Abstract
Inheritance plays a significant role in defining drug response and toxicity. Advances in molecular pharmacology and modern genomics emphasize genetic variation in dictating inter-individual pharmacokinetics and pharmacodynamics. A case in point is the homeostatic ATP-sensitive potassium (KATP) channel, an established drug target that adjusts membrane excitability to match cellular energetic demand. There is an increased recognition that genetic variability of the KATP channel impacts therapeutic decision- making in human disease.
Genetic variations account for 15–30% of inter-individual differences in drug metabolism and as much as 95% of variability in individual drug response.1 Individualization of therapy is aimed at achieving the best therapeutic outcome using patient-stratified genomic information. Integrated pharmacology with genetics provides an attractive strategy poised to decipher the heterogeneity of disease phenotypes and dissect variations in drug response, leading to therapeutic optimization. The information gained through pharmacogenomics holds particular promise in improving drug efficacy while minimizing toxicity, with subgrouping of patients based on genetic variations fostering early and personalized treatment.2
Pharmacogenomics has established genetic variations in drug-metabolizing pathways, transporters, receptors, and signaling cascades as critical in defining pharmacokinetic and/or pharmacodynamic outcomes.3 A therapeutic target that has recently received attention is the KATP channel, widely distributed in tissue beds of high metabolic activity.4 KATP channels exhibit unique energetic decoding capabilities based on a heteromultimeric structure comprised of an inwardly rectifying K+-conducting (Kir) pore and a larger regulatory subunit, an ATPase-harboring ATP-binding cassette protein—the sulfonylurea receptor (SUR). By matching membrane excitability with fluctuations in cellular metabolic demand, KATP channels link energetic flux and cell homeostasis. KATP channels play cytoprotective roles throughout the body, including in the myocardium, vasculature, brain, skeletal muscle, and pancreas.5 Indeed, in the pancreas, antagonism of KATP channel activity with sulfonylurea agents facilitates insulin release and is a first-line treatment in adult-onset diabetes mellitus. KATP channel openers display protective properties, although their clinical use is less common.5 Here, we highlight how the KATP genetic variability influences disease susceptibility, and delineate how this knowledge translates into advances in therapeutic management.
KATP CHANNEL REGULATION OF INSULIN RELEASE
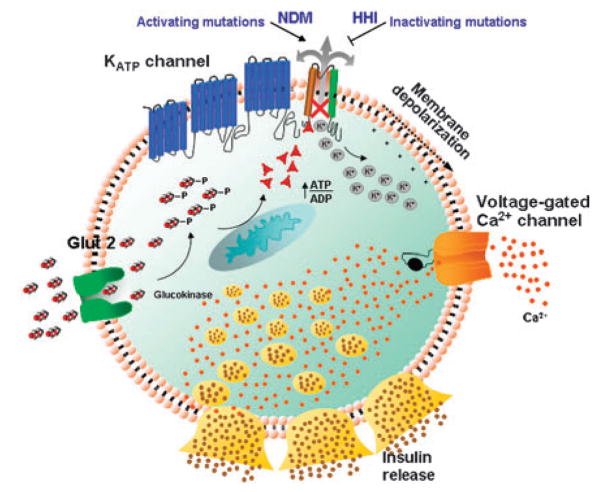
Through decoding of changes in glucose balance in the pancreatic β-cell, KATP channels, composed through association of the Kir6.2 pore with the SUR1 regulatory subunit, regulate insulin release.6,7 Nucleotide fluxes in the submembrane space influence channel function, which sets membrane excitability to ultimately control insulin release (Figure 1). In response to hyperglycemia and high intracellular glucose, channel closure permits membrane depolarization and associated calcium influx, facilitating insulin release. Conversely, an increase in Mg-ADP at the SUR site, favored by a reduction in blood and cellular glucose, leads to channel activation, rendering the membrane hyperpolarized, thereby limiting calcium influx and inhibiting insulin release.
Figure 1.
KATP channels in the pancreatic β-cell control insulin release. Hyperglycemia translates into increased transport of glucose into β-cells, resulting in elevated intracellular ATP promoting closure of KATP channels and membrane depolarization leading to opening of voltage-gated Ca2 +channels and Ca2+influx, which triggers insulin release. Inactivating KATP channel mutations lead to overactivated insulin release and HHI, whereas activating channel mutations induce membrane hyperpolarization, impairing insulin release and resulting in neonatal diabetes mellitus (NDM).
