SUMMARY
Evasion of DNA damage-induced cell death, via mutation of the p53 tumor suppressor or overexpression of prosurvival Bcl-2 family proteins, is a key step toward malignant transformation and therapeutic resistance. We report that depletion or acute inhibition of checkpoint kinase 1 (Chk1) is sufficient to restore γ-radiation-induced apoptosis in p53 mutant zebrafish embryos. Surprisingly, caspase-3 is not activated prior to DNA fragmentation, in contrast to classical intrinsic or extrinsic apoptosis. Rather, an alternative apoptotic program is engaged that cell autonomously requires atm (ataxia telangiectasia mutated), atr (ATM and Rad3-related) and caspase-2, and is not affected by p53 loss or overexpression of bcl-2/xl. Similarly, Chk1 inhibitor-treated human tumor cells hyperactivate ATM, ATR, and caspase-2 after γ-radiation and trigger a caspase-2-dependent apoptotic program that bypasses p53 deficiency and excess Bcl-2. The evolutionarily conserved “Chk1-suppressed” pathway defines a novel apoptotic process, whose responsiveness to Chk1 inhibitors and insensitivity to p53 and BCL2 alterations have important implications for cancer therapy.
INTRODUCTION
The stress-inducible p53 protein acts as a central signal transduction node in the apoptotic response to DNA damage, mainly through its ability to transactivate intrinsic (mitochondrial) and extrinsic (death-receptor) pathway genes (Vousden and Lu, 2002). However, ample evidence supports the existence of p53-independent apoptotic responses to DNA damage. In Drosophila and mouse p53 null embryos, for example, several cell types undergo apoptosis in response to irradiation (IR), but with slower kinetics than p53+/+ cells (Frenkel et al., 1999; Wichmann et al., 2006).
Candidate p53-independent apoptotic pathways have surfaced from in vitro studies. ATM/ATR-activated ABL, Chk1, and Chk2 can upregulate p73 protein levels in genotoxically challenged p53-deficient cells, restoring transactivation of PUMA and other proapoptotic p53 targets (Gong et al., 1999; Roos and Kaina, 2006; Urist et al., 2004; Yuan et al., 1999). p53-independent coupling of DNA damage to mitochondria can also occur through translocation of the nuclear orphan protein Nur77 into the cytosol, activation of nuclear and/or cytosolic caspase-2, or de novo ceramide synthesis by mitochondrial ceramide synthase, all converging on caspase-3 activation (Kolesnick and Fuks, 2003; Li et al., 2000; Lin et al., 2004; Zhivotovsky and Orrenius, 2005). Other p53-independent processes, involving MAPKs (e.g., SAPK/JNKs, p38) and the transcription factors E2F1, NF-κB, and FOXO1 couple DNA damage to caspase-3 activation by upregulating extrinsic pathway genes including CASP8, whose product activates caspase-3 in a mitochondria-dependent (Bcl-2-inhibitable) or -independent manner (Afshar et al., 2006; Huang et al., 2006; Kasibhatla et al., 1998; Yount et al., 2001). Whether the p53-independent pathways identified in vitro operate in vivo remains an active field of investigation.
Radio/chemoresistant p53 mutant human cancer cell lines can be induced to die after genotoxic stress by pharmacologic or RNAi targeting of DNA damage-response (DDR) kinases involved in intra-S and/or G2/M checkpoint control, including ATM, ATR, Chk1, Chk2, Polo-like kinases (Plks) (reviewed in Castedo et al., 2004a), and most recently, the p38/MAPK-activated kinase MAPKAPK2 (MK-2) (Reinhardt et al., 2007). Such treatments might spare cells endowed with wild-type p53, presumably because their intact G1 checkpoint enables them to repair and thus survive DNA damage (Zhou and Bartek, 2004). Although the sensitization of—and selectivity for—p53 mutant cells is at the root of anticancer strategies that target DDR kinases, none of these concepts have been rigorously tested in an animal model, and the underlying cell death mechanism is unclear.
To accelerate the discovery of physiologic p53-independent DDRs, we generated p53 mutant zebrafish lines for use in whole organism-based modifier genetic screens (Berghmans et al., 2005). Zebrafish faithfully recapitulate mammalian intrinsic and extrinsic apoptotic signaling (reviewed in Pyati et al., 2007). The zebrafish p53M214K allele (or p53e7, for mutation in exon 7) affects a conserved amino acid residue within a region of the DNA-binding domain corresponding to a mutational hotspot in human cancer, producing a transactivation-dead p53 variant. Homozygosity for p53e7 recapitulates key traits associated with p53 deficiency in mammalian systems, including a strong tumor-prone phenotype, lack of G1-checkpoint function, and widespread cellular radioresistance (Berghmans et al., 2005).
Here we identify chk1 as a gene whose loss restores IR-induced apoptosis in live p53 mutant zebrafish embryos, and then use in vivo epistasis analyses to dissect the underlying mechanism. Unlike previously identified p53-independent apoptotic pathways, which restore caspase-3 activation downstream of defective p53, Chk1 depletion activates an ATM/ATR-caspase-2 axis that bypasses the mitochondrial and death-receptor pathways. We show that this “Chk1-suppressed” pathway can be triggered in p53-deficient or BCL2-overexpressing human tumor cells, providing a mechanistic rationale for the use of Chk1 inhibitors in cancer therapy.
RESULTS
A Morpholino Screen for Suppressors of p53e7/e7 Radioresistance Identifies chk1
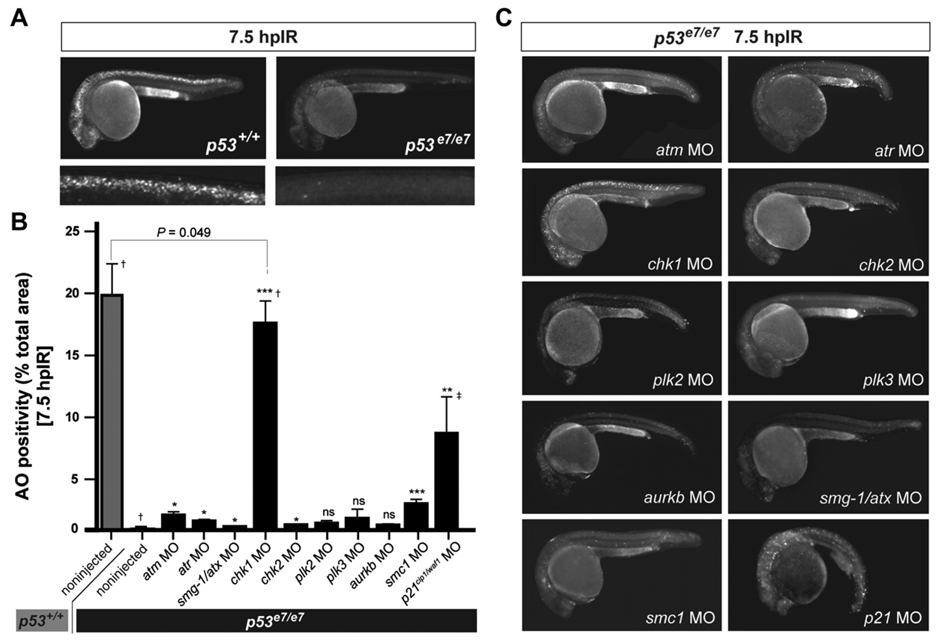
p53e7/e7 mutant zebrafish embryos are refractory to DNA damage-induced cell death, as demonstrated by a nearly complete lack of acridine orange (AO) labeling in the brain and spinal chord of live embryos examined 7.5 hr after whole-body IR delivered at 18 hr postfertilization (hpf) (Figure 1A and Figure 2A). We used morpholino antisense oligonucleotides (MOs) to knock down eight zebrafish S- and G2-checkpoint kinases and two nonkinase checkpoint regulators (p21waf1/cip1 and smc1) in p53e7/e7 mutant embryos. We assessed the ability of each knockdown to restore cell death (AO reactivity) at 7.5 hr post-IR (hpIR).
Figure 1. A Morpholino Screen Identifies chk1 as a Loss-of-Function Suppressor of p53e7/e7-Associated Radioresistance.
(A) Live 25 hpf embryos of the indicated genotypes stained with AO at 7.5 hpIR (12.5 Gy). Anterior, left. Note the complete absence of AO labeling in the brain and spinal cord of the irradiated p53 mutant.
(B) MO screen for loss-of-function suppressors of p53e7/e7-associated radioresistance. Noninjected and 1 cell-stage MO-injected embryos were irradiated at 18 hpf (12.5 Gy). AO uptake by cells was quantified by analyzing images of whole embryos photographed live at 7.5 hpIR (y axis) (images as in C). Injected MOs are indicated along the x axis. Bars are color coded and refer to the genetic background used for injections (gray, p53+/+; black, p53e7/e7). AO staining was quantified in ≥ 8 embryos per knockdown, with 50 or more embryos scored per knockdown (except †> 1000); ‡, embryos showed developmental defects. All data are reported as means ± SEM. Statistical significance versus the noninjected p53e7/e7 response: * p < 0.05; ** p < 0.005; *** p < 0.0005; ns (not significant), (two-tailed Student’s t test).
