Abstract
Background
HCV may increase the risk of hepatopancreaticobiliary tumors other than hepatocellular carcinoma (HCC). Previous case-control studies indicated a possible association between HCV and intrahepatic cholangiocarcinoma (ICC). Little is known about the association between HCV and extrahepatic cholangiocarcinoma (ECC) or pancreatic cancer.
Methods
We conducted a cohort study including 146,394 HCV-infected and 572,293 HCV-uninfected patients who received care at Veterans Affairs health care facilities. Patients with two visits between 1996 and 2004 with HCV infection were included, as were up to four matched HCV-uninfected subjects for each HCV-infected subject. Risks of ICC, ECC, pancreatic cancer, and HCC were assessed using proportional hazards regression.
Findings
In the 1.37 million person-years of follow-up, which began 6 months after the baseline visit, there were 75 cases of ECC, 37 cases of ICC, 617 cases of pancreatic cancer, and 1679 cases of HCC. As expected, the risk of HCC associated with HCV was very high (hazard ratio [HR], 15.09; 95% confidence interval [95% CI], 13.44, 16.94). Risk for ICC was elevated with HCV infection 2.55; 1.31, 4.95), but risk for ECC was not significantly increased (1.50; 0.60, 1.85). Adjustments for cirrhosis, diabetes, inflammatory bowel disease, hepatitis B, alcoholism, and alcoholic liver disease did not reduce the risk for ICC below twofold. The risk of pancreatic cancer was slightly elevated (1.23; 1.02, 1.49), but was attenuated after adjusting for alcohol use, pancreatitis, and other variables.
Conclusions
Findings indicated HCV infection conferred a more than twofold elevated risk of ICC. Absence of an association with ECC was consistent in adjusted and unadjusted models. A significant association with pancreatic cancer was erased by alcohol use and other variables.
Keywords: hepatitis c virus; neoplasms, hepatic; neoplasms, pancreatic; cholangiocarcinomas; choledochal cyst
INTRODUCTION
Hepatitis C virus (HCV) causes chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC). The prevalence of HCV infection in the United States (U.S.) general population is estimated to be 1.6%, and an estimated 4.1 million infected persons live in the U.S. (1). HCV infection is more common in U.S. military veterans who use the Veterans Affairs (VA) medical system, where approximately 5% are infected with HCV (2).
Uncertainty persists over whether HCV causes cancers other than HCC, including intrahepatic cholangiocarcinoma (ICC), which is increasingly common, or extrahepatic cholangiocarcinoma (ECC). Apart from sclerosing cholangitis, risk factors for ICC are poorly understood (3). Several case-control studies have found HCV prevalence among persons with ICC as high as 36%, with odds ratios between 2 and 17, but little or no increase in the risk of ECC (4-7). However, these studies had limitations that precluded drawing definitive conclusions. Some studies have been too small to provide information regarding HCV’s association with cholangiocarcinoma subtypes. Hospital-based studies were also subject to selection bias. We recently evaluated risk factors for ICC in a population-based case-control retrospective study using Surveillance, Epidemiology, and End Results (SEER) and Medicare linked data and found HCV to be associated with a significantly increased risk of ICC but not ECC (8). However, that study was limited by the possibility of ascertainment bias related to differential HCV testing between cases and controls. In one cohort study from Denmark, the risk of cholangiocarcinoma was elevated 10-fold in patients with cirrhosis; however, the study did not report the etiology of cirrhosis (e.g., HCV) or the whether the cholangiocarcinoma was intrahepatic or extrahepatic(9). To our knowledge, there have been no cohort studies of HCV-infected persons to evaluate the risk of cholangiocarcinoma. Lastly, although one U.S. case-control study described a significant association between pancreatitis and HCV (10), little is known about the association between HCV and the risk of subsequent pancreatic cancer (11;12).
To characterize the association between HCV infection and the cholangiocarcinomas and pancreatic cancer as well as confirm its association with HCC, we conducted a retrospective cohort study including 146,394 HCV-infected U.S. veterans—the largest cohort of HCV-infected individuals assembled to date—and 572,293 HCV-uninfected U.S. veterans. We previously examined this cohort to determine HCV infection’s association with several hematological and solid organ malignancies (13).
METHODS
Data Sources
Data were collected on 718,687 inpatients and outpatients at VA medical facilities between October 1, 1988, and September 30, 2004, as previously described (13). The institutional review boards of the National Cancer Institute and Baylor College of Medicine and its affiliated hospitals approved this retrospective study, waiving informed consent requirements and releasing health information necessary for the analysis. Sources included inpatient records from more than 150 VA hospitals in the Patient Treatment File and outpatient records from any VA facility in the Outpatient Clinic File. Both contain demographic data, encounter dates, and up to 10 diagnoses identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) codes. Patients whose outpatient visits did not include physician care or oversight were excluded. We collected information about deaths from the Beneficiary Identification Records Locator System (14;15).
