian training sessions, five CS+ and five CS) presentations were given (resulting in a ∼40-min session) and in the remaining training sessions four CS+ and four CS− presentations were given (resulting in a ∼32-min session). The number of head entries into the liquid receptacle and locomotor activity levels were recorded. Double-mutant mice and their WT controls received 17 sessions of Pavlovian training, whereas the remaining four groups of mice received 15 sessions.
Next, the levers were inserted into the chambers for instrumental training. Responding on the active lever resulted in the immediate presentation of liquid reward for a 5-s period. Responding on a second (control) lever had no programmed consequence. Initially, mice were trained on 30-min sessions to respond on a continuous (FR1) schedule of reinforcement such that each active lever-press resulted in reward delivery. Once high levels of responding were observed (two to three sessions), mice were trained on 30-min sessions to respond on increasingly leaner variable interval schedules of reinforcement such that responses on the active lever were rewarded on average every 15 s, then every 30 s and finally every 60 s. Training continued until high and stable levels of active lever-responding were established for all groups (15 sessions for the double mutants and 12 session for the Ser845 mutants and their corresponding WT mice). However, in an attempt to overcome lower rates of responding in the Ser831 mutants, we gave 15 extra sessions to mutant and WT mice in this condition. In addition, for similar reasons we gave a 6-h overnight session between sessions 22 and 23.
After robust and reliable performance was established in the instrumental training sessions, the ability of the Pavlovian CS+ (and CS−) to modulate instrumental responding was evaluated during a final 32-min PIT test session. As in the instrumental training phase, both levers were available during the entire session. The test session began with a an initial period with no CS presentations (2 min in duration in the double and Ser845 genotype conditions and 8 min in duration in the Ser831 condition). In this last group, this extinction period was longer in an attempt to establish comparable baseline instrumental response levels prior to the CS presentations. At the conclusion of the extinction period, five CS+ and five CS− presentations occurred in random order, separated by fixed 2-min ITIs. No rewards were delivered in the test session, after either lever-presses or CS presentations. The impact of CS+ and CS− presentations on instrumental responding on the reinforcement lever was determined by comparing lever response rates during the CS+ period with response rates during the CS− period and baseline (ITI) levels of responding. Responding on the control lever, liquid receptacle entry responses and locomotor activity were also recorded during the test session.
Data analysis
The results were analysed for each mutation condition (double mutation, Ser831 or Ser845) separately, using anova followed by Bonferroni-corrected post hoc t-tests when appropriate. Performance during the Pavlovian (receptacle entries) sessions was analysed for effects of genotype (WT vs. mutant), CS (CS+ vs. CS−) and session using three-way anova. Performance during the instrumental sessions was analysed for each lever separately (reinforced/active or non-reinforced/inactive) for effects of genotype (WT vs. mutant) and session using two-way anova. In the PIT test session, active lever responses, control lever responses and receptacle entries were each analysed with genotype × stimulus two-way anovas.