KIR6.2 (KCNJ11) MUTATIONS AND NEONATAL DIABETES
Activating mutations in the Kir6.2 pore-encoding gene (KCNJ11) have been identified in both transient and permanent neonatal diabetes mellitus.8–11 These mutations are familial or more often sporadic in nature.8 KCNJ11-activating mutations result in reduced channel sensitivity to ATP, in the presence of glucose, favoring an open channel state and membrane hyperpolarization,6 translating into impaired insulin release and neonatal diabetes mellitus seen in the absence of β-cell auto-antibodies.8 KCNJ11-activating mutations, such as the R201H polymorphism (the most common permanent neonatal diabetes mellitus-causing mutation), result in ATP-insensitive channels that respond to sulfonylureas with channel closure and insulin release.8 Heterozygous mutations in the N terminus of KCNJ11 (F35L and F35V) that affect the KATP channel pore increase whole-cell current owing to reduced inhibition by ATP in the presence of Mg2+, and increase the probability for the open channel state in the absence of ATP, resulting in neonatal diabetes (Figure 2). Channels in the heterozygous state are characterized by strong tolbutamide blockade, which translates into a favorable clinical response to sulfonylureas in patients with F35V mutations, allowing insulin therapy to be discontinued.12
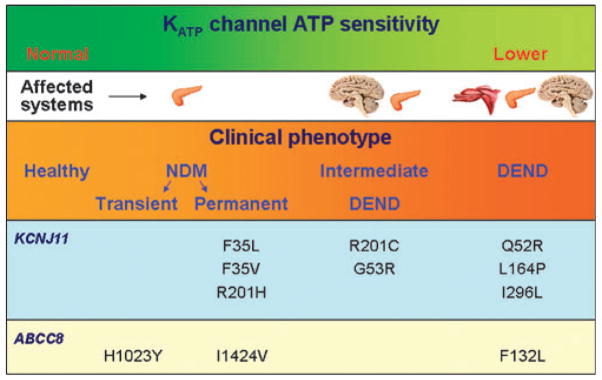
Figure 2.
The KATP channel sensitivity to ATP determines clinical outcome. Genetic variation in KCNJ11 and ABCC8-encoded Kir6.2 and SUR1 subunits translates into varying degrees of disease severity correlating with altered sensitivity of the ATP channel. Representative polymorphisms in KCNJ11 and ABCC8 lead to phenotypes that range from transient forms of neonatal diabetes mellitus (NDM) to the more severe developmental delay, epilepsy, and permanent neonatal diabetes (DEND) syndrome.
Of the KCNJ11 gene polymorphisms in neonates with diabetes that failed to respond to oral sulfonylureas, mutations Q52R and I296L were associated with the triad of developmental delay, epilepsy, and neonatal diabetes, i.e., the developmental delay, epilepsy, and permanent neonatal diabetes (DEND) syndrome, whereas mutations G53R and R201C were associated with a less severe phenotype of intermediate DEND manifested as neonatal diabetes with milder developmental delay without epilepsy.6 In vitro inhibition of KATP channel current with tolbutamide, the prototypic sulfonylurea, is less pronounced in the mutant Q52R and I296L channels, supporting clinical data.6 The I296L mutation, associated with DEND syndrome, markedly increases KATP channel current by decreasing the sensitivity of the channel to ATP, altering normal channel kinetics stabilizing the open state, and possibly through allosteric effects on ATP binding and/or transduction.13 Other mutations, such as Q52R and V59G, also cause a large reduction in ATP sensitivity.13,14 Mutations that produce a small decrease in ATP sensitivity, such as R201H in the heterozygous state, are associated with a limited phenotype,6,8 whereas those with markedly decreased sensitivity to ATP, such as I296L, Q52R, and V59G, are associated with DEND syndrome, resulting in a more severe phenotype affecting, beyond the pancreas, skeletal muscles and the central nervous system, in line with the broad roles of KATP channels in the body.13,14
SUR1 (ABCC8) MUTATIONS AND NEONATAL DIABETES
Conformations in the ABCC8 gene-encoded SUR1, induced by the interaction of Mg-nucleotides with this regulatory channel subunit, dictate KATP channel gating.15 SUR1 also serves as a receptor for sulfonylurea drugs, which result in ATP-independent KATP channel inhibition. Critical to its role in channel behavior, polymorphisms of ABCC8 gene manifest as disorders of glucose metabolism.