Definition of Study Cohorts
Using these sources, we identified 146,394 HCV-infected subjects and 572,293 HCV-uninfected subjects, as previously described (13). In brief, those identified as infected had diagnosis codes specifying HCV infection (070.41, 070.44, 070.51, 070.54, and V02.62) on two visits, including one outpatient visit. The baseline date for each subject was defined as the second visit on which the HCV code was recorded. Those identified as uninfected had no HCV infection diagnosis codes in the medical record on or before the matched subject’s baseline date. Inclusion criteria required uninfected subjects to be the same sex and age (+/- 1 year) as the matched subject, to have had a VA visit within 30 days of the matched subject’s baseline date and also a prior visit, and to have their visits match in type (outpatient or inpatient) those of the matched subject with HCV. Exclusion criteria and validation tests of cohort selection are described elsewhere (13).
Identification of Outcomes of Interest
Outcomes of interest, identified by ICD-9 code and recorded in the hospitalization or outpatient files, were ECC (156.1, 156.2, 156.8, 156.9), pancreatic adenocarcinoma (157.0, 157.1, 157.2, 157.3, 157.8, 157.9), ICC (155.1), and HCC (155.0). Only those cases of ECC, pancreatic cancer, ICC, and HCC detected 6 months after the baseline date were counted, so all calculations were based on cases diagnosed 6 months post baseline until the study’s end (September 30, 2004).
To improve the specificity of this approach, we used an algorithm in defining the outcomes of interest. Specifically, we required for ECC the absence of codes for pancreatic cancer, ICC, or HCC; for pancreatic cancer, the absence of codes for ECC, HCC, or ICC; for ICC, absence of HCC; and for HCC, absence of ICC or ECC. Patients with records indicating the diagnosis of HCC, ICC, ECC, or pancreatic cancer within a year (before or after) of the HCV diagnosis date were excluded. To validate the use of our algorithm in detecting ICC and ECC, we reviewed the medical records at the Michael E. DeBakey VA Medical Center in Houston of 12 patients with ICC and 48 with ECC identified by ICD-9 codes from VA administrative datasets, as well as 29 HCV-infected cases with pancreatic cancer identified from this study’s cohort and determined their classification based on a combination of clinical, radiological, and pathological criteria. Klatskin’s tumors were classified as extrahepatic cholangiocarcinoma. We compared the PPV for any codes with those of the developed algorithm as outlined above. For ICC, the PPV for any code was 75% (9/12) and for our algorithm was 86% (6/7). Similarly, for ECC, the PPV for any code was 69% (33/48), and for our algorithm it was 79% (27/34). Lastly, the PPV for codes of pancreatic cancer was 70% (17/28). We previously conducted similar validation studies for HCC (16).
Potential Confounders
Potential confounders were also ascertained in patients with and without HCV by the presence of diagnostic codes at any time starting in fiscal year 1988 for inpatient data or fiscal year 1997 for outpatient data and the end of the study (September 30, 2004). These included hepatitis B infection (070.22, 070.23, 070.32, 070.33, V02.61), alcoholic liver disease (571.0–571.3), cirrhosis (571.2, 571.5, 571.6), alcoholism (291, 303.0, 303.9, 305.0, V0401—V0405); acute pancreatitis (577.0), chronic pancreatitis (577.1), inflammatory bowel disease (555, 556), cholelithiasis (574.0, 574.1), choledocholithiasis (574.5), choledochal cyst (751.69), or record of a cholecystectomy (51.22, 51.23). We also examined the incidence of two conditions strongly associated with tobacco smoking, namely chronic obstructive pulmonary disease (COPD) (ICD-9: 491.2x, 493.2x, 496.xx), and lung cancer (ICD-9: 162.x) detected 6 months following baseline to examine the possibility of smoking as a potential confounder.
Statistical Analysis
We calculated incidence rates per 100,000 person-years for ICC, ECC, pancreatic cancer, and HCC by HCV status. We then compared risk in the two cohorts using Cox proportional hazards regression, adjusting for the matching factors (age, sex, baseline visit date, and type of visit). We also adjusted for race, era of military service (pre-Vietnam, Vietnam, post-Vietnam), number of inpatient and outpatient visits prior to baseline (a measure of use of VA medical services), and specific potential confounders for each malignancy (e.g., acute pancreatitis for pancreatic cancer, and cirrhosis for ICC). We generated Kaplan-Meier curves to illustrate the cumulative incidence of ECC, HCC, ICC, and pancreatic cancer in the two cohorts (HCV-infected and HCV-uninfected) beginning 6 months after baseline.
To gauge the robustness the findings, we conducted several sensitivity analyses. First, we repeated the regression analyses including all events post baseline, not just those that occurred after the first 6 months. We also repeated the regression analyses adjusting for potential confounders as defined only by the presence of diagnostic codes at or before the entry date into the cohort. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of the cohort, which included 146,394 subjects with HCV and 572,293 subjects without HCV (1:3.9 ratio), are presented in Table 1. All but 3.3% of the study population were men, and most subjects (56.2%) were 50 years of age or older (mean, 52 years). The majority of the cohort was white; however, there were more African Americans and subjects of unknown race in the HCV-infected group. Less than 20% of subjects had baseline dates before fiscal year 2000, and about two thirds were Vietnam era veterans. The HCV-uninfected group had more annual VA hospitalizations within the 5 years before the baseline date (mean 1.6 vs. 1.2) and more outpatient visits during the previous year (mean 19.5 vs.10.8). Beginning 6 months after baseline, subjects in the cohorts were followed for a mean of 2.3 years, with a total follow-up time of 280,676 person-years in the HCV-infected cohort and 1,095,911 person-years in the HCV-uninfected cohort.