Results
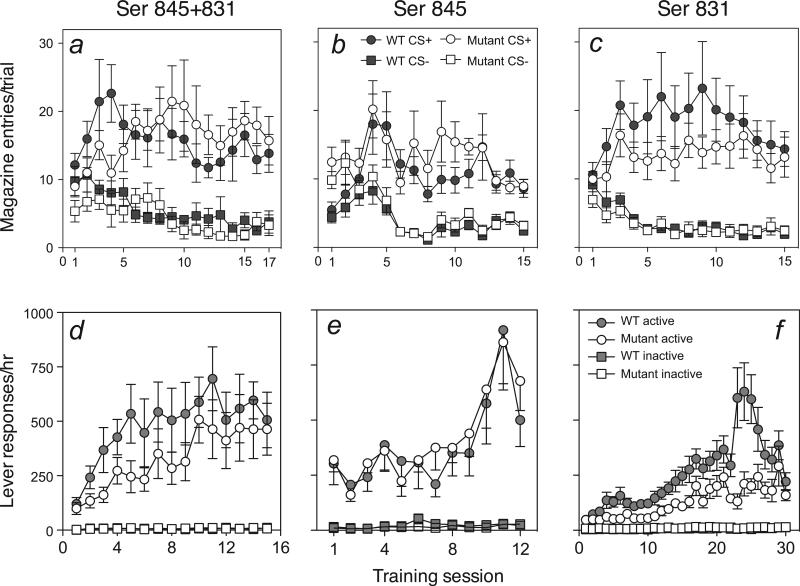
Mice first received Pavlovian conditioning sessions in which one auditory cue (CS+) was associated with reward and a second auditory cue (CS−) was presented without reward. Phosphorylation site mutations had no significant effects on the acquisition of this Pavlovian discrimination. The top panels of Fig. 1 show performance during the Pavlovian training phase for all three genotype conditions. In all cases, Pavlovian training resulted in a progressively greater number of receptacle entries during the CS+ periods relative to entries during the non-reward CS− periods (F-values > 2.8, P-values < 0.01 for the effects of session × CS). Importantly, the entries into the magazine did not vary as a function of genotype in any of the three mutation conditions [F-values < 1.2, P-values > 0.3 for main effects of genotype (mutant vs. WT) and F-values < 1 for all genotype interaction effects].
Fig. 1.
Pavlovian discrimination and instrumental training results for GluR1 phosphorylation site mutants. (a–c) Results of the Pavlovian discrimination training sessions (number of food receptacle entries during the CS+ and CS− periods) in mice with knock-in mutations of both Ser845 and Ser831 phosphorylation sites or littermate WT control mice. (d–f ) Total number of instrumental responses on the reinforced (active) and non-reinforced (inactive) levers on increasingly leaner variable interval schedules of reinforcement. The error bars show ± SEM.
Next, the mice were trained to selectively press one of two available levers in an instrumental training phase. In the first two sessions, each response on the designated active lever was rewarded, whereas responses to the other, control lever were not. Active lever-press rates during these two sessions did not differ with genotype (Table 1). The mice then received extensive additional lever-press training in which reward was delivered after active lever-presses on variable interval schedules of reinforcement. During this training, lever-press responding of the double-mutation mice and the Ser845 single-mutation mice did not differ significantly from responding of their corresponding WT controls (Fig. 1d and e). In both conditions, responding on the active lever increased progressively over sessions (F-values > 7.6, P-values < 0.001 for the effects of session) and there were no effects of genotype (F-values < 1.5, P-values > 0.2) or session × genotype interactions (F-values < 1). By contrast, Ser831 mutant and their WT control mice showed a difference in instrumental response levels across sessions (Fig. 1f). Two-way anova showed a significant effect of session (F = 15.6, P < 0.001) as well as a main effect of genotype (F1,21 = 9.5, P < 0.01) and an interaction between these variables (F = 4.1, P < 0.001). These effects of genotype were due to a higher rate of instrumental lever-responding in WT mice, although this difference dissipated towards the end of training. These mice received an extended, overnight session (not shown) between sessions 22 and 23 and additional training sessions in an unsuccessful attempt to stabilize and equalize responding across mutant and WT mice. Indeed, although the WT mice showed a large increase in response rate after the overnight session, mutant mice were unaffected by that additional training.
Table 1.