As with KCNJ11, activating mutations in ABCC8 are associated with both transient and permanent neonatal diabetes.11 ABCC8 gene polymorphisms L213R and I1424V are seen in permanent neonatal diabetes, and C435R, L582V, H1023Y, R1182Q, and R1379C in patients with transient neonatal diabetes.7 Patch clamp reveals that I1424V or H1023Y polymorphisms produce overactivate channels by Mg-nucleotide-dependent stimulatory effects under physiological conditions.7 This is thought to cause membrane hyperpolarization with resultant reduced calcium influx and decreased insulin release. A significant finding is the sensitivity of mutant I1424V and H1023Y channels to the sulfonylurea tolbutamide.7 A heterozygous activating mutation of ABCC8 gene, F132L, has also been identified in a patient with DEND syndrome, although most cases of DEND syndrome have been associated with mutations in KCNJ11 affecting the Kir6.2 pore function. F132L was found as a de novo mutation, and associated with a marked reduction in the sensitivity of KATP channels to inhibition by Mg-ATP, resulting in activation of channel current and inhibition of insulin release. The sulfonylurea tolbutamide has a somewhat reduced effectiveness in inhibiting heterozygous F132L channels in vitro.16 This mutation could account for weakness of neurogenic origin in DEND, as SUR1 is expressed in neurons and not in muscle.16 Also, a recently identified mutation in SUR1, L225P, demonstrates increased Mg-nucleotide stimulation of the channel, resulting in permanent neonatal diabetes mellitus without affecting sulfonylurea sensitivity.17
CLINICAL APPLICATION OF KATP CHANNEL ANTAGONISTS IN NEONATAL DIABETES
The ability of sulfonylureas to successfully inhibit KATP channel activity by an ATP-independent mechanism, bypassing nucleotide-dependent channel gating, forms the basis for the clinical application of these drugs in patients with neonatal diabetes owing to mutations affecting the ATP sensitivity of the channel. Recently, the efficacy of therapeutic amendment from insulin to sulfonylurea-based treatment was assessed in cases of neonates with diabetes owing to Kir6.2 mutations.6 Ninety percent of subjects had a successful therapeutic response to an oral sulfonylurea, such as glyburide.6 These patients usually have minimal, if any, detectable circulating insulin levels and require exogenous insulin to prevent hyperglycemia and ketoacidosis. Sulfonylureas were not only effective in achieving an acceptable level of glycated hemoglobin, a parameter used to assess glycemic control, but they also sustained the euglycemic response in patients with Kir6.2 mutations.6 Independently, other studies have also established the success of sulfonylureas in achieving a clinical response in patients with diabetes owing to Kir6.2 mutations affecting the ATP sensitivity of the channel.8,18 Oral sulfonylurea treatment thus forms an attractive alternative to lifelong exogenous injections of insulin in these patients. It should be noted that Kir6.2 mutations, such as Q52R, I296L, and L164P, known to affect channel kinetics resulting in increased open-state probability do not display a therapeutic response to sulfonylureas clinically.13,14
As with Kir6.2 mutations, SUR1 mutations with retained KATP channel sensitivity to sulfonylureas demonstrate a favorable therapeutic outcome with sulfonylurea treatment in patients with transient and permanent neonatal diabetes, a drug class effect.6,7 The successful therapeutic response to sulfonylureas in patients with neonatal diabetes requires, however, a larger dose than the current recommended regimen for adult type 2 diabetic patients.6,19 As neonatal diabetes could be caused by several genetic defects other than mutations affecting KATP channels (e.g., mutations in the glucokinase gene or FOXP3), sulfonylureas are not universally effective for all cases of neonatal diabetes. The therapeutic implications thus mandate an individualized molecular diagnosis in patients with diabetes diagnosed in the neonatal stage or in the first 6 months of life, regardless of current age.6
KATP MUTATIONS AND HYPERINSULINEMIC HYPOGLYCEMIA OF INFANCY
In contrast to KATP channel activating gene mutations responsible for neonatal diabetes, loss-of-function gene defects have been implicated in hyperinsulinemic hypoglycemia of infancy (HHI).20 HHI, characterized by hypoglycemia, is associated with severe outcomes, including seizures and mental retardation.20 Polymorphisms/mutations in either ABCC8 or KCNJ11 resulting in loss of KATP channel function can manifest in either an autosomal recessive or dominant form of HHI.21 Although familial forms have been described, HHI for the most part is sporadic.22–25 SUR1 mutations account for ~50% of HHI,26,27 and could manifest as HHI with underlying defects associated with defective channel biogenesis owing to deficit in protein trafficking (Class I) or generation of nonfunctional channels, despite the presence of channel proteins at the plasma membrane (Class II).28 Class I mutations produce more severe phenotypes, whereas Class II mutations are associated with milder forms of the disease owing to a residual response to Mg-ADP24, although no precise genotype–phenotype correlation exists in HHI.28 Mutations affecting Kir6.2 function causing loss-of-function defect are less common causes of HHI.23,29
CLINICAL APPLICATION OF KATP CHANNEL OPENERS IN HHI
Although not uniformly effective, the KATP channel opener diazoxide provides the mainstay for HHI treatment.21 The mechanism underlying diazoxide effectiveness is inhibition of insulin release through KATP channel activation owing to interaction with the SUR1 subunit. Diazoxide hyperpolarizes the membrane by promoting K+ efflux through KATP channels and reduces insulin secretion by limiting calcium influx into β-cells. To be effective, diazoxide requires intact KATP channels at the level of the β-cell plasma membrane. In fact, diazoxide is found most effective in HHI caused by mutations in the glycolytic enzyme glucokinase, the mitochondrial enzyme glutamate dehydrogenase, and short-chain L-3-hydroxyacyl-CoA dehydrogenase, where KATP channels are intact.28 Thus, patient stratification based on molecular diagnosis of HHI has important therapeutic implications.