Table 1.
Baseline Characteristics of Hepatitis C Virus-infected and Hepatitis C Virus-uninfected Veterans
| Characteristic | HCV-infected Cohort (N=146,394) |
HCV-uninfected Cohort (N=572,293) |
||
|---|---|---|---|---|
| Age in years, n (%) | ||||
| < 40 | 5,160 | (3.5) | 20,416 | (3.6) |
| 40–44 | 18,476 | (12.6) | 71,740 | (12.5) |
| 45–49 | 41,161 | (28.1) | 157,787 | (27.6) |
| 50–54 | 45,243 | (30.9) | 177,085 | (30.9) |
| 55–59 | 18,213 | (12.4) | 72,750 | (12.7) |
| 60–75+ | 18,141 | (20.9) | 72,515 | (12.7) |
| Mean age in years (SD) | 51.9 | (8.4) | 52.0 | (8.5) |
| Sex, n (%) | ||||
| Male | 141,695 | (96.8) | 553,659 | (96.7) |
| Race, n (%) | ||||
| White | 76,382 | (52.2) | 341,181 | (59.6) |
| African American | 35,625 | (24.3) | 117,998 | (20.6) |
| Other | 1,221 | (0.8) | 6,145 | (1.1) |
| Unknown | 33,166 | (22.7) | 106,969 | (18.7) |
| Baseline date, n (%) | ||||
| FY 1997–1999 | 25,066 | (17.1) | 98,799 | (17.3) |
| FY 2000 | 17,036 | (11.6) | 66,742 | (11.7) |
| FY 2001 | 23,144 | (15.8) | 90,084 | (15.7) |
| FY 2002 | 26,025 | (17.8) | 101,284 | (17.7) |
| FY 2003 | 27,305 | (18.7) | 106,431 | (18.6) |
| FY 2004 | 27,818 | (19.0) | 108,953 | (19.0) |
| Era of military service* | ||||
| Pre-Vietnam | 15,908 | (10.9) | 58,272 | (10.2) |
| Vietnam | 97,332 | (66.5) | 358,023 | (62.6) |
| Post-Vietnam and other | 33,135 | (22.6) | 155,952 | (27.3) |
| Selected comorbidities: | ||||
| Alcoholic liver disease | 15,448 | (10.6) | 17,232 | (3.0) |
| Alcoholism | 37,665 | (25.7) | 101,997 | (17.8) |
| Cirrhosis | 14,187 | (9.7) | 9,202 | (1.6) |
| Diabetes mellitus | 8,106 | (5.5) | 44,021 | (7.7) |
| Inflammatory bowel disease | 1,129 | (0.8) | 5,445 | (1.0) |
| Pancreatitis, acute | 4,244 | (2.9) | 11,886 | (2.1) |
| Pancreatitis, chronic | 2,764 | (1.9) | 6,898 | (1.2) |
| Cholelithiasis | 6,039 | (4.1) | 15,020 | (2.6) |
| Choledocholithiasis | 538 | (0.4) | 1,602 | (0.3) |
Data were missing on 19 hepatitis C virus-infected and 46 hepatitis C virus-uninfected veterans.
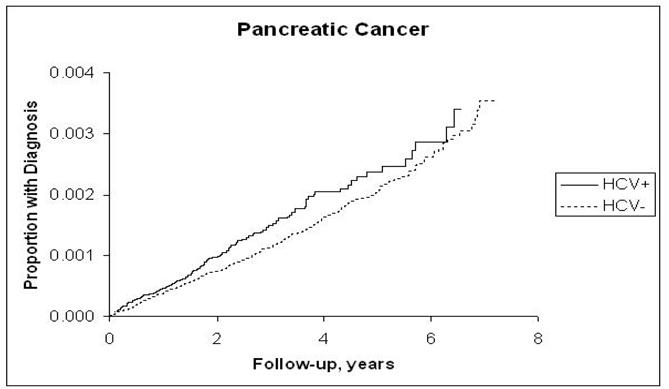
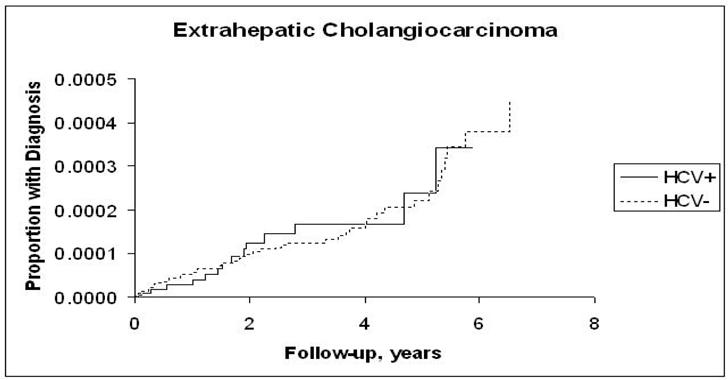
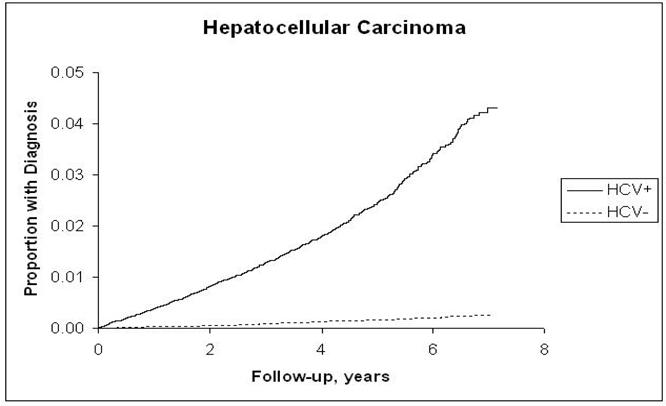