Response rates (responses/min) on the reinforcement lever for liquid reward under FR1 schedule conditions in WT and GluR1 phosphorylation mutant mice
| Condition | Genotype | Session | Active lever | Control lever |
|---|---|---|---|---|
| Double | WT | 1 | 0.74 ± 0.08 | 0.33 ± 0.17 |
| 2 | 0.81 ± 0.07 | 0.22 ± 0.12 | ||
| Double | Mutant | 1 | 0.65 ± 0.11 | 0.22 ± 0.12 |
| 2 | 0.8 ± 0.03 | 0.31 ± 0.14 | ||
| Ser845 | WT | 1 | 0.59 ± 0.12 | 0.14 ± 0.07 |
| 2 | 0.88 ± 0.17 | 0.24 ± 0.06 | ||
| Ser845 | Mutant | 1 | 0.62 ± 0.1 | 0.23 ± 0.11 |
| 2 | 0.87 ± 0.25 | 0.3 ± 0.14 | ||
| Ser831 | WT | 1 | 0.81 ± 0.16 | 0.19 ± 0.04 |
| 2 | 0.93 ± 0.18 | 0.11 ± 0.03 | ||
| Ser831 | Mutant | 1 | 0.71 ± 0.12 | 0.11 ± 0.03 |
| 2 | 0.78 ± 0.2 | 0.14 ± 0.08 |
KO, knock-out; WT, wild-type; CS, conditioned stimulus.
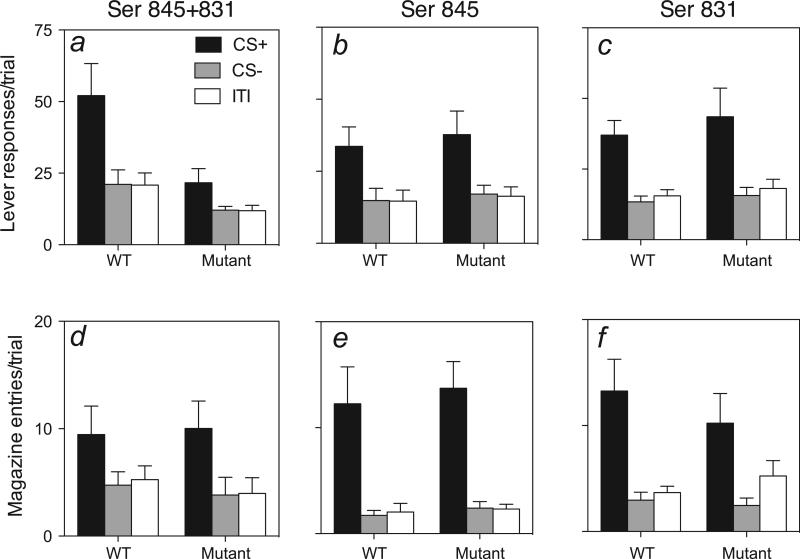
Figure 2 shows the critical results, those of the PIT test, in which the Pavlovian CSs were presented while the mice were pressing the lever. This test session was conducted in extinction; no rewards were presented, either after lever-presses or during CS delivery. The top panels show lever-press responding in the PIT test, in the double- and each of the single-phosphorylation-site mutation conditions (top panels). First, in all three cases, WT mice showed substantial PIT, i.e. CS+ presentations markedly increased active lever-press response rates (over 200%) relative to baseline (ITI) or CS− levels. CS− had no effect on baseline lever-pressing. Second, double-mutant mice failed to show significant PIT. Third, mice with mutations of only one of the phosphorylation sites, i.e. the Ser831 and Ser845 mice, showed normal PIT. These assertions are supported by the results of two-way anovas. For the double-mutant condition (Fig. 2a), anova revealed a significant effect of stimulus (F2,24 = 12.7, P < 0.001), genotype (F1,24 = 6.9, P < 0.05) and stimulus × genotype interaction (F2,24 = 3.5, P < 0.05). Whereas WT mice showed the characteristic CS+ potentiated response levels over baseline and CS− periods (post hoc comparisons, P-values < 0.05), the double-mutant mice did not. By contrast, neither single-site mutation (Ser845 or Ser831; Fig. 2b and c) produced deficits in PIT compared with their WT controls. In both of these conditions, anovas showed significant effects of stimulus (F-values > 32.8, P-values < 0.0001) but no main or interaction effects of genotype (F-values < 1). Additionally, post hoc tests (P-values < 0.05) showed that, in both of these groups of mutant mice, CS+ (but not CS−) presentations elevated response levels by similar magnitudes as seen in WT mice.