(C) Fluorescent images of AO-labeled, live p53 mutants injected with indicated MOs and representative of the phenotypes quantified in (B).
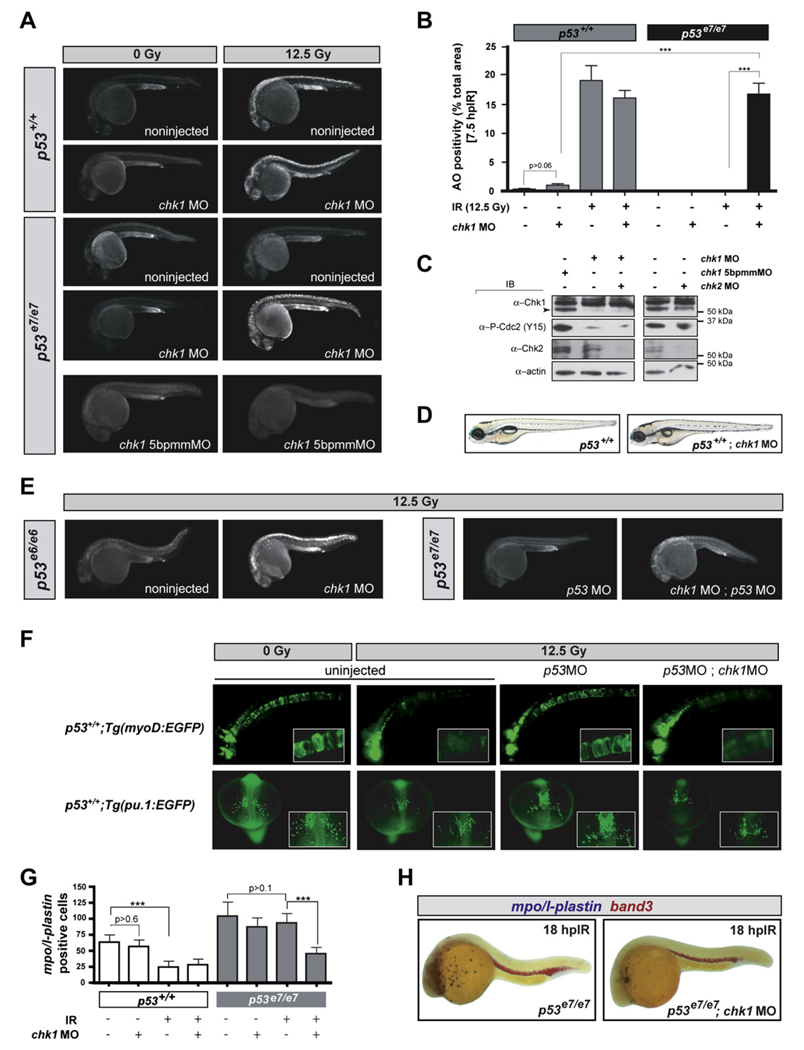
Figure 2. chk1 Knockdown Radiosensitizes p53 Mutants but Is Otherwise Compatible with Normal Zebrafish Development.
(A) Fluorescent images of representative embryos of indicated genotypes +/− chk1 MO after 0 or 12.5 Gy IR; 5bpmmMO (5 base pair mismatch MO).
(B) Quantified AO responses of indicated genotypes with or without IR (12.5 Gy) and chk1 MO. Gray bars, p53+/+ background; black bars, p53e7/e7 background. AO staining was quantified in ≥ 8 embryos per condition, with > 1000 embryos scored. All data are reported as means ± SEM *** p < 0.0001 (two-tailed Student’s t test).
(C) Western blots comparing the levels of Chk1, Chk2, and phosphorylated Cdc2 (Tyr15) in protein lysates from 25.5 hpf embryos injected with the indicated MOs.
(D) Nonirradiated p53+/+;chk1MO larva photographed live at 5 days postfertilization (dpf) show no apparent developmental defects but is slightly delayed (smaller swim bladder). Such larvae survived to adulthood.
(E) Fluorescent images of representative irradiated embryos of indicated genotypes. p53e6 is the N168K mutation, corresponding to human residue 200. p53MO, MO against the p53 5′UTR.
(F) Fluorescent images of live transgenic embryos injected with the indicated MOs at the 1-cell stage and expressing EGFP in the notochord (top row, embryos photographed at 24 hpf) or in myeloid progenitors (bottom row, embryos photographed at 16.5 hpf). Tg(myoD:EGFP) and Tg(pu. 1:EGFP) embryos were treated with or without IR (12.5 Gy) at 18 hpf and 10 hpf, respectively. Insets, higher magnification views of GFP-expressing cells. Top row, lateral views, anterior to the left. Bottom row, dorsal views, anterior facing down.
(G) Quantification of myeloid cells in 28 hpf embryos generated as indicated (x axis) and processed as in (H). Gray bars, p53+/+ background; black bars, p53e7/e7 background. mpo/l-plastin staining was quantified in ≥ 15 embryos per condition. Data are reported as means ± SD ** p < 0.001, *** p < 0.0001 (two-tailed Student’s t test). Note that while the numbers of mpo/l-plastin-positive cells are reduced ~3-fold in IR-treated versus untreated p53+/+ embryos; they are unchanged in treated versus untreated p53e7/e7 embryos. Also note that chk1 knockdown induces an average 2-fold reduction in myeloid cell numbers in the p53e7/e7 background after IR.
(H) Images of representative 28 hpf embryos of indicated genotypes processed for in situ hybridization of mpo and l-plastin riboprobes (blue, differentiated granulocytes and monocytes) and band 3 (red, erythrocytes). Note the specific reduction in number of granulocytes/monocytes.
Single knockdowns of all genes tested, excluding plk2, plk3, and aurkb, radiosensitized p53 mutants with variable efficiency (Figures 1B and 1C). Whereas atm, atr, smg-1/atx, and chk2 deficiencies restored only minor AO reactivity averaging 1%–5% of the p53+/+ response, chk1 knockdown resulted in a staining pattern that closely resembled wild-type (87.7% of the p53+/+ response, p < 0.0001; see also Figures 2A and 2B). Enhanced IR-induced cytotoxicity resulted specifically from chk1 knockdown because (1) injections of a chk1 mismatch MO failed to radiosensitize p53 mutants (Figure 2A, bottom panels); (2) the chk1 MO resulted in a robust reduction of the endogenous Chk1 protein pool, correlating with impaired Chk1 activity (Figure 2C); and (3) a specific inhibitor of human Chk1, but not inhibitors of ATM or Chk2, phenocopied the effects of chk1 MO (see Figure 7). As would be expected from Chk1 loss, p53e7/e7;chk1MO embryos lacked the IR-induced G2/M checkpoint (Figures S1A–S1D). chk1 MO also fully radiosensitized p53e6 (p53N168K) homozygotes (Berghmans et al., 2005) and p53 morphants (Langheinrich et al., 2002) lacking p53 protein (Figure 2E), including in mesodermal derivatives (Figures 2F–2H). Together, these results provide in vivo evidence that Chk1 depletion is sufficient to restore IR sensitivity to p53 mutant cells.
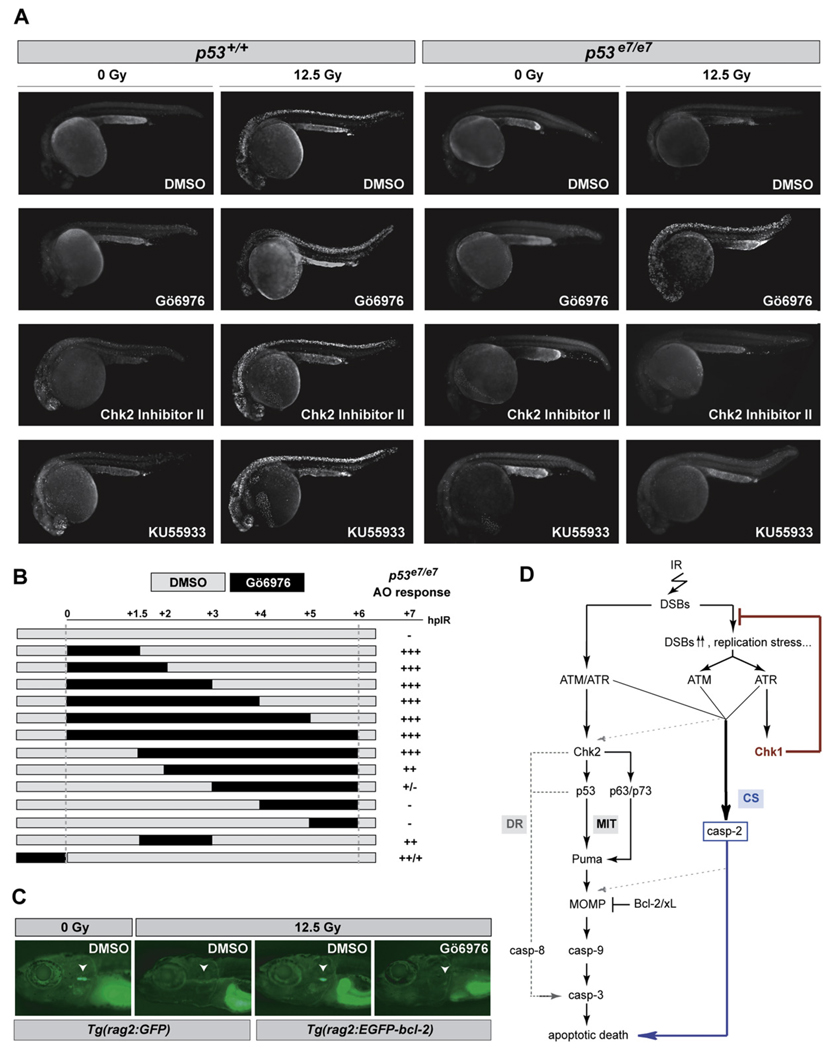
Figure 7. Effects of Gö6976 in Zebrafish In Vivo Models of p53 Loss and bcl-2 Gain.