The incidence rates of ICC, ECC, pancreatic cancer, and HCC and the hazard ratios associated with HCV infection for each are presented in Table 2. Risk for ICC in the HCV-infected cohort although low (4 per 100,000 P-Y) was more than double that in the HCV-uninfected cohort (HR = 2.55; 95% CI, 1.31, 4.95) (Figure 1,a), and the risk for pancreatic cancer although high (40 per 100,000 P-Y) was less strongly but still significantly elevated in the HCV-infected cohort (HR = 1.23; 95% CI, 1.02, 1.49) (Figure 1.b). The proportion of patients with recorded cirrhosis was significantly higher (7/14, 50%) among HCV positive ICC cases than HCV negative ICC (3/23, 13%). Risk for ECC was not significantly elevated (HR = 1.03; 95% CI, 0.60, 1.85) (Figure 1.c). In contrast, HCC, included as a reference measure, had the highest incidence in HCV-infected patients (381.6/100,000 person-years), reflecting a 15-fold risk (HR = 15.09; 95% CI, 13.44, 16.94) (Figure 1.d). After further adjustments for race, era of military service, and use of VA services prior to baseline, the point estimate of the hazard ratios for ICC did not change substantially and remained elevated (HRs = 2.31–2.38). Similarly, there was no substantial change in the hazard ratio for pancreatic cancer, which also remained minimally but significantly elevated. The risk for ECC was not significantly associated with HCV after adjustment for matching variables or other factors.
Table 2.
Associations of Intrahepatic cholangiocarcinoma, Extrahepatic Cholangiocarcinoma, Pancreatic Carcinoma, and Hepatocellular Carcinoma with Hepatitis C Virus Infection among Veterans
| Outcome | Events/100,000 person-years (events) |
Hazard Ratios Comparing HCV-infected with HCV-uninfected Veterans |
|||
|---|---|---|---|---|---|
| HCV-infected cohort (N = 146,394) |
HCV-uninfected cohort (N = 572,293) |
HR (95%CI) Adjusted for Matching Variables‡ |
HR (95%CI) Adjusted for Matching Variables‡ and Previous VA Use |
HR (95%CI) Adjusted for Matching Variables, ‡ Previous VA Use, Race, and Era of Military Service |
|
| Intrahepatic cholangiocarcinoma |
4.0 (14) | 1.6 (23) | 2.55 (1.31, 4.95) | 2.38 (1.22, 4.65) | 2.31 (1.18, 4.54) |
| Extrahepatic cholangiocarcinoma |
4.3 (15) | 4.2 (60) | 1.05 (0.60, 1.85) | 1.24 (0.70, 2.20) | 1.25 (0.70, 2.22) |
| Pancreatic carcinoma |
40.3 (140) | 33.5 (477) | 1.23 (1.02, 1.49) | 1.32 (1.09, 1.60) | 1.26 (1.04, 1.53) |
| Hepatocellular carcinoma |
381.6 (1310) | 25.9 (369) | 15.09 (13.44, 16.94) | 14.80 (13.17, 16.63) | 14.75 (13.12, 16.59) |
Note: Hazard ratio, HR; HCV, hepatitis C virus; VA, Veterans Affairs; HCC, hepatocellular carcinoma; ECC, extrahepatic cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma.
Matching variables include age, sex, baseline visit date, and type of visit (inpatient or outpatient) for the baseline visit and a preceding visit.
Figure 1.