Fig. 2.
Results of the PIT test for GluR1 phosphorylation site mutants. (a–c) Number of responses/trial on the previously reinforced (active) lever during the no-cue periods (ITI), non-reward-paired stimulus (CS−) and the (previously) reward-paired stimulus (CS+) presentation periods. (d–f) Number of entries/trial into the (previously reward-delivering) magazine during the ITI, CS− and CS+ presentation periods. The error bars show +SEM.
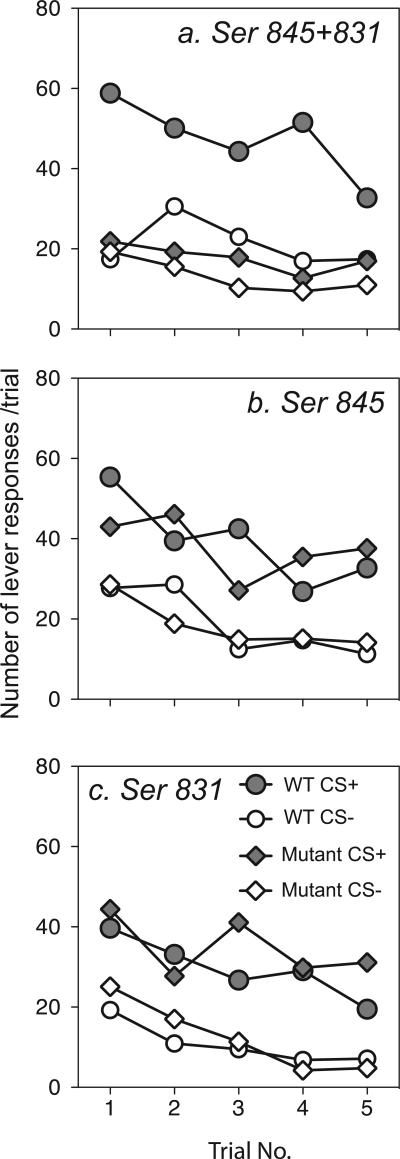
To assess whether these effects were the result of genotypic differences in extinction rates during the test for PIT, Fig. 3 shows responding on the previously reinforced lever during CS+ vs. CS− presentation periods. As these data illustrate, CS+ presentations elevated lever responding in mice with single-phosphorylation-site mutations (Fig. 3b and c) and this CS+ potentiation effect appeared to persist for most of the duration of the 40-min PIT test session. By contrast, mice with mutations of both GluR1 phosphorylation sites (Fig. 3a) failed to show a similar CS+ potentiating effect on lever-pressing and this genotype difference was evident even early in the test sessions for PIT. In other words, the overall lack of a PIT effect in these mice appeared to be not merely due to a more rapid rate of extinction of the CS+ effect on lever responding in mutant vs. WT mice.
Fig. 3.
Time course of lever responding during the PIT test for GluR1 phosphorylation site mutants (a–c). Number of responses/trial on the previously reinforced lever during individual 2-min presentation periods of the non-reward-paired stimulus (CS−) and the (previously) reward-paired stimulus (CS+).
Responding on the non-rewarded (control) lever (Table 2) occurred at low rates and did not show PIT. Indeed, in both single-phosphorylation-site mutant conditions and in their corresponding WTs there was a small but significant (F-values > 6.8, P-values < 0.0001) decrease in responding on the control lever during CS+ periods relative to baseline response levels, suggesting competition between the responding to the previously reinforced and non-reinforced levers. There were no significant main or interaction effects of genotype (F-values < 2.1, P-values > 0.2).
Table 2.