(A) Fluorescent images of AO-labeled embryos of indicated genotypes photographed at 25.5 hpf. Embryos were exposed to 0 or 12.5 Gy IR and to the indicated drugs at 18 hpf. Gö6976, specific Chk1 inhibitor (1 µM). Chk2 Inhibitor II, specific Chk2 inhibitor (10 µM); KU55933, specific ATM inhibitor (10 µM). Note the range of toxicities in nonirradiated p53+/+ embryos treated with KU55933 or Chk2 Inhibitor II, with strong AO labeling preferentially localized in the brain and eyes (first column, third and fourth rows), as opposed to the Gö6976-treated embryo (first column, second row). Inversely, note the strong IR-induced AO labeling in the Gö6976-treated p53 mutant (last column, second row), but the lack of staining in the mutants treated with KU55933 or Chk2 Inhibitor II (last column, third and fourth rows).
(B) Temporal requirement for Chk1 loss with respect to IR. p53 mutant embryos were exposed to Gö6976 for the indicated times. AO staining was quantified on a scale from “−” to “+++”with “−” representing the p53 mutant response and “+++” the response of sibling mutants treated with Gö6976 for 6 hr (~500-fold greater response).
(C) Fluorescent images of 9 dpf zebrafish larvae carrying the indicated transgene. Larvae were treated with 0 Gy or 15 Gy IR at 5 dpf and were exposed to Gö6976 (or DMSO as control) for a total of 5 days starting at 4 dpf. White arrowhead indicates the position of the thymus. Note the absence of detectable GFP in the Gö6976-treated Tg(rag2:EGFP-bcl-2) irradiated larva.
(D) Simplified model for the vertebrate apoptotic response to DNA damage, highlighting the p53-independent pathway normally blocked by IR-activated Chk1 (CS, for Chk1-suppressed pathway), which is distinct from the classical intrinsic (mitochondrial, MIT) and extrinsic (death-receptor, DR) pathways. See text for details.
Transient Chk1 Depletion Is Viable in the Absence of IR
Chk1 is essential for fly and mouse development, with homozygous null mutants succumbing to major cell cycle defects (Fogarty et al., 1997; Liu et al., 2000). We therefore tested whether the cytotoxicity of chk1 knockdown in zebrafish p53 mutants was strictly IR dependent. Indeed, chk1 depletion had no apparent effect on normal zebrafish development and viability, in either the p53+/+ or p53e7/e7 background (Figures 2A and 2D; compare bars 1 and 2 in Figure 2B). Western blots performed with an anti-zebrafish Chk1 antibody revealed a substantial knockdown of the protein (Figure 2C). Yet chk1 morphants harbored residual levels of Chk1 activity, as shown by weak but persistent levels of phosphorylated Cdc2 (Figure 2C). These results demonstrate that transient depletion, as opposed to persistent total loss (Liu et al., 2000), of Chk1 function, is tolerable by vertebrate cells in vivo and compatible with long-term organismal viability. Crucially, however (as already shown above), such transient downregulation is sufficient to restore the IR-induced cell-death response in p53 mutants (Figure 1B, 1C, Figure 2A, and 2B).
Irradiated p53e7/e7;chk1MO Embryos Undergo Caspase-3-Independent Cell-Autonomous Apoptosis
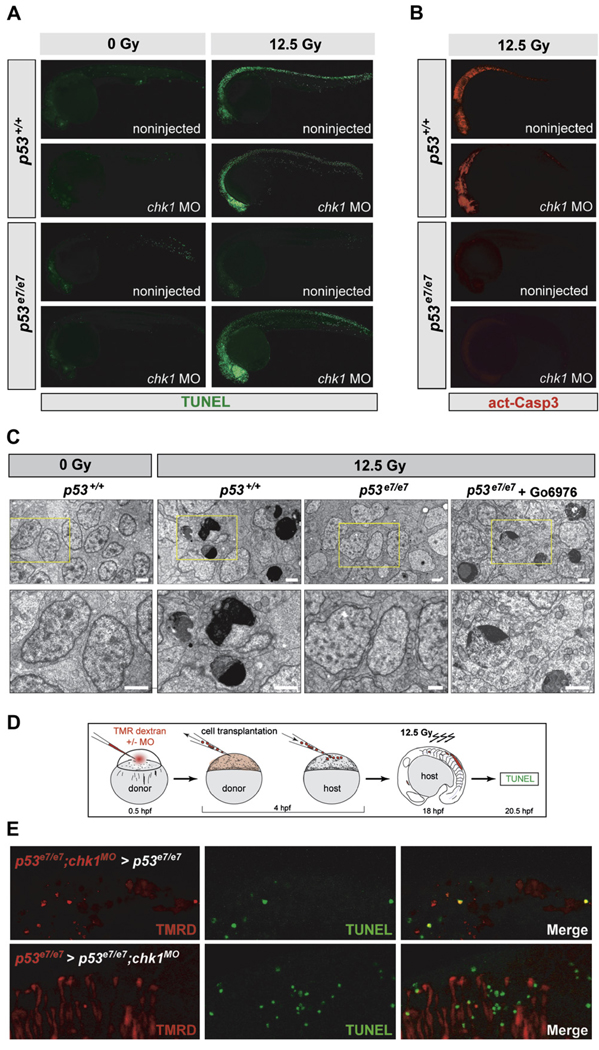
Chk1 knockdown might restore a wild-type response to IR (that is, classical intrinsic apoptosis) (Kratz et al., 2006) or trigger a different cell-death program in p53 mutants. To distinguish between these possibilities, we first analyzed two hallmarks of apoptosis: TUNEL-positive DNA fragmentation and cleaved caspase-3 (as well as electron micrographs) in embryos fixed at 7.5 hpIR. AO labeling of irradiated p53e7/e7;chk1MO embryos (Figure 1C and Figure 2A) correlated with high levels of TUNEL labeling throughout the CNS, similar to findings in irradiated p53+/+ embryos (Figure 3A). Multiple cells in the irradiated CNS of p53+/+ and Chk1-depleted p53e7/e7 embryos also showed similar ultrastructural manifestations of apoptosis (e.g., chromatin compaction/segregation and cytoplasmic condensation; Figures 3C and S2). Surprisingly, however, while irradiated p53+/+ embryos exhibited strong immunostaining for active caspase-3, irradiated p53e7/e7;chk1MO embryos did not and showed no increase in active caspase-3 levels compared to p53 single mutants, which were devoid of both TUNEL and active caspase-3 (Figures 3A and 3B). Thus, the p53-independent cell death-inducing DDR triggered by Chk1 depletion is a caspase-3-independent apoptotic pathway (see also Figure S2F).
Figure 3. IR-induced p53-Independent Apoptosis after Chk1 Loss Occurs Cell Autonomously and Independently of Caspase-3.
(A) Fluorescent images of 25 hpf embryos (anterior, left). TUNEL reactivity after IR (0 or 12.5 Gy) recapitulates live AO labeling (see Figure 2A).
(B) Embryos from the same experiment immunostained with an antiactivated-Caspase-3 antibody. Note the absence of immunoreactivity in the irradiated p53e7/e7;chk1MO embryo.
(C) Electron micrographs (sagittal sections) of the CNS in embryos of indicated genotypes after 0 or 12.5 Gy IR. Gö6976 is a specific Chk1 inhibitor (see Figure 5–Figure 7). Lower row, 2.5× closeups on the areas boxed in yellow in upper panels. Note multiple cells with stereotypical chromatin compaction/segregation in columns 2 and 4, as opposed to healthy nuclei in columns 1 and 3. Organelles and plasma membrane are intact in the shown Chk1-inhibited irradiated p53 mutant cell, as expected from an apoptotic (as opposed to necrotic) event. See Figure S2 for more details. Scale bar, 2 µM.
(D) Experimental procedure for the generation of the genetic chimeras shown in (E).
(E) 5-µm-thick confocal sections of spinal cords in irradiated chimeras. TMR Dextran (red) marks the donor cells. TUNEL shown in green. First row, cells from a p53e7/e7 embryo that was injected with the chk1 MO at the 1-cell stage (p53e7/e7;chk1MO embryo) transplanted into a p53e7/e7 host. Second row, p53e7/e7 cells transplanted into a p53e7/e7;chk1MO host.