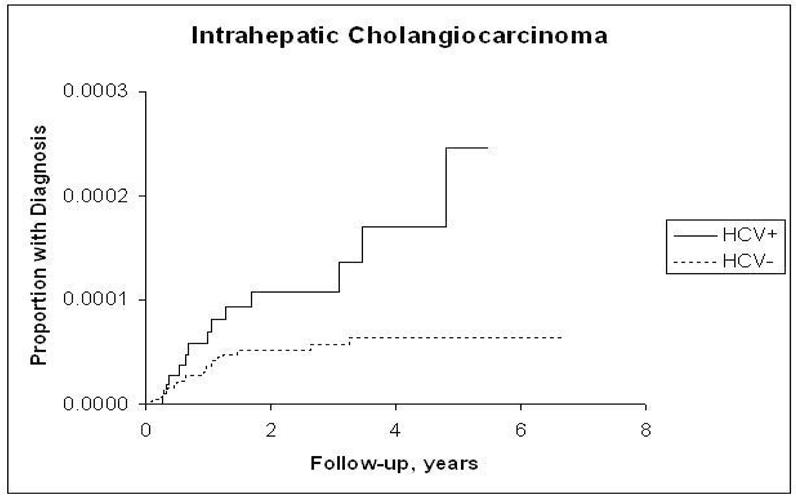
a: Kaplan-Meier Estimates of the Cumulative Incidence of Intrahepatic Cholangiocarcinoma by Hepatitis C Virus Status
b: Kaplan-Meier Estimates of the Cumulative Incidence of Pancreatic Cancer by Hepatitis C Virus Status
c: Kaplan-Meier Estimates of the Cumulative Incidence of Extrahepatic Cholangiocarcinoma by Hepatitis C Virus Status
d: Kaplan-Meier Estimates of the Cumulative Incidence of Hepatocellular Carcinoma by Hepatitis C Virus Status
To further verify our findings, we performed additional analyses adjusting for single variables or combinations of variables that might explain associations (Table 3). These analyses confirmed the strong association of HCV to ICC and HCC and the absence of an association with ECC. The association of HCV and pancreatic cancer was similar in magnitude but became nonsignificant when we controlled for variables related to alcohol (alcoholism, alcoholic liver disease, cirrhosis), pancreatitis (presence of acute or chronic pancreatitis), cholelithiasis or choledocholithiasis, HBV, or primary sclerosing cholangitis. Lastly, we performed similar analyses with confounders, as identified in Table 3, but changing their definition to incorporate codes in the records at or before baseline; the repeat analyses produced similar findings to the ones presented above (not shown).
Table 3.
Hazard Ratios of Intrahepatic Cholangiocarcinoma, Extrahepatic Cholangiocarcinoma, Pancreatic Carcinoma, and Hepatocellular Carcinoma Among Hepatitis C—infected and Hepatitis C—uninfected Veterans
| Variables for which Hazard Ratios Were Adjusted in Addition to Matching Variables* |
Intrahepatic Cholangio- carcinoma (N = 37) |
Extrahepatic Cholangio- carcinoma (N = 75) |
Pancreatic Carcinoma (N = 617) |
Hepatocellular Carcinoma (N = 1,679) |
|---|---|---|---|---|
| Matching variables only |
2.55 (1.31, 4.95) | 1.05 (0.60, 1.85) | 1.23 (1.02, 1.49) | 15.09 (13.44, 16.94) |
| Acute pancreatitis | 2.39 (1.23, 4.66) | 1.02 (0.58, 1.79) | 1.18 (0.97, 1.42) | 14.97 (13.34, 16.81) |
| Chronic pancreatitis | 2.53 (1.30, 4.92) | 0.99 (0.56, 1.75) | 1.18 (0.98, 1.42) | 15.02 (13.38, 16.86) |
| Alcoholism | 2.49 (1.28, 4.86) | 1.06 (0.60, 1.87) | 1.21 (1.00, 1.46) | 14.92 (13.29, 16.75) |
| Alcoholic liver disease |
2.07 (1.03, 4.16) | 0.72 (0.40, 1.31) | 1.18 (0.98, 1.44) | 12.89 (11.45, 14.50) |
| Cholecystectomy | 2.55 (1.31, 4.95) | 1.05 (0.60, 1.85) | 1.23 (1.02, 1.49) | 15.09 (13.44, 16.94) |
| Choledochal cyst | 2.48 (1.27, 4.83) | 1.05 (0.60, 1.85) | 1.23 (1.02, 1.48) | 15.09 (13.44, 16.93) |
| Choledocholithiasis | 2.48 (1.28, 4.83) | 1.04 (0.59, 1.83) | 1.21 (1.00, 1.46) | 15.07 (13.42, 16.91) |
| Cholelithiasis | 2.45 (1.26, 4.77) | 0.98 (0.56, 1.74) | 1.18 (0.97, 1.42) | 14.75 (13.14, 16.56) |
| Cirrhosis | 2.76 (1.41, 5.41) | 0.60 (0.32, 1.12) | 1.12 (0.92, 1.37) | 12.56 (11.15, 14.14) |
| Diabetes | 2.54 (1.31, 4.94) | 1.04 (0.59, 1.83) | 1.24 (1.03, 1.49) | 15.13 (13.48, 16.99) |
| Hepatitis B virus (HBV) |
2.13 (1.05, 4.29) | 0.95 (0.53, 1.71) | 1.19 (0.98, 1.44) | 14.81 (13.18, 16.63) |
| Inflammatory bowel disease (IBD) |
2.54 (1.31, 4.93) | 1.05 (0.60, 1.85) | 1.23 (1.02, 1.49) | 15.10 (13.45, 16.94) |
| Primary sclerosing cholangitis (PSC) |
2.32 (1.19, 4.52) | 1.05 (0.60, 1.86) | 1.20 (0.99, 1.45) | 15.06 (13.42, 16.91) |
| Diabetes + cirrhosis | 2.75 (1.40, 5.40) | 0.59 (0.32, 1.10) | ||
| IBD + PSC | 2.33 (1.20, 4.55) | 1.05 (0.60, 1.85) | ||
| HBV + cirrhosis | 2.30 (1.13, 4.68) | 0.56 (0.30, 1.06) | ||
| Cirrhosis + alcoholic liver disease + alcoholism |
2.20 (1.09, 4.44) |
Matching variables include age, sex, baseline visit date, and type of visit (inpatient or outpatient) for the baseline visit and a preceding visit.