Response rates on the non-reinforced (control) lever during the test for PIT in WT and GluR1 phosphorylation mutant mice
| Condition | Genotype | CS+ | CS− | Baseline |
|---|---|---|---|---|
| Double | WT | 0.4 ± 0.31 | 2.0 ± 1.05 | 0.97 ± 0.4 |
| Mutant | 0.17 ± 0.14 | 0.52 ± 0.12 | 0.56 ± 0.14 | |
| Ser845 | WT | 1.15 ± 0.43 | 1.83 ± 0.77 | 1.99 ± 0.75 |
| Mutant | 0.77 ± 0.19 | 1.87 ± 0.74 | 1.86 ± 0.51 | |
| Ser831 | WT | 0.75 ± 0.38 | 1.08 ± 0.53 | 1.24 ± 0.38 |
| Mutant | 0.61 ± 0.28 | 1.25 ± 0.42 | 1.12 ± 0.32 |
KO, knock-out; WT, wild-type; CS, conditioned stimulus.
Figure 2 (bottom panels) also shows receptacle entry responses for the three genotype conditions during the test sessions for PIT. As in Pavlovian training, receptacle entries were more frequent during CS+ than during baseline or CS− periods in all three genotype conditions. Two-way anovas revealed significant main effects of stimulus (F-values > 9.5, P-values < 0.001) but not of genotype or stimulus × genotype interaction (F-values < 1). Thus, unlike with lever-pressing, but consistent with the results of Pavlovian training, double-mutant mice showed no deficit in receptacle responding during CS+.
There were no significant differences in overall activity levels in the experimental chambers, as assessed by a commercial activity-monitoring system, during the PIT test as a function of any mutation (counts/min: Ser831, WT = 160.3 ± 22.1 and mutant = 170.9 ± 20.2; Ser845, WT = 175.7 ± 15.7 and mutant = 180.3 ± 47.5; Ser831 + Ser845, WT = 194.3 ± 24.7 and mutant = 156.1 ± 38.0), suggesting that the differences in lever-pressing found were not due to mutation-specific changes in mobility.
Finally, we conducted an overall anova of PIT performance of all mice in these studies, including all three mutation types and their WT controls. This three-way anova for the effects of mutation type (double, 845 and 833), genotype (WT and mutant) and stimulus (CS+,CS− and ITI) revealed a significant main effect of stimulus (F = 58.4, P < 0.0001) and, most important, a significant three-way interaction effect of mutation × genotype × stimulus (F = 3.13, P < 0.05). This interaction further supports our claim that the effects of the mutations on PIT differed depending on the altered phosphorylation site(s). There were no significant effects of genotype or mutation alone, or of any of the other interactions among these factors. The results of this analysis should be viewed with caution, however, because the experiments that evaluated each of the mutations were conducted separately and there were some procedural differences among them. Mice with the Ser845 mutation and their WT controls were trained with milk reward, whereas mice in the other two conditions were trained with sucrose reward. Similarly, mice with the Ser831 mutation were given extra training and additional extinction in comparison with the remaining mice. However, we do not think that the different effects on PIT that we observed can be attributed to these procedural differences. Notably, the Ser845 and Ser831 mutants, which differed in both reward and training parameters, both showed normal PIT, whereas the double-mutant mice, which received the same sucrose reward as the Ser831 mutants but the same training parameters as the Ser845 mutants, exhibited deficits in PIT.