To determine the cell autonomy of the Chk1-antagonized pathway, we generated genetic chimeras (Figures 3D and S3). While p53e7/e7;chk1MO cells grafted into p53e7/e7 hosts often stained TUNEL-positive after IR (39%, n = 102), neighboring host cells did not (Figure 3E, upper panels). In the reciprocal experiment, p53e7/e7 cells transplanted into p53e7/e7;chk1MO hosts remained TUNEL negative within an otherwise TUNEL-positive environment (Figure 3E, lower panels). Therefore, IR-induced TUNEL reactivity of transplanted cells strictly depends on Chk1 dosage, occurs irrespective of the cellular environment, and has very little (if any) influence on neighboring cells. The Chk1-suppressed apoptotic DDR pathway thus functions in a cell-autonomous manner.
Chk1 Blocks a Mitochondria and Death Receptor-Independent Apoptotic Pathway Involving ATM, ATR, and Caspase-2
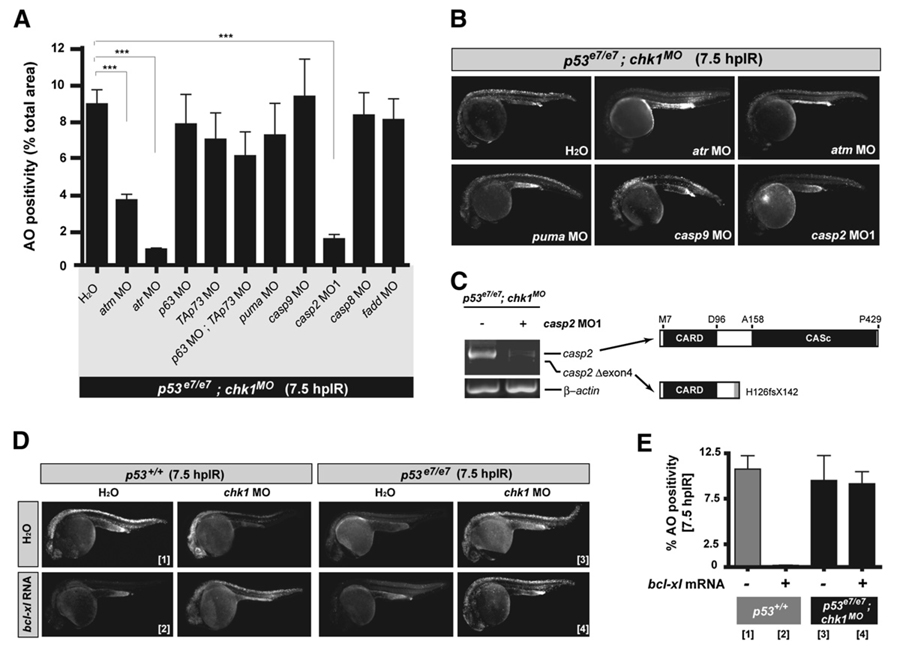
To molecularly characterize the newly identified apoptotic pathway, we capitalized on the unique advantages of zebrafish embryos for in vivo epistasis analyses. Specifically, we knocked down or forced the expression of candidate pathway contributors in p53e7/e7;chk1MO embryos and assessed the effects on IR-induced cell death using the AO assay.
atm and atr single knockdowns severely impaired chk1 knockdown-mediated radiosensitization of zebrafish p53 mutants, indicating that ATM and ATR are nonredundantly required to activate the pathway after DNA damage (Figures 4A and 4B; 60% and 90% reductions in cell death levels, respectively). In contrast, single or combined knockdowns of p63 and/or p73 led to (at best) a ~30% reduction in AO staining compared to control p53e7/e7;chk1MO embryos (Figure 4A). This attenuation was reminiscent of the effects of chk2 knockdown (Figure S4A, compare bars 3 and 5) and may reflect a role for p53-independent Chk2-p63/p73 apoptotic pathways (Bernassola et al., 2005; Urist et al., 2004) in a subset of cell deaths in irradiated p53e7/e7;chk1MO embryos. It is unlikely that these effects result from weaker MO efficiencies, because the chk2, p63, and p73 MOs lead to stronger gene knockdowns than the atm and atr MOs (Figures 2C and S5A–S5C; also see Rentzsch et al., 2003). The inability of Chk2, p63, and p73 to account for the majority of cell death events in irradiated p53e7/e7;chk1MO embryos implies that ATM and ATR operate predominantly within a novel apoptotic pathway, which we have designated “Chk1-suppressed pathway” (CS in Figure 7D).
Figure 4. Genetic Dissection of the Zebrafish Chk1-Suppressed Apoptotic Pathway.
(A) Quantified AO labeling in spinal cords of 12.5 Gy-exposed p53e7/e7;chk1MO embryos injected with H2O (bar on the far left) or the indicated MOs (x axis). AO staining was quantified in ≥8 embryos per MO with a total of ≥100 embryos scored. All data are means ± SEM *** p < 0.0001 (two-tailed Student’s t test).
(B) Fluorescent images of representative embryos from the experiments shown in (A).
(C) At left, RT-PCR of casp2 transcripts from embryos either injected or not injected with casp2 MO. At right, schematics of caspase-2 protein variants (top, wild-type protein; bottom, predicted protein translated from exon 4-deleted transcripts).
(D) Fluorescent images of embryos of the indicated genotypes with or without IR (12.5 Gy at 18 hpf), chk1 MO, or bcl-xl mRNA. Numbers in brackets refer to the corresponding bars in (E).
(E) Quantified AO responses (n ≥ 8) for embryos of indicated genotypes +/− bcl-xl mRNA. Gray bars, p53+/+ background; black bars, p53e7/e7;chk1MO background. Numbers in brackets refer to the representative-embryo images in (D). Data are means ± SEM.
To test whether the mitochondrial apoptotic axis contributes to the Chk1-suppressed pathway, we first knocked down the proapoptotic BH3-only family member Puma. puma depletion did not significantly affect AO labeling of irradiated p53e7/e7; chk1MO embryos (Figures 4A and 4B) at a puma MO concentration that is otherwise sufficient to completely block IR-induced apoptosis in p53+/+ zebrafish embryos (Figure S6) (Kratz et al., 2006). Similarly, a dose of bcl-xl mRNA that completely blocked cell death 7.5 hpIR in wild-type embryos failed to affect the AO reactivity of irradiated p53e7/e7;chk1MO embryos (Figures 4D and 4E; p53+/+ + bcl-xl, 0.035% of the mean p53+/+ response; p53e7/e7;chk1MO + bcl-xl, ~95% of the mean p53e7/e7;chk1MO response). casp9 knockdown also lacked an effect (Figures 4A, 4B, and S5E). Thus, two major regulators of mitochondrial membrane permeabilization (Puma and Bcl-xL), as well as the main initiator and executioner caspases acting downstream of mitochondria (caspase-9 and caspase-3, see Figure 3), are dispensable for the Chk1-suppressed apoptotic pathway.
The death-receptor axis bypasses the requirement for mitochondria and caspase-9, suggesting that it could contribute to the Chk1-suppressed pathway. In addition, a link between Chk1 loss and caspase-8 activation has recently been observed (Xiao et al., 2005). Even so, the death-receptor pathway converges on caspase-3 activation via caspase-8 (Hengartner, 2000). This caspase-3 recruitment contrasts with the caspase-3 independence of the pathway we identified, which, together with the established cell autonomy of the new pathway (Figure 3E), argues against a role for DNA damage-induced extrinsic signaling downstream of chk1 depletion. Indeed, the AO reactivity of p53e7/e7;chk1MO;casp8MO zebrafish embryos did not differ from that of p53e7/e7;chk1MO specimens (Figures 4A and S5D). Blocking death-receptor signaling with a fadd (Fas Associated protein with Death Domain) MO (Eimon et al., 2006) also failed to affect AO staining (Figure 4A). Thus, extrinsic signaling—like mitochondrial signaling—does not appear to play an important role downstream of chk1 loss.
The sole caspase whose depletion blocked the Chk1-suppressed pathway was caspase-2, a poorly characterized yet highly conserved caspase with features of both initiator and executioner caspases (Zhivotovsky and Orrenius, 2005). In three separate experiments, p53e7/e7;chk1MO;casp2MO1 embryos consistently showed a mean 6-fold decrease in AO labeling compared with p53e7/e7;chk1MO embryos (~16% of the mean p53e7/e7;chk1MO response, p < 0.0001; Figures 4A and 4B). casp2 MO1, which targets the splice donor site of intron 4, led to marked reductions in casp2 mRNA levels and to aberrant residual transcripts lacking exon 4 (Figure 4C). A second casp2 MO reduced IR-induced death in p53e7/e7;chk1MO embryos (Figure S5F and S5G), and a mismatch version of casp2 MO1 had no effect (data not shown). Altogether, these epistasis analyses identify a novel atm/atr-casp2 apoptotic program as a key mechanism through which Chk1 depletion radiosensitizes p53 mutant zebrafish embryos without recruiting the classical mitochondrial and death-receptor pathways (Figure 7D).