The incidence rates (IR) for COPD and lung cancer were very similar between the two groups with or without HCV infection (2,656 per 100,000 P-Y vs. 2,668 per 100,000 P-Y for COPD) and (285 per 100, 000 P-Y vs. 293 per 100,000 P-Y for lung cancer). Therefore the incidence rate ratio was virtually equal to one for both conditions; 1.00 (0.98-1.03) for COPD and 0.99 (0.93-1.06) for lung cancer. Given this equal incidence of these two tobacco-smoking conditions, adjustment for these conditions in the HCV-pancreatic cancer analyses was unnecessary.
COMMENT
In this cohort study of 718,687 U.S. military veterans—the largest study ever conducted on the risk conferred by HCV infection for cholangiocarcinoma—we found in 146,394 veterans with HCV infection a significant 2.55-fold increase in the risk of ICC and a 15-fold increase in the risk of HCC but no evidence that HCV infection increases the risk of ECC. A small increase (HR = 1.23) detected in the risk of pancreatic cancer could have been due to confounding by alcohol use or other factors.
Cholangiocarcinoma is a highly fatal cancer of the biliary tree. In the U.S., approximately 5,000 new cases of cholangiocarcinoma are diagnosed annually, and these cases are equally distributed between ICC and ECC (17). Data from the SEER program indicate that the incidence of ICC tripled between 1975 and 1999 before declining slightly afterward, whereas the rates of ECC have been steady (18).
To our knowledge this is the first time a significant association has been detected between HCV and ICC in a large cohort study. This higher level of evidence supports and extends prior observations of an association from mostly hospital-based case-control studies. In a Japanese hospital-based study, investigators found that 36% of 50 patients with ICC but only 3% of 205 controls (other surgical patients who did not have primary liver cancer) were HCV seropositive (odds ratio [OR] = 16.87; 95% CI, 5.69, 50.00) (7). Korean investigators performed a case-control study comparing 41 cases of ICC with 406 controls without cancer and found that 13.8% of cases (3.5% of controls) were anti-HCV and 12.5% of cases (2.3% of controls) were HBsAg positive (6). An Italian case-control study (21 cases and 686 controls) found a positive association between ICC and HCV but not HBV (4). In that study, the prevalence of anti-HCV was 23.1% in cases compared with 6.1% in controls. The adjusted OR for ICC was 9.7 (95% CI, 1.6, 58.9) for anti-HCV. The previous case-control studies provide consistent evidence for a statistical association between HCV and ICC; however, case-control studies risk bias by their nature. Selection bias is a problem when controls without cancer are not representative of the base population from which cases were identified, and ascertainment bias exists when cases are more likely than controls to be tested for HCV. These biases were likely present and could have skewed the results toward finding a significant association. Further, the temporal relationship, the incidence rate of ICC, and the risk of ICC could not have been adequately examined with a case-control design.
Despite the doubling in relative risk, the absolute risk of ICC as measured by the incidence rate is quiet low (4 per 100,000) as compared to HCC (382 per 100,000) in this study. The only other cohort study we found showed a relatively high incidence of ICC among patients with HCV-related cirrhosis (5). The Japanese investigators reported that 2.3% of 600 patients with HCV-related cirrhosis developed ICC during an average follow-up of 7.2 years. That study, however, had the disadvantage of lacking internal controls free of HCV infection, and, thus, it was unable to derive relative risk estimates. Therefore, in aggregate, evidence points to an association between HCV and ICC, an association supported further by our cohort study findings.
HCV is a strong risk factor for HCC, and hepatocytes and cholangiocytes have the same progenitor cell; therefore, it could be postulated that HCV could induce carcinogenesis in both cell types by the same mechanism. In at least one study, HCV RNA has been detected in ICC tissue, which further supports the potential role of HCV infection in the pathogenesis of cholangiocarcinoma (3;19;20). Preliminary evidence indicates that HCV core protein (HCV C protein) may promote the cellular proliferation of hilar cholangiocarcinoma cells and inhibit apoptosis (19). It may be that effects are indirect as well, with HCV damaging the liver, causing cirrhosis, and thereby increasing ICC risk. Torbenson et al. reviewed 1058 cases of liver explants in patients with HCV as well as HCV uninfected control groups; cases of chronic biliary tract disease were excluded. Dysplasia of the intrahepatic bile ducts was seen in 19/1058 (1.8%) of cases and was associated with chronic HCV infection and alcohol use; 10 of 19 cases (52.6%) of dysplasia were in patients with chronic HCV, and 4 of 19 (21.1%) were in patients with HCV with a history of alcohol use (20).
