1. Procedures
Caution! Decomposition of excess PCl3 (step A) should be carried out carefully in a fume-hood because hydrogen chloride is evolved in the highly exothermic hydrolysis reaction.
A. (R)-(−)-(1,1′-Binaphthalene-2,2′-dioxy)chlorophosphine [1]2
A 250-mL, single-necked round-bottomed flask, equipped with a magnetic stirring bar, a reflux condenser, a three-way flow-controlled stopcock is flame-dried and purged with nitrogen. All joints are greased. The nitrogen outlet is connected to a gas scrubber trap (Note 1). The flask is charged with (R)-(+)-1,1′-bi(2-naphthol) (7.0 g, 24.5 mmol) (Note 2) and phosphorus trichloride (20.5 mL, 32.3 g, 235 mmol, 9.6 equiv) (Note 3) followed by 1-methyl-2-pyrrolidinone (20 μL, 0.2 mmol, 0.008 equiv) (Note 4). The flask is placed in an oil bath preheated to 92 °C. The solid dissolves within 5 min. The flask is swirled to dissolve the solid on the side. During this period, HCl evolves rapidly and the solution becomes yellow. The solution is kept at reflux for 10 min, then the oil bath is removed and the reaction mixture is allowed to cool to ambient temperature. The remaining phosphorus trichloride and HCl are removed under reduced pressure (7 mmHg) (Note 5) for 2 h, leaving a foamy wax. After venting the flask with nitrogen, diethyl ether (30 mL) (Note 6) is added to dissolve the solid. The solvent and traces of phosphorus trichloride are removed under reduced pressure (7 mm Hg) for 2 h, leaving a pale-yellow solid. The flask is connected to high vacuum (0.05 mm Hg) for 12 h to afford 9.08 g (106 %) of the desired product as off-white powder containing traces of diethyl ether (Note 7 and 8).
B. (R)-2,2′-Binaphthoyl-(S,S)-di-(1-phenylethyl)aminoylphosphine [3]3
A 250-mL, three-necked flask equipped with a rubber septum, nitrogen inlet, and a temperature probe (Note 9) is flame-dried and purged with nitrogen. The flask is charged with anhydrous THF (35 mL) (Note 10) and (−)-bis-[(S)-1-phenylethyl]amine (2, 1.69 mL, 7.39 mmol) (Note 11). The solution is cooled to −78 °C in a dry ice-acetone bath. n-Butyllithium (4.62 mL, 7.39 mmol, 1.6 M solution in hexanes, 1.0 equiv) (Note 12) is added dropwise over a 15 min period maintaining the internal temperature below −68 °C until the addition is complete, resulting in a pale-pink solution (Note 13). The contents of the flask are warmed to −30 °C in about 30 min and immediately cooled to −78 °C for an additional 1 h, which results in the solution becoming dark pink. A solution of (R)-(−)-1,1′-binaphthyl-2,2′-dioxychlorophosphine (1, 2.85 g, 8.13 mmol, 1.1 equiv) in dry THF (10 mL) is then introduced dropwise via syringe maintaining the solution temperature below −68 °C. The solution is maintained at −78 °C for an additional 2 h before it is warmed to ambient temperature and allowed to stir for 12 h. The solvent is removed by rotary evaporation (23 °C, 20 mmHg) to afford a pale-yellow oil mixed with a white solid (Note 14). The residue is dissolved in 5 mL of dichloromethane, then is loaded onto a silica gel column (150 g, 50 cm × 5 cm) and is eluted with 2 L of pentane/dichloromethane, 4: 1 (Note 15). Removal of the solvent from the eluate by rotary evaporation (23 °C, 20 mmHg) followed high vacuum (~ 0.05 mm Hg) affords 3.43 g (86%) of (R)-2,2′-binaphthoyl-(S,S)-di(1-phenylethyl)aminoyl-phosphine (3) as a white foam, mp 106–110 °C (Note 16). This product can be crushed into a powder and weighed as needed.
2. Notes
The gas scrubber trap contains 200 mL of aq. 6 N KOH solution.
(R)-(+)-1,1′-Bi-(2-naphthol), 99%, was purchased from Alfa Aesar and was used as received.
Phosphorus trichloride, Reagentplus®, 99%, was obtained from Sigma-Aldrich Company and was fractionally distilled under nitrogen before use. Excess PCl3 ensures complete dissolution of the binaphthol.
1-Methyl-2-pyrrolidinone 99.5% was purchased from Sigma-Aldrich Company and was used as received. The submitters employed a procedure that did not use 1-methyl-2-pyrrolidone and the reaction time was 16 h.
Three liquid nitrogen traps were used.
Diethyl ether (Fisher, BHT stabilized, HPLC grade) was dried by percolation through two columns packed with neutral alumina under a positive pressure of argon.
(R)-(−)-1,1′-Binaphthyl-2,2′-dioxychlorophosphine when stored in a dry nitrogen atmosphere is stable in excess of six months at ambient temperature.