Discussion
Mice with single mutations of either the Ser831 or Ser845 GluR1 phosphorylation sites alone showed normal and substantial enhancement of instrumental lever-pressing by a Pavlovian cue for food (PIT). Thus, phosphorylation at either site was sufficient for the acquisition and expression of incentive motivation reflected in the display of PIT. By contrast, mice with mutations at both sites showed no evidence of PIT. At the same time, none of the mutant lines showed deficits in food receptacle entries during presentations of the reinforced CS, suggesting that even the double mutation produced no general impairment in the formation of Pavlovian cue-reinforcer associations. Although both groups that had mutations of the Ser831 site showed apparent deficits in the initial performance of instrumental lever-press responding, we do not think that any such deficits contributed to our PIT findings. First, none of the mutants differed from their WT controls in their baseline or CS− rates of lever-pressing during the PIT test session or by the end of instrumental training. Second, the apparent deficits in lever-press responding were statistically significant only in mice with the Ser831 site alone mutated, and these mice showed no deficit in PIT. Likewise, the double-mutation mice, which showed substantial deficits in PIT, displayed only non-significant reduced instrumental responding compared with their WT controls in acquisition. Thus, the display of PIT appeared to be unrelated to patterns of instrumental responding during acquisition.
Our results complement those of Crombag et al. (2008b), who found that these same double Ser831 + Ser845 mutation mice also showed evidence of another example of learned incentive motivation, i.e. conditioned reinforcement. Although WT control mice acquired an instrumental nose-poke response that produced presentations of CS+ in the absence of primary reward, mutant mice were no more likely to perform nosepokes that earned CS+ than nose-pokes that earned CS− presentations. Thus, phosphorylation of GluR1 at these two sites may be critical to a range of incentive motivation learning effects. Although little is known of the role of GluR1 phosphorylation in plasticity in brain regions known to be critical for these types of incentive learning (including regions in the amygdala), double mutants like those used here show substantial impairments in LTP and LTD in hippocampus (Lee et al., 2003) and visual cortex (Seol et al., 2007) as well as altered thresholds for GluR1 synaptic incorporation during LTP and for norepinephrine-induced memory modulation in contextual fear conditioning (Hu et al., 2007).
Notably, there is a considerable amounta of other data that indicate substantial dissociation between neural mechanisms of single-outcome PIT and conditioned reinforcement. Although studies in rats and primates demonstrate that most examples of incentive learning depend on the integrity of amygdala function, different aspects of that learning are mediated by different amygdalar subregions and extra-amygdalar circuitry. For example, with experimental protocols using a single primary reinforcer, lesions of nuclei within the basolateral amygdala impair the ability of a food-paired cue to reinforce new instrumental or Pavlovian learning but have no effect on PIT. By contrast, under those same conditions, rats with lesions of CeA display impairments in PIT but show no deficits in conditioned reinforcement (Hatfield et al., 1996; Everitt et al., 2000; Hall et al., 2001; Holland & Gallagher, 2003) or in outcome-selective PIT (Corbit & Balleine, 2005; see below).
Such dissociations have also been noted at the cellular level. For example, Mead & Stephens (2003a,b) found that mice with deletion of gria1, which codes for GluR1, showed impaired conditioned reinforcement but normal single-reinforcer PIT, whereas mice with deletion of gria2, which codes for the AMPA receptor GluR2, showed impaired PIT but unimpaired conditioned reinforcement. These investigators related these observations to the relative distributions of these two AMPA receptor subtypes within the amygdala, i.e. GluR1 is more common in basolateral amygdala than in CeA, whereas the opposite is true of GluR2/3. Furthermore, they noted that, in gria1 knock-out mice, the absence of GluR1 subunit resulted in a redistribution of GluR2/3 subunit from dendritic processes to the cell body in basolateral amygdala, suggesting a failure of GluR2/3 to be inserted into functional receptors, but no such redistribution in CeA.
On the surface, it is puzzling that we found that preventing phosphorylation of GluR1 at Ser831 and Ser845 together eliminated PIT, whereas deletion of the entire GluR1 subunit in the studies of Mead & Stephens (2003a,b) had no effect on PIT. However, those two alterations may have very different consequences for plasticity. For example, although Mead & Stephens (2003b) noted redistribution of Glu2/3 receptor subunits in amygdala in gria1 knock-outs and Zamanillo et al. (1999) found redistribution of GluR2/3 in hippo-campal pyramidal and granular cells, double-mutant mice lacking both Ser831 and Ser845 phosphorylation sites show normal distribution of GluR1 and GluR2 subunits in hippocampus (Lee et al., 2003). We do not know if this is the case in regions known to affect PIT. Nevertheless, compensatory mechanisms that may be engaged in the absence of the entire GluR1 subunit may be less pronounced when that subunit is available for incorporation but not subject to normal phosphorylation processes.