Our results do not support an association between HCV infection and ECC. This study and other case-control investigations previously conducted—one hospital based (22) and another population based (8)—suggested no significant change in the risk of ECC with HCV infection. Therefore, results from all three studies are consistent and describe risk factor profiles indicating ICC and ECC as two distinct malignancies.
This is the first study to formally examine the association between HCV and pancreatic cancer. The mixed findings in this study merit additional investigation. The hazard ratios for pancreatic cancer were consistently increased (HR: 1.23–1.32) after controlling for matching variables, previous VA use, race, and era of military service. The associations were attenuated, however, and no longer statistically significant when we controlled for variables related to alcohol use, pancreatitis, or choledocholithiasis, cholelithiasis, or primary sclerosing cholangitis. Alcohol use as well as chronic pancreatitis are known risk factors for pancreatic cancer as well as associated with HCV, and thus these factors could have been confounders of the association between HCV and pancreatic cancer. We were limited in our ability to adjust for tobacco smoking, a known risk factor for pancreatic cancer. Tobacco use is likely to be common among veterans, however there are no to data to support unequal tobacco use among veterans based on their HCV status; such a difference would have to be present for there to be confounding. Because the diagnostic code for smoking is vastly underutilized, we did not use it for this purpose. However, We have identified two conditions strongly linked to tobacco smoking: lung cancer and COPD. We calculated the incidence rates for each condition in a similar method to that employed for the main cancer outcomes, where we considered only cases diagnosed 6 months or longer than the index date for HCV. The incidence rates for these two conditions were very similar between the two groups with or without HCV infection and therefore adjustment for these conditions in the HCV-pancreatic cancer analyses was unnecessary.
The biological reason for an association between HCV and pancreatic cancer is unclear. A previous reported that patients diagnosed with acute hepatitis C also suffer from acute pancreatitis (10). In addition, serum levels of pancreatic enzymes have been shown to increase with the progression of liver disease in patients diagnosed with viral hepatitis (11-12).
Findings in this study also confirmed the high risk of HCC conferred by HCV infection (HR = 15.09). These are consistent with findings of a published meta-analysis, where the pooled odds ratio from 32 case-control studies was 17.3 (95% CI, 13.9–21.6) for anti-HCV/HCV RNA positivity in the setting of HBsAg negativity (23), and confer internal validity to the study. We did not examine HBV in the current study because we have previously shown that HBV related ICD-9 codes are poorly predictive of the serological status in medical record (24).
Strengths of the study include its large sample size and relatively long follow-up, which allowed the examination of otherwise rare malignancies. The study examined users of the VA health care system, which tends to be relatively stable and provides standardized access to veterans, independent of socioeconomic status. Use of a HCV infection—free veterans population as an internal control group, rather than the general population, was meant to ensure the comparability of the two groups with regard to features other than HCV. In addition, we conducted internal checks, including tests of the predictive value of codes used to denote HCV infection (13), and the careful verification of outcomes of interest—ICC and ECC-- through a limited individual chart review. This review validated the use of logical algorithms to identify these conditions as well as adding assurance about the codes themselves. We previously conducted a similar chart validation study for HCC and reported a PPV of 94% (16).
In the analyses, we obtained consistent results after considering a broad range of confounders, which encourages confidence in our findings. Efforts were made to clearly establish the existence of HCV infection before diagnosis of malignancy, and therefore we considered only cancers diagnosed 6 months following the HCV index date. Furthermore, in additional testing, the risks of several negative control cancers, including prostate cancer, colon cancer, and melanoma, which have no plausible relation to HCV infection, were not elevated, confirming the reliability of our database (13).
Limitations to our study are in general those imposed by work with administrative datasets. Reliance on ICD-9 codes and large administrative databases for identifying HCV infection was necessary because laboratory data are not collected systematically nationwide. While our internal chart review study validated this approach, some misclassification was still present given that only a small proportion of cancer cases were diagnosed in the single center where the chart review was conducted. Other factors that may have affected our results include the reality that cancer rates are higher in veterans than in the general population (25;26), the study group was overwhelmingly male, and exclusive reliance on VA records fails to capture all outcomes. Other limitation included the possibility of differential ascertainment of potential confounders in cancer cases vs. non-cases. However, when we conducted a sensitivity analysis that considered only confounders present at baseline, the results were similar to those in the primary analysis. Lastly, we did not capture cancer outcomes that were diagnosed outside the VA. The potential effect of missing information is difficult to predict
In conclusion, among 146,394 HCV-infected U.S. military veterans we found a 2.55-fold elevated risk of ICC. Our study is the largest epidemiologic study ever conducted to evaluate the relationship between HCV and ICC, ECC, and pancreas cancer. We adjusted our analyses for multiple confounding variables and found that the relationship between HCV and ICC remained significantly positive and that the association between HCV and ECC remained negative. Results also suggested a possible relationship to pancreatic cancer that requires further study.