KATP CHANNELS AND CARDIOVASCULAR DISEASE
As membrane-based metabolic sensors regulating cardiomyocyte excitability, myocardial KATP channels are critical in cardiac adaptation to ischemia, in the “flight-or-fight” response, and in heart failure.4 Mishandling of myocardial calcium balance under stress is an established mediator in the pathogenesis of cardiomyopathy, with calcium loading recognized as a major elicitor of myocyte maladaptive remodeling that gradually progresses to contractile dysfunction and ultimately decompensates into congestive heart failure.30 To this end, KATP channels have emerged as novel protectors against cardiac maladaptation to stress, with hearts deficient in functional KATP channels found susceptible to calcium- dependent maladaptive remodeling, progressing to organ failure and death.31 Mutations in ABCC9, the gene encoding the SUR2A protein, have been found in patients with idiopathic heart failure and rhythm disturbances likely caused by dysfunctional phenotype of the mutant channel with metabolic sensing deficit.32,33 KATP channel-pore polymorphisms (P266T and R371H) have also been linked to sudden cardiac death.34 Patch-clamp studies in these cases demonstrate lack of allosteric modulation between intracellular pH and ATP, with decreased expression and altered current ratios of mutant channels likely compromising the beneficial effect of KATP channel during stress and thus contributing to the sudden cardiac death syndrome.34 Implication of KATP channel in the genetics of heart failure and rhythm disorders identifies potassium channel openers as novel therapeutics in these patients.
SUMMARY
Polymorphisms in KATP channel genes, underscoring the channel’s diverse distribution and critical role in several organ systems, have profound implications in both disease manifestation and dictating therapeutic response. With advances in our understanding of how specific polymorphisms impact channel behavior, new strategies to exploit this information would refine therapeutic management in the context of translating discovery at the bench to personalized medicine at the bedside.35 With established importance in disorders of glucose metabolism and emerging significance in cardiovascular pathology, KATP channel dysfunction emerges as a novel channelopathy ripe for pharmacogenomic investigation.
Acknowledgments
This work was supported by grants from the National Institutes of Health, Ted Nash Long Life Foundation, and Marriott Heart Disease Research Program, Marriott Foundation. SS is supported by the NIH T32-GM008685 Training Grant in Clinical Pharmacology at Mayo Clinic.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Eichelbaum M, Ingelman-Sundberg M, Evans WE. Pharmacogenomics and individualized drug therapy. Annu Rev Med. 2006;57:119–137. doi: 10.1146/annurev.med.56.082103.104724. [DOI] [PubMed] [Google Scholar]
- 2.Giacomini KM, et al. The pharmacogenetics research network: from SNP discovery to clinical drug response. Clin Pharmacol Ther. 2007;81:328–345. doi: 10.1038/sj.clpt.6100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinshilboum RM, Wang L. Pharmacogenetics and pharmacogenomics: development, science, and translation. Annu Rev Genomics Hum Genet. 2006;7:223–245. doi: 10.1146/annurev.genom.6.080604.162315. [DOI] [PubMed] [Google Scholar]
- 4.Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jahangir A, Terzic A. KATP channel therapeutics at the bedside. J Mol Cell Cardiol. 2005;39:99–112. doi: 10.1016/j.yjmcc.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson ER, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 7.Babenko AP, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 8.Gloyn AL, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 9.Gloyn AL, et al. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005;14:925–934. doi: 10.1093/hmg/ddi086. [DOI] [PubMed] [Google Scholar]