The lack of effects of the single-site mutations is also of interest. First, we found no effect of Ser845 mutation on PIT and Crombag et al. (2008b) found no deficits in conditioned reinforcement in Ser845 mutants. Nevertheless, GluR1 phosphorylation at Ser845 has been found to be critical to many examples of synaptic plasticity, including the induction of hippocampal LTP and LTD (Esteban et al., 2003; Oh et al., 2006) and the occurrence of timing-dependent associative LTP and LTD in visual cortex (Seol et al., 2007). Examination of the role of Ser845 in neural plasticity in amygdala, striatum and other regions critical to incentive learning would thus be of great interest.
Second, we found no impairment in PIT in Ser831 mutants, in which GluR1 phosphorylation by CaMKII is absent. Interestingly, unlike the present findings, Crombag et al. (2008b) found impairment of conditioned reinforcement in Ser831 mutants similar to that observed in double mutants, thus implicating Ser831 phosphorylation by CaMKII as a critical player in conditioned reinforcement. Thus, like rats with lesions of basolateral amygdala, Ser831 mutants showed deficits in conditioned reinforcement but not in PIT. In this regard, it would be of interest to determine if Ser831 mutants would also show deficits in reinforcer-selective PIT, which is also eliminated by lesions of basolateral amygdala rather than of CeA (Blundell et al., 2001; Corbit & Balleine, 2005). In this latter version of PIT procedures, in which two or more CSs, reinforcers and instrumental responses are used, PIT occurs selectively when the CS superimposed on instrumental responding is paired with the same reinforcer that was previously earned by that response. Notably, mice with a mutated form of CaMKII expressed in the striatum showed impairments in reinforcer-selective PIT (Wiltgen et al., 2007). Again, studies into the role of Ser831, which is phosphorylated by CaMKII, in neural plasticity in amygdala, striatum and other regions critical to incentive learning would be of great value.
An issue that remains unresolved in the present study is whether the PIT deficit shown by the double mutants reflects deficits in acquisition of learned incentive motivational properties to the CSs during the Pavlovian training phase, as we have suggested, or deficits in the expression of that incentive motivation as enhanced lever-pressing in the final PIT test. Although a role for GluR1 phosphorylation in the acquisition of incentive properties to CSs is consistent with its known role in synaptic plasticity (Boehm & Malinow, 2005), GluR1 phosphorylation has other consequences that might also affect neuronal activity at the time of expression in the PIT test (e.g. Roche et al., 1996; Varga et al., 2004). Notably, using a mouse model in which a mutated form of CaMKII could be conditionally suppressed or expressed in striatum (by administration of doxycycline), Wiltgen et al. (2007) found that striatal CaMKII function at the time of the PIT test, and not at the time of Pavlovian CS-food training, was critical to the observation of (outcome selective) PIT. Accordingly, Wiltgen et al. (2007) attributed their results to altered neuronal excitability at the time of test rather than to altered plasticity at the time of learning. Examination of PIT after rescue of Ser831 and/or Ser845 phosphorylation after Pavlovian learning but before PIT testing in our mice would be similarly informative.