The Chk1-Suppressed Apoptotic Pathway Is Conserved In Human Cancer Cells
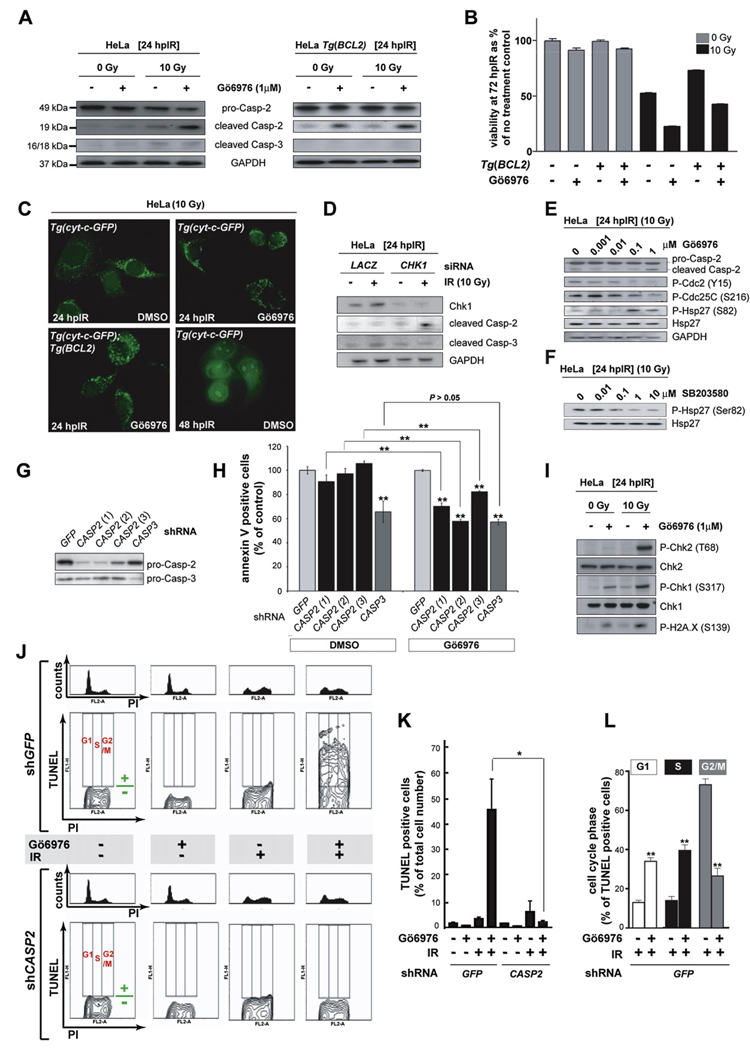
We next investigated whether the DNA damage-induced apoptotic pathway suppressed by Chk1 in zebrafish is conserved in human cancer cells defective in p53 signaling. To inhibit Chk1 in these cells, we used the indolocarbazole small molecule Gö6976 (Kohn et al., 2003), which has greater specificity than the commonly used Chk1 inhibitor UCN-01 (reviewed in Kawabe, 2004 and see below). In HeLa cells (in which the p53 protein pool is depleted by HPV-18 E6), caspase-2 cleavage was readily apparent at 24 hpIR in the presence of Gö6976 (Figure 5A). This effect was synergistic because neither IR nor Gö6976 alone caused substantial increases in cleaved caspase-2 levels compared to basal levels observed in control cells. In addition, caspase-2 cleavage tightly correlated with a strong radiosensitizing effect (~50% increase in cell death; Figure 5B, compare bars 5 and 6; see also Figure 5J, 5K, and Figure 6C). By contrast, the levels of cleaved caspase-3 in Gö6976-treated cells at 24 hpIR were negligible and did not differ from those observed in irradiated cells not exposed to the inhibitor (Figure 5A). Furthermore, both caspase-2 cleavage and concomitant cellular radiosensitization were insensitive to overexpression of human BCL2, whereas caspase-3 cleavage was completely removed in this context (Figures 5A and 5B, compare bars 7 and 8). Synergistic activation of caspase-2 by Gö6976 and IR did not elicit or involve cytochrome c release from the mitochondria at 24 hpIR (Figure 5C). Together, these findings demonstrate that Chk1 inhibition and IR synergize to activate caspase-2 and trigger BCL2- and mitochondria-independent cell death in p53-defective human cells, consistent with our zebrafish data.
Figure 5. The Chk1-Suppressed Pathway Is Conserved in HeLa Cells.
(A) Western blots comparing the levels of caspase-2 (pro and cleaved forms) and cleaved caspase-3 at 24 hpIR in lysates from HeLa cells carrying or not carrying a BCL2 transgene (Tg[BCL2]) and treated with or without IR (10 Gy) or Chk1 inhibitor (Gö6976, 1 µM).
(B) Analysis of HeLa cell survival at 72 hpIR (0 Gy versus 10 Gy) in the presence or absence of Gö6976 and/or BCL2. Gö6976 radiosensitizes the cells ~2-fold regardless of the BCL2 transgene (compare bars 5 and 6, and bars 7 and 8). Note that BCL2 is functional (i.e., radioprotective) in these experiments (compare lanes 5 and 7). Data are means ± SEM.
(C) Fluorescent images of HeLa Tg(Cyt-c-GFP) cells with or without Tg(BCL2) or Gö6976 at 24 or 48 hpIR (10 Gy). Note the punctate GFP patterns in all 24 hpIR samples and the diffuse GFP pattern in the 48 hpIR sample.
(D) Levels of cleaved caspase-2 and caspase-3 at 24 hpIR (10 Gy) in HeLa cells transfected with LACZ or CHK1 siRNAs at 72 hr before IR.
(E) Western blots comparing the activities of Chk1 (Cdc2 phosphorylation at Tyr15 and CDC25C phosphorylation at Ser216) and MK-2 (Hsp-27 phosphorylation at Ser82) following exposure to IR and increasing concentrations of Gö6976.
(F) MK-2 phosphorylates Hsp-27 in HeLa cells. Western blot of lysates from irradiated HeLa cells exposed to increasing concentrations of the p38MAPK specific inhibitor SB203580 (Reinhardt et al., 2007), showing a dose-dependent reduction in phosphorylated Hsp-27.
(G) Knockdown efficiencies of the indicated shRNAs as measured by western blots with anticaspase-2 and anticaspase-3 antibodies.
(H) Effects of GFP, CASP2, and CASP3 shRNAs on apoptotic cell numbers at 48 hpIR as measured by AnnexinV (+) / PI (−) staining of HeLa cells treated with 10 Gy with or without Gö6976 (1 µM). For each shRNA, the average apoptotic cell number (given as % of GFP shRNA control) is shown. All data are means ± SD ** p < 0.01 (two-tailed Student’s t test). Asterisks on top of bars refer to comparisons with GFP shRNA.
(I) Synergistic activation of ATM and ATR by Gö6976 and IR. Western blots comparing the activities of ATM (Chk2 phosphorylation at Thr68) and ATR (Chk1 phosphorylation at Ser317) after 0 or 10 Gy IR with or without Gö6976 (1 µM). Levels of DNA damage were detected with an antiphospho-H2A.X antibody. (J) Cell-cycle distribution of HeLa cells undergoing Chk1-suppressed apoptosis. HeLa cells harboring GFP or CASP2 shRNAs and treated with or without 10 Gy IR with or without Gö6976 (1 µM), as indicated, were fixed at 48 hpIR and stained for TUNEL and PI. For each shRNA line, upper panels show PI-single histograms and lower panels show PI/TUNEL double-staining images. Cell-cycle phases and threshold for TUNEL positivity are indicated in red and green, respectively, in each no-treatment control images.
(K) Quantification of the TUNEL stains shown in (J). Data are means ± SEM * p < 0.05 (two-tailed Student’s t test).
(L) Quantified data from experiment in (J) expressed as means ± SEM ** p < 0.002 (two-tailed Student’s t test). White bars indicate cells dying in G1 phase. Black bars indicate cells dying in S phase. Grey bars, cells dying in G2 phase.
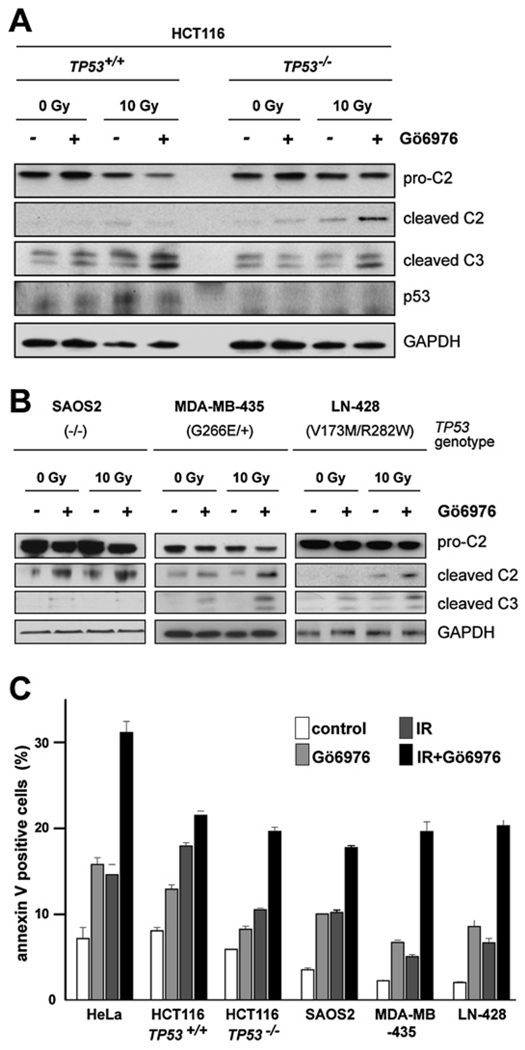
Figure 6. Influence of Genetic Background on Gö6976-Mediated Radiosensitization of Human Cancer Cells.