These findings associating HCV and ICC but not ECC support those of prior smaller epidemiologic studies as well as previously described carcinogenic mechanisms related to HCV infection. From a clinical perspective, early intervention strategies, including screening HCV-positive individuals earlier or more rigorously, may improve the outcomes for both HCC and ICC. Additional epidemiological studies of ICC are needed, and new evaluations of the effects of early interventions, including HCV treatment, on the molecular carcinogenesis of ICC are warranted.
Acknowledgments
Sources of support Supporting this work was the Intramural Program of the National Cancer Institute, National Institutes of Health, and the Michael E. DeBakey Veterans Affairs Medical Center, Department of Veterans Affairs, Houston, Texas.
Footnotes
Study conception and design: TPG, LH, OL, HES, EAE Acquisition of data: TPG, LH, HES Analysis and interpretation of data: TPG, LH, OL, HES, EAE Drafting of the manuscript: TPG, LH, OL, EAE Critical revision of the manuscript for important intellectual content: TPG, OL, HES, EAE Statistical analysis: TPG, LH, EAE Obtaining funding: TPG, HES, EAE Administrative, technical, or material support: TPG, HES, EAE Study supervision: TPG, EAE
Reference List
- (1).Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- (2).Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88–96. doi: 10.1002/hep.20502. [DOI] [PubMed] [Google Scholar]
- (3).Perumal V, Wang J, Thuluvath P, Choti M, Torbenson M. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol. 2006;37(9):1211–1216. doi: 10.1016/j.humpath.2006.04.012. [DOI] [PubMed] [Google Scholar]
- (4).Donato F, Gelatti U, Tagger A, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12(10):959–964. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
- (5).Kobayashi M, Ikeda K, Saitoh S, et al. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88(11):2471–2477. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- (6).Shin HR, Lee CU, Park HJ, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25(5):933–940. doi: 10.1093/ije/25.5.933. [DOI] [PubMed] [Google Scholar]
- (7).Yamamoto S, Kubo S, Hai S, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95(7):592–595. doi: 10.1111/j.1349-7006.2004.tb02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5(10):1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sorensen HT, Friis S, Olsen JH, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28(4):921–925. doi: 10.1002/hep.510280404. [DOI] [PubMed] [Google Scholar]
- (10).Alvares-Da-Silva MR, Francisconi CF, Waechter FL. Acute hepatitis C complicated by pancreatitis: another extrahepatic manifestation of hepatitis C virus? J Viral Hepat. 2000;7(1):84–86. doi: 10.1046/j.1365-2893.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- (11).Katakura Y, Yotsuyanagi H, Hashizume K, et al. Pancreatic involvement in chronic viral hepatitis. World J Gastroenterol. 2005;11(23):3508–3513. doi: 10.3748/wjg.v11.i23.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Yoffe B, Bagri AS, Tran T, Dural AT, Shtenberg KM, Khaoustov VI. Hyperlipasemia associated with hepatitis C virus. Dig Dis Sci. 2003;48(8):1648–1653. doi: 10.1023/a:1024744613671. [DOI] [PubMed] [Google Scholar]
- (13).Giordano TP, Henderson L, Landgren O, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297(18):2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- (14).Fisher SG, Weber L, Goldberg J, Davis F. Mortality ascertainment in the veteran population: alternatives to the National Death Index. Am J Epidemiol. 1995;141(3):242–250. doi: 10.1093/oxfordjournals.aje.a117426. [DOI] [PubMed] [Google Scholar]
- (15).Page WF, Mahan CM, Kang HK. Vital status ascertainment through the files of the Department of Veterans Affairs and the Social Security Administration. Ann Epidemiol. 1996;6(2):102–109. doi: 10.1016/1047-2797(95)00126-3. [DOI] [PubMed] [Google Scholar]
- (16).Davila JA, Weston A, Smalley W, El-Serag HB. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007;41(8):777–782. doi: 10.1097/MCG.0b013e3180381560. [DOI] [PubMed] [Google Scholar]
- (17).Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- (18).Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- (19).Chen RF, Li ZH, Zou SQ, Chen JS. Effect of hepatitis C virus core protein on modulation of cellular proliferation and apoptosis in hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2005;4(1):71–74. [PubMed] [Google Scholar]
- (20).Torbenson M, Yeh MM, Abraham SC. Bile duct dysplasia in the setting of chronic hepatitis C and alcohol cirrhosis. Am J Surg Pathol. 2007;31(9):1410–1413. doi: 10.1097/PAS.0b013e318053d122. [DOI] [PubMed] [Google Scholar]
- (21).Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- (22).Shaib YH, El-Serag HB, Nooka AK, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102(5):1016–1021. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- (23).Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75(3):347–354. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- (24).Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- (25).Harris RE, Hebert JR, Wynder EL. Cancer risk in male veterans utilizing the Veterans Administration medical system. Cancer. 1989;64(5):1160–1168. doi: 10.1002/1097-0142(19890901)64:5<1160::aid-cncr2820640533>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- (26).Namboodiri KK, Harris RE. Hematopoietic and lymphoproliferative cancer among male veterans using the Veterans Administration Medical System. Cancer. 1991;68(5):1123–1130. doi: 10.1002/1097-0142(19910901)68:5<1123::aid-cncr2820680540>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]