Understanding how Pavlovian signals for reward influence instrumental behaviors is important not only for understanding normal motivated behavior but also for understanding pathological states of motivation such as addiction. It is now well appreciated that environmental stimuli that have become associated with the effects of addictive drugs play an important role in perpetuating (and possibly escalating) drug-taking behavior and triggering relapse in abstinent addicts. Together with previous research (e.g. Bossert et al., 2006; Feltenstein & See, 2007), the present results suggest that this ability of reward-associated cues to influence and modulate such drug-directed behavior relies, in part, on glutamatergic neurotransmission and, more specifically, on AMPA receptor activation. Given the now well-documented changes in glutamatergic neurotransmitter systems that occur as a function of chronic drug exposure (reviewed in Vanderschuren & Kalivas, 2000), it is conceivable that drug-induced changes in incentive motivational processes that contribute to addiction rely, in part, on changes in activity at AMPA GluR1 receptors.
Acknowledgement
This research was supported by Center Grants P40-RR-017688.
Abbreviations
- AMPA
alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- CaMKII
calcium calmodulin-dependent protein kinase II
- CeA
central nucleus of the amygdala
- CS
conditioned stimulus
- ITI
intertrial interval
- LTD
long-term depression
- LTP
long-term potentiation
- PIT
Pavlovian-instrumental transfer
- WT
wild-type
References
- Barria A, Derkach V, Soderling T. Identification of the Ca2+ / calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J. Biol. Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J. Neurosci. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochem. Soc. Trans. 2005;33:1354–1356. doi: 10.1042/BST0331354. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;10:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J. Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Galarce EM, Holland PC. Pavlovian influences on gola-directed behavior in mice: the role of cue-reinforcer relations. Learn. Mem. 2008a;15:299–303. doi: 10.1101/lm.762508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Lee HK, Holland PC, Gallagher M, Huganir RL. A necessary role for GluR1 serine 831 phosphorylation in appetitive incentive learning. Behav. Brain Res. 2008b;191:178–183. doi: 10.1016/j.bbr.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. Eur. J. Neurosci. 2007;25:1557–1567. doi: 10.1111/j.1460-9568.2007.05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Hall J, Parkinson JA, Robbins TW. Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In: Aggleton J, editor. The Amygdala: a functional analysis. Oxford University Press; New York: 2000. pp. 353–390. [Google Scholar]
- Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol. Learn. Mem. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur. J. Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J. Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol. Behav. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. Involvement of AMPA receptor GluR2 subunits in stimulus-reward learning: evidence from glutamate receptor gria2 knock-out mice. J. Neurosci. 2003a;23:9500–9507. doi: 10.1523/JNEUROSCI.23-29-09500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. Selective disruption of stimulus-reward learning in glutamate receptor gria1 knock-out mice. J. Neurosci. 2003b;23:1041–1048. doi: 10.1523/JNEUROSCI.23-03-01041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murschall A, Hauber W. Inactivation of the ventral tegmental area abolished the general excitatory influence of Pavlovian cues on instrumental performance. Learn. Mem. 2006;13:123–126. doi: 10.1101/lm.127106. [DOI] [PubMed] [Google Scholar]
- Nagy A, Moens C, Ivanyi E, Pawling J, Gertsenstein M, Hadjantonakis AK, Pirity M, Rossant J. Dissecting the role of N-myc in development using a single targeting vector to generate a series of alleles. Cur. Biol. 1998;8:661–664. doi: 10.1016/s0960-9822(98)70254-4. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Bateup H, Qi H, Takamiya K, Huganir RL, Spedding M, Roth BL, McEwen BS, Greengard P. Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur. J. Neurosci. 2007;26:3509–3517. doi: 10.1111/j.1460-9568.2007.05952.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Varga AW, Yuan L-L, Anderson AE, Schrader LA, Wu G-Y, Gatchel JR, Johnston D, Sweatt JD. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J. Neurosci. 2004;24:3643–3654. doi: 10.1523/JNEUROSCI.0154-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Law M, Ostland S, Mayford M, Balleine BW. The influence of Pavlovian cues on instrumental performance is mediated by CaMKII activity in the striatum. Eur. J. Neurosci. 2007;25:2491–2497. doi: 10.1111/j.1460-9568.2007.05487.x. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]