(A) Western blots comparing the levels of caspase-2 (pro and cleaved forms) and cleaved caspase-3 in 24-hpIR lysates from TP53+/+ and TP53−/− HCT116 cells that were treated with or without IR (10 Gy) or Gö6976 (1 µM).
(B) Analysis as in (A) of the SAOS2 (left), MDA-MB-435 (middle), and LN-428 (right) lines.
(C) Apoptotic cell numbers at 48 hpIR as measured by Annexin V (+) / PI (−) staining of the indicated cell lines treated with 0 or 10 Gy IR with or without Gö6976 (1 µM). Data are means ± SEM. See Figure S9 for a CHK1 shRNA-mediated phenocopy of Gö6976 in TP53−/− HCT116 cells.
Before testing whether caspase-2 is required for cell-death induction, we verified the specificity of Gö6976 as an inhibitor of Chk1. CHK1 siRNA, but not a LACZ control siRNA, induced caspase-2 cleavage in concert with IR at 24 hr posttreatment but did not stimulate caspase-3 processing at this stage, in accord with the effects of Gö6976 (Figure 5D). Furthermore, while Gö6976 inhibited Chk1 in a dose-dependent manner, it did not impair MK-2 activity (Figure 5E), in contrast with UCN-01 (Reinhardt et al., 2007).
To test whether caspase-2 is required for Gö6976-mediated HeLa cell killing after IR, we used three independent CASP2 shRNAs that produced strong and specific knockdowns (Figure 5G). Each shRNA significantly reduced apoptosis induction at 48 hr after IR + Gö6976 treatment, but not after IR treatment alone (Figure 5H; see also Figures 5J and 5K). In contrast, the reduction in apoptosis observed upon CASP3 knockdown at 48 hr (stage at which caspase-3 is eventually cleaved) was independent of Gö6976, as CASP3 shRNA led to a similar attenuation after IR treatment alone (Figure 5H). The severity of the apoptotic blockades caused by the CASP2 shRNAs (20%–45% reductions, p < 0.01 for each) correlated with their respective knockdown efficiencies (Figures 5G and 5H). Altogether, these results demonstrate that caspase-2—but not caspase-3—is specifically required for the increase in IR-induced apoptosis observed in Chk1-inhibited human cancer cells, similar to its requirement in irradiated p53e7/e7;chk1MO zebrafish embryos.
If the ATM/ATR-caspase-2 apoptotic axis in zebrafish is well conserved in human cells, ATM and ATR should be activated after Chk1 inhibition in irradiated HeLa cells, similar to caspase-2. Indeed, IR + Gö6976 treatment led to synergistic increases in phosphorylated Chk2 at Thr68 (readout for ATM activity) and phosphorylated Chk1 at Ser317 (readout for ATR activity) (Figure 5I). Elevated ATM and ATR activities correlated with increased levels of DNA damage in the IR + Gö6976-treated cells, as indicated by an increased abundance of phosphorylated H2A.X (Figure 5I). Even though Chk2 was strongly activated in this context (Figure 5I), a specific CHK2 siRNA failed to block caspase-2 activation (Figures S4B and S4C). This result substantiates our prediction (based on epistasis analyses in zebrafish) that the Chk1-suppressed pathway is Chk2 independent. Taken together, our experiments in HeLa cells show that apoptosis after IR + Gö6976 treatment of human cells involves ATM and ATR activation, is independent of Chk2, Bcl-2, mitochondria, and caspase-3, but requires caspase-2 activation and function (Figure 7D). Thus, the zebrafish Chk1-suppressed pathway is evolutionarily conserved in human cancer cells.
Chk1 Inhibition Induces a Sustained Increase in S Phase Apoptosis after IR
MK-2 depleted Tp53−/− MEFs (mouse embryonic fibroblasts) undergo DNA damage-induced apoptosis exclusively during mitosis (Reinhardt et al., 2007). In contrast, pH3/TUNEL double-labeling of irradiated p53e7/e7;chk1MO zebrafish embryos indicates that Chk1-suppressed apoptosis operates predominantly during the cell-cycle interphase (Figure S7). To further address this question in HeLa cells, we used TUNEL/PI double labeling, such that PI fluorescence intensity indicated the cell-cycle status of TUNEL-positive cells. The Chk1-suppressed pathway was readily detected in this assay as a dramatic, entirely caspase-2-dependent increase in TUNEL-positive cells after IR + Gö6976 treatment (Figures 5J and 5K). Moreover, the cell-cycle distribution of TUNEL-positive cells was significantly different upon IR + Gö6976 treatment compared to IR alone. Whereas only a minority of TUNEL-positive cells were in G1 or S phase in the presence of normal Chk1 activity (~15% each), these fractions increased 2.5-fold upon Chk1 inhibition (~35% and ~40% of TUNEL-positive cells, respectively; p < 0.002 for each) (Figure 5L). Thus, in human cells, the Chk1-suppressed pathway operates predominantly during the S and G1 phases of the cell cycle. Importantly, Gö6976-induced S phase apoptosis increased with time and the effect was sustained for at least 72 hpIR (Figure S8), indicating an important role for Chk1 in preventing DNA damage-induced apoptosis during DNA replication (see Discussion).
Chk1 Inhibition Sensitizes Multiple Cancer Cell Lines to IR-Induced Apoptosis
We next asked whether the Chk1-suppressed pathway could be triggered in human cancer cell lines other than HeLa, including TP53+/+ and TP53−/− HCT116 colon carcinoma cells (Bunz et al., 1998), the SAOS2 osteosarcoma line (p53 null), the MDA-MB-435 breast cancer line (heterozygous for the G266E mutation), and the V173M/R282W, transheterozygous LN-428 glioblastoma line (Ishii et al., 1999). All TP53 null or mutant lines tested displayed increases in caspase-2 cleavage and apoptosis after IR + Gö6976 treatment (Figures 6A–6C). While these observations substantiate the results in HeLa cells, we noted several differences. First, TP53+/+ HCT116 cells failed to engage the Chk1-suppressed pathway, as evidenced by their inability to cleave caspase-2 after IR + Gö6976 treatment (see also Discussion). Instead, caspase-3 was activated in a p53-dependent manner (likely via classical mitochondrial signaling), followed by a modest increase in apoptosis. Intriguingly, TP53 mutant MDA-MB-435 and LN-428 cells also engaged caspase-3 cleavage after IR + Gö6976 treatment (Figure 6B). This caspase-3 cleavage could result from either p53-independent apoptotic processes operating in parallel with the newly identified pathway (such as the Chk2-p63/73 axis), or from caspase-2 itself triggering the classical intrinsic or extrinsic apoptotic pathways (dashed arrows in Figure 7D) (Castedo et al., 2004b; Nutt et al., 2005; Shin et al., 2005; Tu et al., 2006; Zhivotovsky and Orrenius, 2005). However, it is unlikely that any of these alternative pathways substitute for the Chk1-suppressed pathway in HeLa, SAOS2, or TP53−/− HCT116 lines, in which caspase-3 cleavage is undetectable or minimal after IR + Gö6976 treatment (Figure 5A, Figure 6A, and 6B).
Gö6976 Selectively Radiosensitizes Zebrafish In Vivo Models of p53 Loss and bcl-2 Gain
To investigate the effects of Gö6976 in vivo, we evaluated it—together with specific Chk2 and ATM inhibitors—in the zebrafish system. Drug toxicity was monitored by scoring the AO reactivity of inhibitor-treated, but nonirradiated, p53+/+ embryos (left column in Figure 7A). Unless otherwise indicated, the inhibitors were applied at 17 hpf (1 hr before IR) for a total of 6 hr.
Whereas relatively toxic doses of KU55933 (an ATM inhibitor) (Hickson et al., 2004) and Chk2 Inhibitor II (Arienti et al., 2005) only modestly radiosensitized p53 mutants, a nontoxic dose of Gö6976 (1 µM) restored a complete apoptotic response to IR (Figure 7A). The effects of Gö6976 were almost fully penetrant, with 95% of Gö6976-treated p53 mutants (n = 210) showing a marked IR-induced apoptotic response. In fact, as short as a 1.5 hr exposure to Gö6976 immediately after IR was sufficient to phenocopy the 0–24 hpf chk1 depletion obtained via chk1 MO (Figure 7B). Similar to chk1 morphants, nonirradiated p53+/+ embryos treated with Gö6976 developed into normal adults without overt signs of spontaneous tumorigenesis or other pathologies.
The BCL2/XL independence of the Chk1-suppressed pathway (Figure 4D and Figure 5B) suggests that Chk1 inhibitors could prove valuable in radio/chemosensitizing malignancies that overexpress BCL2 family members, including follicular lymphoma. Tg(rag2: EGFP-bcl-2) larvae are characterized by highly radioresistant T and B cells at 9 dpf (Langenau et al., 2005). Systemic treatment with Gö6976 suppressed T cell radioresistance in a mean 58% of these larvae (n = 26) compared to none of the DMSO-treated larvae (n = 28), without any apparent adverse effects (Figure 7C). Together with our human cell culture studies, the in vivo analysis of Gö6976 in zebrafish supports the concept that human tumors with mutational alteration of p53 or its attendant downstream pathway—in other words, most human cancers—would be selectively sensitized by Chk1 inhibitors to DNA damage-induced apoptosis.