- 10.Landau Z, et al. Sulfonylurea-responsive diabetes in childhood. J Pediatr. 2007;150:553–555. doi: 10.1016/j.jpeds.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan SE, et al. Mutations in KATP channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930–1937. doi: 10.2337/db07-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proks P, Girard C, Baevre H, Njolstad PR, Ashcroft FM. Functional effects of mutations at F35 in the NH2-terminus of Kir6.2 (KCNJ11), causing neonatal diabetes, and response to sulfonylurea therapy. Diabetes. 2006;55:1731–1737. doi: 10.2337/db05-1420. [DOI] [PubMed] [Google Scholar]
- 13.Proks P, et al. A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep. 2005;6:470–475. doi: 10.1038/sj.embor.7400393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proks P, et al. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc Natl Acad Sci USA. 2004;101:17539–17544. doi: 10.1073/pnas.0404756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alekseev AE, et al. ATP-sensitive K+ channel channel/enzyme multimer: metabolic gating in the heart. J Mol Cell Cardiol. 2005;38:895–905. doi: 10.1016/j.yjmcc.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proks P, et al. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet. 2006;15:1793–1800. doi: 10.1093/hmg/ddl101. [DOI] [PubMed] [Google Scholar]
- 17.Masia R, et al. A mutation in the TMD0-L0 region of sulfonylurea receptor-1 (L225P) causes permanent neonatal diabetes mellitus (PNDM) Diabetes. 2007;56:1357–1362. doi: 10.2337/db06-1746. [DOI] [PubMed] [Google Scholar]
- 18.Zung A, Glaser B, Nimri R, Zadik Z. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J Clin Endocrinol Metab. 2004;89:5504–5507. doi: 10.1210/jc.2004-1241. [DOI] [PubMed] [Google Scholar]
- 19.Sagen JV, et al. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes. 2004;53:2713–2718. doi: 10.2337/diabetes.53.10.2713. [DOI] [PubMed] [Google Scholar]
- 20.Hussain K, Cosgrove KE. From congenital hyperinsulinism to diabetes mellitus: the role of pancreatic β-cell KATP channels. Pediatric Diabetes. 2005;6:103–113. doi: 10.1111/j.1399-543X.2005.00109.x. [DOI] [PubMed] [Google Scholar]
- 21.Dekelbab BH, Sperling MA. Recent advances in hyperinsulinemic hypoglycemia of infancy. Acta Paediatr. 2006;95:1157–1164. doi: 10.1080/08035250600640414. [DOI] [PubMed] [Google Scholar]
- 22.Thomas PM, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- 23.Nestorowicz A, et al. A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes. 1997;46:1743–1748. doi: 10.2337/diab.46.11.1743. [DOI] [PubMed] [Google Scholar]
- 24.Huopio H, et al. Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest. 2000;106:897–906. doi: 10.1172/JCI9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magge SN, et al. Familial leucine-sensitive hypoglycemia of infancy due to a dominant mutation of the β-cell sulfonylurea receptor. J Clin Endocrinol Metab. 2004;89:4450–4456. doi: 10.1210/jc.2004-0441. [DOI] [PubMed] [Google Scholar]
- 26.Dunne MJ, Cosgrove KE, Shepherd RM, Aynsley-Green A, Lindley KJ. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev. 2004;84:239–275. doi: 10.1152/physrev.00022.2003. [DOI] [PubMed] [Google Scholar]
- 27.Glaser B, Thornton P, Otonkoski T, Junien C. Genetics of neonatal hyperinsulinism. Arch Dis Child Fetal Neonatal Ed. 2000;82:F79–F86. doi: 10.1136/fn.82.2.F79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996;5:1809–1812. doi: 10.1093/hmg/5.11.1809. [DOI] [PubMed] [Google Scholar]
- 30.Kane GC, et al. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 31.Yamada S, et al. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bienengraeber M, et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson TM, et al. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4:110–116. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui N, et al. Elimination of allosteric modulation of myocardial KATP channels by ATP and protons in two Kir6.2 polymorphisms found in sudden cardiac death. Physiol Genomics. 2006;25:105–115. doi: 10.1152/physiolgenomics.00106.2005. [DOI] [PubMed] [Google Scholar]
- 35.Waldman SA, Christensen NB, Moore JE, Terzic A. Clinical pharmacology: the science of therapeutics. Clin Pharmacol Ther. 2007;81:3 –6. doi: 10.1038/sj.clpt.6100032. [DOI] [PubMed] [Google Scholar]