The product displayed the following physicochemical properties: 1H NMR (400 MHz, CDCl3) δ: 8.02 (d, J = 8.80 Hz, 2 H), 7.97 (dd, J1 = 8.20 Hz, J2 = 3.40 Hz, 2 H), 7.55 (dd, J1 = 8.80 Hz, J2 = 0.80 Hz, 1 H), 7.52–7.45 (m, 3 H), 7.45–7.41 (m, 2 H), 7.35–7.29 (m, 2 H); 13C NMR (100.6 MHz, CDCl3) δ: 147.8, 147.2, 132.7, 132.4, 131.9, 131.5, 130.9, 130.1, 128.5, 127.0, 126.9, 126.7, 126.5, 125.7, 125.5, 124.44, 124.38, 123.1, 121.6, 121.1; 31P NMR (101.33 MHz, CDCl3) δ: 178.5.
A digital thermometer (Omega Instruments Digicator Model 400A with J-type thermocouple leads) was used.
Reagent-grade tetrahydrofuran (Fisher, BHT stabilized, HPLC grade) was dried by percolation through two columns packed with neutral alumina under a positive pressure of argon.
The submitters employed (−)-bis-[(S)-1-phenylethyl]amine from Sigma-Aldrich Company. Because the free base was not available, the checkers prepared (−)-bis-[(S)-1-phenylethyl]amine from (−)-bis-[(S)-1-phenylethyl]amine hydrochloride (97%; Sigma-Aldrich Company) using the following procedure. A 500-mL, round-bottomed flask equipped with a magnetic stir bar is charged with (−)-bis-[(S)-1-phenylethyl]amine hydrochloride (3.3 g, 12.6 mmol), aq. 6 N KOH solution (60 mL, 360 mmol, 28 equiv) and methyl tert-butyl ether (MTBE, 60 mL, ACS grade, Sigma-Aldrich Company). The mixture is stirred vigorously for 6 h. The biphasic mixture is poured into a 500-mL separatory funnel and the aqueous layer is separated. The aqueous layer is extracted with MTBE (2 × 40 mL). The organic extracts are combined, dried over anhydrous MgSO4 (10 g), filtered through a sintered glass funnel (6 cm × 6 cm), which is subsequently rinsed with MTBE (20 mL). The solvent is removed via rotary evaporation (23 °C, 20 mmHg). The product is purified by Kugelrohr distillation (air bath temperature 150 °C, 0.15 mmHg) to provide 2.8 g (98%) of the free base as a clear colorless liquid with the following physicochemical properties: 1H NMR (400 MHz, CDCl3) δ: 7.37–7.22 (m, 10 H), 3.52 (q, J = 6.6 Hz, 2 H), 1.59 (s (broad), 1 H), 1.29 (d, J = 6.6 Hz, 6 H); 13C NMR (100.6 MHz, CDCl3) δ: 145.8, 128.4, 126.6, 55.0, 25.0; IR (CHCl3) cm−1: 3082, 3061, 3025, 2960, 2924, 2863, 1602, 1492, 1469, 1451, 1368, 1202; MS (EI, 70 eV) m/z (%): 225 (2), 210 (58), 148 (3), 120 (10), 105 (100) 77 (17); HRMS (EI) calcd for C16H19N: 225.1517. Found: 225.1520. Anal. Calcd. for C16H19N: C, 85.28; H, 8.50; N, 6.22. Found: C, 84.90; H, 8.60; N, 6.36. [α]D −170 (c 3.5, MeOH).
n-Butyllithium was obtained as a solution in hexanes from Sigma-Aldrich Company and was doubly titrated by the method of Gilman.
The initial addition of the n-BuLi may cause a rapid rise in temperature. Addition may need to be temporarily suspended upon onset of the exotherm.
The submitters removed the small amount of solid by filtering the reaction mixture through Celite (30 g) and rinsing with diethyl ether (3 × 80 mL) before the rotary evaporation.
Silica gel (Merck, grade 9385, mesh 230–400) was purchased from Sigma-Aldrich Company. Product was isolated from sixteen 50-mL fractions. The product, Rf = 0.10 (silica gel, pentane/dichloromethane, 4: 1), was visualized by UV and KMnO4.