DISCUSSION
We have identified an evolutionarily conserved apoptotic process distinct from the mitochondrial and death-receptor axes. This ATM/ATR-caspase-2 pathway is triggered by DNA damage in cells in which Chk1 activity is simultaneously compromised. The pathway is insensitive to p53 loss and BCL2/XL gain—two of the most common genetic abnormalities in human cancers—can be targeted with Chk1 inhibitors and assessed on the basis of caspase-2 cleavage.
The ATM/ATR-caspase-2 pathway is triggered by the combined effects of IR and Chk1 inhibition, but not by either stimulus alone. Our data show increased levels of γH2A.X and synergistic activation of ATM and ATR in irradiated cells lacking Chk1, indicating that Chk1 acts upstream of ATM and ATR to moderate the accumulation of DNA damage. This might suggest that increasing IR doses would eventually substitute for Chk1 inhibitor treatment by matching a DNA-damage threshold necessary for caspase-2 activation. However, even very high levels of DNA damage induced by IR doses of up to 150 Gy (Gray) did not robustly induce apoptosis in zebrafish p53 mutants with functional Chk1 (Figure S10). Thus, the ATM/ATR-caspase-2 pathway cannot mount a nonspecific response to excess damage, but rather is obligatorily tied to Chk1 activity. An involvement of Chk1’s essential or damage-dependent checkpoint functions during DNA replication (Bartek et al., 2004; Lam et al., 2004; Sorensen et al., 2003; Syljuasen et al., 2005) seems likely given the sustained rise in S phase apoptosis observed in IR + Chk1 inhibitor-treated HeLa cells. A role for replication stress in triggering the ATM/ATR-caspase-2 pathway gains support from observations that Chk1-depleted cells exposed to replication inhibitors undergo p53- and Chk2-independent apoptosis during S phase (Rodríguez and Meuth, 2006). Also, caspase-2 is the sole caspase whose proform resides in the nucleus (Zhivotovsky et al., 1999), where it is stabilized by cyclin D3, a positive regulator of the G1/S transition (Mendelsohn et al., 2002). We propose that tight control of the ATM/ATR-caspase-2 pathway by Chk1 contributes to the decision to live (arrest and repair) or die in replicating cells suffering DNA damage.
ATM and ATR, while both necessary for activation of the Chk1-suppressed pathway, are individually insufficient for this function (Figure 4A). ATM and ATR might phosphorylate different substrates, each being essential for caspase-2 activation and susceptible to Chk1 regulation. However, neither caspase-2 nor its proposed activators, including PIDDosome components PIDD (p53-induced protein with death domain) and RAIDD (RIP-associated ICH-1/CED-3 homologous protein with a death domain) (Tinel and Tschopp, 2004), belong to the list of 700 potential ATM/ATR substrates (Matsuoka et al., 2007). A more likely interpretation is that ATM and ATR serve different sensory functions, with ATM responding primarily to IR-induced double-strand breaks while ATR predominantly senses signals resulting from reduced Chk1 activity, such as replication stress (Cuadrado et al., 2006; Syljuasen et al., 2005).
The ATM/ATR-caspase-2 pathway may serve as a mechanism that ensures the demise of cells carrying potentially harmful DNA lesions in the absence of proper genome-surveillance activity (as provided by Chk1) (Lam et al., 2004). Such a function might help explain why CHK1 mutations, despite fueling genomic instability (Lam et al., 2004), are paradoxically rare in human cancers (Bartek and Lukas, 2003). Our demonstration that the Chk1-suppressed pathway can operate in both the absence and presence of p53, as revealed in irradiated p53+/+;chk1MO;bcl-xl embryos and in irradiated p53+/+;Tg(rag2:EGFP-bcl-2) larvae treated with Gö6976, disqualifies it as a “backup” program (Roos and Kaina, 2006) operating only in cells that lack p53. Rather, we propose that it constitutes an alternative, perhaps primitive, response to DNA injury that evolved independently of the p53 network. Intriguingly, however, TP53+/+ and TP53−/− HCT116 cells differed in their response to IR + Gö6976 treatment, in that caspase-2 but not caspase-3 cleavage was actively inhibited in the TP53+/+ cells, via an apparent downregulation of procaspase-2 levels (Figure 6A; see also Baptiste-Okoh et al., 2008). Thus, a form of crosstalk might have evolved to link these p53-dependent and -independent apoptotic pathways, similar to that described for caspase-dependent and -independent pathways (Colell et al., 2007).
Chk1 inhibitors can radio/chemosensitize p53-deficient human tumor cells in vitro, leading to clinical trials of their activity in cancer patients (Kawabe, 2004; Tse et al., 2007; Zhou and Bartek, 2004). Because of the embryonic lethality of Chk1−/− mice, however, it has remained unclear whether the potency and selectivity of radio/chemosensitization observed in vitro will apply in vivo. Our findings in zebrafish using the Chk1 inhibitor Gö6976 and chk1 morphants, which retain residual levels of Chk1 activity, indicate that levels of Chk1 inhibition not toxic to normal cells are sufficient to sensitize p53 mutant cells to IR-induced apoptosis within a living vertebrate. Our results also identify cleavage of caspase-2 as a candidate biomarker for Chk1-targeting treatments. The isolation of such specific biomarkers remains a pressing challenge in the development and optimal use of targeted cancer therapeutics (Cully et al., 2006; Tse et al., 2007). Finally, our results unexpectedly predict that in addition to tumors with altered p53 activity, those with other types of prosurvival alterations that block mitochondrial signaling downstream of p53, such as BCL2-expressing follicular lymphomas, would respond favorably to combination therapy with Chk1 inhibitors.
EXPERIMENTAL PROCEDURES
Zebrafish Stocks
The homozygous viable p53M214K and p53N168K mutant lines (p53e7 and p53e6, respectively, in this article) (Berghmans et al., 2005), and the Tg(rag2:GFP) (Langenau et al., 2003), Tg(rag2:EGFP-bcl-2) (Langenau et al., 2005), Tg(pu. 1:GFP) (Hsu et al., 2004), and Tg(myoD:EGFP) (Yang et al., 2004) transgenic lines were used and maintained at 28.5°C by standard methods. For experimental purposes, irradiated p53e6/e6 embryos were incubated for 6 hr at 37°C (restrictive temperature for N168K).
Morpholino Screen and Epistasis Analyses Using the Live AO Assay
MOs were obtained from Gene Tools, LLC. MO sequences, target sites, working concentrations, knockdown efficiencies, selected references, and injection procedures, as well as detailed protocols for AO staining of live embryos and the ImageJ-based quantification method, are listed in Table S1, Figure S5, and the Supplemental Experimental Procedures.
Human Cancer Cell Lines, siRNAs, and shRNAs
The HeLa, SAOS2, MDA-MB-435, and LN-428 cell lines, the TP53+/+ and TP53−/− HCT116 isogenic pair (Bunz et al., 1998), and the Cyt-c-GFP transgenic, 2H18 HeLa-derived lines (Goldstein et al., 2000), carrying or not carrying a BCL2 transgene, were cultured in DMEM medium (GIBCO) supplemented with 15% fetal bovine serum (FBS). siRNAs were transfected in HeLa cells using Hiperfect (QIAGEN) according to the manufacturer’s instructions. Cells were exposed to IR +/− Gö6976 at 48 or 72 hr posttransfection. shRNA knockdown analyses were performed as previously described (Moffat et al., 2006). See Supplemental Data for more details, siRNA and shRNA sequences, and all other experimental procedures.
ACKNOWLEDGMENTS
We thank John R. Gilbert for editorial review. We gratefully acknowledge George Kourkoulis and Barry Baker for zebrafish care, Bert Vogelstein and Rosalind Segal for the HCT116 and LN-428 cell lines; William Hahn for the CASP2(1), CASP3, and GFP shRNAs; Thomas Diefenbach and Louise Trakimas for assistance with confocal and electron microscopy; and Yebin Ahn and Peter Schow for assistance with FCM analysis. This work was supported by NIH grants HL-88664 (S.S., A.T.L.) and AI47891 (D.R.G.), the Susan G. Komen Breast Cancer Foundation (R.D.K.), and the A-T Children’s Project (S.K.).
Footnotes
SUPPLEMENTAL DATA
Supplemental Data include ten figures, one table, Supplemental Experimental Procedures, and Supplemental References and can be found online at http://www.cell.com/cgi/content/full/133/5/864/DC1/.
REFERENCES
- Afshar G, Jelluma N, Yang X, Basila D, Arvold ND, Karlsson A, Yount GL, Dansen TB, Koller E, Haas-Kogan DA. Radiation-induced caspase-8 mediates p53-independent apoptosis in glioma cells. Cancer Res. 2006;66:4223–1232. doi: 10.1158/0008-5472.CAN-05-1283. [DOI] [PubMed] [Google Scholar]
- Arienti KL, Brunmark A, Axe FU, McClure K, Lee A, Blevitt J, Neff DK, Huang L, Crawford S, Pandit CR, et al. Checkpoint kinase inhibitors: SAR and radioprotective properties of a series of 2-arylbenzimidazoles. J. Med. Chem. 2005;48:1873–1885. doi: 10.1021/jm0495935. [DOI] [PubMed] [Google Scholar]
- Baptiste-Okoh N, Barsotti AM, Prives C. Caspase 2 is both required for p53-mediated apoptosis and downregulated by p53 in a p21-dependent manner. Cell Cycle. 2008;7 doi: 10.4161/cc.7.9.5805. Published online February 19, 2008. [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell Biol. 2004;5:792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CD, Morris JP, Liu TX, Schulte-Merker S, Kanki JP, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc. Natl. Acad. Sci. USA. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernassola F, Oberst A, Melino G, Pandolfi PP. The promyelocytic leukaemia protein tumour suppressor functions as a transcriptional regulator of p63. Oncogene. 2005;24:6982–6986. doi: 10.1038/sj.onc.1208843. [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G. Cell death by mitotic catastrophe: a molecular definition. Oncogene. 2004a;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Yakushijin K, Horne D, Medema R, Kroemer G. The cell cycle checkpoint kinase Chk2 is a negative regulator of mitotic catastrophe. Oncogene. 2004b;23:4353–4361. doi: 10.1038/sj.onc.1207573. [DOI] [PubMed] [Google Scholar]
- Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, Fitzgerald P, Guio-Carrion A, Waterhouse NJ, Li CW, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Cuadrado M, Martinez-Pastor B, Fernandez-Capetillo O. “ATR activation in response to ionizing radiation: still ATM territory.”. Cell Div. 2006;1:7. doi: 10.1186/1747-1028-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- Eimon PM, Kratz E, Varfolomeev E, Hymowitz SG, Stern H, Zha J, Ashkenazi A. Delineation of the cell-extrinsic apoptosis pathway in the zebrafish. Cell Death Differ. 2006;13:1619–1630. doi: 10.1038/sj.cdd.4402015. [DOI] [PubMed] [Google Scholar]
- Fogarty P, Campbell SD, Abu-Shumays R, Phalle BS, Yu KR, Uy GL, Goldberg ML, Sullivan W. The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 1997;7:418–426. doi: 10.1016/s0960-9822(06)00189-8. [DOI] [PubMed] [Google Scholar]
- Frenkel J, Sherman D, Fein A, Schwartz D, Almog N, Kapon A, Goldfinger N, Rotter V. Accentuated apoptosis in normally developing p53 knockout mouse embryos following genotoxic stress. Oncogene. 1999;18:2901–2907. doi: 10.1038/sj.onc.1202518. [DOI] [PubMed] [Google Scholar]
- Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG, Jr, Levrero M, Wang JY. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- Hsu K, Traver D, Kutok JL, Hagen A, Liu TX, Paw BH, Rhodes J, Berman JN, Zon LI, Kanki JP, et al. The pu.1 promoter drives myeloid gene expression in zebrafish. Blood. 2004;104:1291–1297. doi: 10.1182/blood-2003-09-3105. [DOI] [PubMed] [Google Scholar]
- Huang H, Regan KM, Lou Z, Chen J, Tindall DJ. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science. 2006;314:294–297. doi: 10.1126/science.1130512. [DOI] [PubMed] [Google Scholar]
- Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC, Van Meir EG. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999;9:469–479. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol. Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- Kawabe T. G2 checkpoint abrogators as anticancer drugs. Mol. Cancer Ther. 2004;3:513–519. [PubMed] [Google Scholar]
- Kohn EA, Yoo CJ, Eastman A. The protein kinase C inhibitor Gö6976 is a potent inhibitor of DNA damage-induced S and G2 cell cycle checkpoints. Cancer Res. 2003;63:31–35. [PubMed] [Google Scholar]
- Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- Kratz E, Eimon PM, Mukhyala K, Stern H, Zha J, Strasser A, Hart R, Ashkenazi A. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ. 2006;13:1631–1640. doi: 10.1038/sj.cdd.4402016. [DOI] [PubMed] [Google Scholar]
- Lam MH, Liu Q, Elledge SJ, Rosen JM. Chk1 is haploinsufficient for multiple functions critical to tumor suppression. Cancer Cell. 2004;6:45–59. doi: 10.1016/j.ccr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Langenau DM, Traver D, Ferrando AA, Kutok JL, Aster JC, Kanki JP, Lin S, Prochownik E, Trede NS, Zon LI, et al. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299:887–890. doi: 10.1126/science.1080280. [DOI] [PubMed] [Google Scholar]
- Langenau DM, Jette C, Berghmans S, Palomero T, Kanki JP, Kutok JL, Look AT. Suppression of apoptosis by bcl-2 overexpression in lymphoid cells of transgenic zebrafish. Blood. 2005;105:3278–3285. doi: 10.1182/blood-2004-08-3073. [DOI] [PubMed] [Google Scholar]
- Langheinrich U, Hennen E, Stott G, Vacun G. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr. Biol. 2002;12:2023–2028. doi: 10.1016/s0960-9822(02)01319-2. [DOI] [PubMed] [Google Scholar]
- Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cul XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- Mendelsohn AR, Hamer JD, Wang ZB, Brent R. Cyclin D3 activates Caspase 2, connecting cell proliferation with cell death. Proc. Natl. Acad. Sci. USA. 2002;99:6871–6876. doi: 10.1073/pnas.072290599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Nutt LK, Margolis SS, Jensen M, Herman CE, Dunphy WG, Rathmell JC, Kornbluth S. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell. 2005;123:89–103. doi: 10.1016/j.cell.2005.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyati UJ, Look AT, Hammerschmidt M. Zebrafish as a powerful vertebrate model system for in vivo studies of cell death. Semin. Cancer Biol. 2007;17:154–165. doi: 10.1016/j.semcancer.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11:175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch F, Kramer C, Hammerschmidt M. Specific and conserved roles of TAp73 during zebrafish development. Gene. 2003;323:19–30. doi: 10.1016/j.gene.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Rodríguez R, Meuth M. Chk1 and p21 cooperate to prevent apoptosis during DNA replication fork stress. Mol. Biol. Cell. 2006;17:402–412. doi: 10.1091/mbc.E05-07-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Shin S, Lee Y, Kim W, Ko H, Chol H, Kim K. Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. EMBO J. 2005;24:3532–3542. doi: 10.1038/sj.emboj.7600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen CS, Syljuasen RG, Falck J, Schroeder T, Ronnstrand L, Khanna KK, Zhou BB, Bartek J, Lukas J. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, Helleday T, Sehested M, Lukas J, Bartek J. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol. Cell. Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clin. Cancer Res. 2007;13:1955–1960. doi: 10.1158/1078-0432.CCR-06-2793. [DOI] [PubMed] [Google Scholar]
- Tu S, McStay GP, Boucher LM, Mak T, Beere HM, Green DR. In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat. Cell Biol. 2006;8:72–77. doi: 10.1038/ncb1340. [DOI] [PubMed] [Google Scholar]
- Urist M, Tanaka T, Poyurovsky MV, Prives C. p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev. 2004;18:3041–3054. doi: 10.1101/gad.1221004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat. Rev. Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Wichmann A, Jaklevic B, Su TT. Ionizing radiation induces caspase-dependent but Chk2- and p53-independent cell death in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2006;103:9952–9957. doi: 10.1073/pnas.0510528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Xue J, Sowln TJ, Rosenberg SH, Zhang H. A novel mechanism of checkpoint abrogation conferred by Chk1 downregulation. Oncogene. 2005;24:1403–1411. doi: 10.1038/sj.onc.1208309. [DOI] [PubMed] [Google Scholar]
- Yang HW, Kutok JL, Lee NH, Piao HY, Fletcher CD, Kanki JP, Look AT. Targeted expression of human MYCN selectively causes pancreatic neuroendocrine tumors in transgenic zebrafish. Cancer Res. 2004;64:7256–7262. doi: 10.1158/0008-5472.CAN-04-0931. [DOI] [PubMed] [Google Scholar]
- Yount GL, Afshar G, Ries S, Korn M, Shalev N, Basila D, McCormick F, Haas-Kogan DA. Transcriptional activation of TRADD mediates p53-independent radiation-induced apoptosis of glioma cells. Oncogene. 2001;20:2826–2835. doi: 10.1038/sj.onc.1204393. [DOI] [PubMed] [Google Scholar]
- Yuan ZM, Shioya H, Ishiko T, Sun X, Gu J, Huang YY, Lu H, Kharbanda S, Weikchselbaum R, Kufe D. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature. 1999;399:814–817. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B, Orrenius S. Caspase-2 function in response to DNA damage. Biochem. Biophys. Res. Commun. 2005;331:859–867. doi: 10.1016/j.bbrc.2005.03.191. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B, Samali A, Gahm A, Orrenius S. Caspases: their intracellular localization and translocation during apoptosis. Cell Death Differ. 1999;6:644–651. doi: 10.1038/sj.cdd.4400536. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat. Rev. Cancer. 2004;4:216–225. doi: 10.1038/nrc1296. [DOI] [PubMed] [Google Scholar]