The product displayed the following physicochemical properties: 1H NMR (400 MHz, CDCl3) δ: 7.93 (d, J = 8.80 Hz, 2 H), 7.88 (dd, J1 = 7.80 Hz, J2 = 4.20 Hz, 2 H), 7.58 (d, J = 8.80 Hz, 1 H), 7.43 (d, J = 8.80 Hz, 1 H), 7.40–7.34 (m, 3 H), 7.28 (d, J = 8.00 Hz, 1 H), 7.25–7.16 (m, 2 H), 7.15–7.06 (m, 10 H), 4.49 (m, 2 H), 1.72 (d, J = 6.8 Hz, 6 H); 13C NMR (100.6 MHz, CDCl3) δ: 150.1, 150.0, 149.5, 142.8, 132.8, 132.7, 131.4, 130.4, 130.2, 129.4, 128.3, 128.1, 127.9, 127.7, 127.1, 127.07, 126.6, 126.0, 125.9, 124.7, 124.4, 124.1, 124.0, 122.4, 122.3, 121.73, 121.71, 52.3, 52.2, 21.9; 31P NMR (101.3 MHz, CDCl3) δ: 145.6; IR (thin film) cm−1: 3069, 2971, 1617, 1590, 1506, 1495, 1375, 1326, 1231, 1203, 1134, 1070. MS (ES, 70 eV) m/z (%): 539 (1), 538 (2), 450 (6), 434 (100), 315 (8), 268 (15), 239 (7), 105 (28). Anal. Calcd. for C36H30NO2P: C, 80.13; H, 5.60; N, 2.60. Found: C, 80.37; H, 5.63; N, 2.89. [α]D −500.7 (c 1.8, EtOH).
Safety and Waste Disposal Information
All hazardous materials should be handled and disposed of in accordance with “Prudent Practices in the Laboratory”; National Academy Press; Washington, DC, 1995.
3. Discussion
Phosphoramidite ligands derived from biaryldiphenols were originally introduced by Feringa for the asymmetric Cu-catalyzed conjugate addition of dialkylzinc reagents to enones.3,4a These ligands have been found to have extensive applications in asymmetric catalysis. Representative examples since the publication of Feringa’s review4b include, intramolecular Heck reactions,5 Cu-catalyzed SN2′ substitutions of allylic halides with Grignard reagents6 and addition of organo-aluminum reagents to enones (Cu-catalyzed) 7 and aldehydes (Ni-catalyzed)8, Rh9- and Ir10-catalyzed asymmetric hydrogenation of enamides, Ir-catalyzed allylic alkylations,11 aminations,12 and etherifications,13 Rh-catalyzed arylation of enones14 and imines,15 asymmetric Pd-catalyzed diamination of conjugated dienes,16 Rh-catalyzed [2+2+2] cycloaddition of alkenyl isocyanates and terminal alkynes,17 asymmetric hydrovinylation of vinylarenes,18 strained alkenes,18b,19 and 1,3-dienes.20
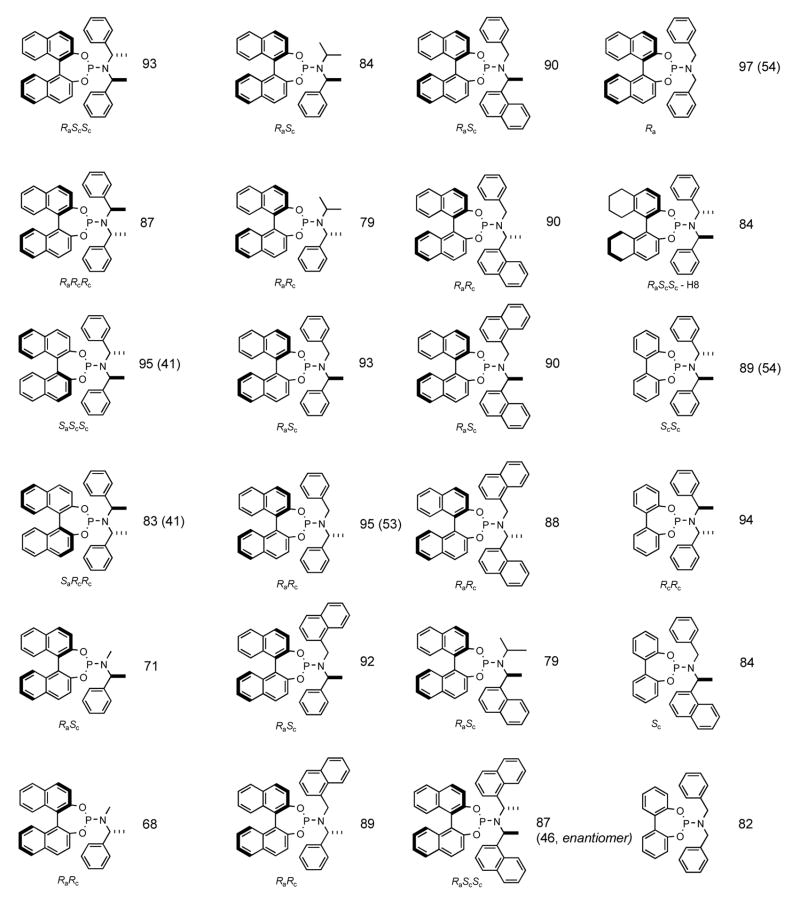
In general, two procedures starting with phosphorus(III) chlorides have been employed for the synthesis of phosphoramidites.21 Feringa used a phosphoryl chloride [(RO)2P-Cl], prepared by the base-mediated reaction of a diol with PCl3 followed by nucleophilic substitution of the chloride with a secondary amine, or the corresponding lithium amide.3 Alternatively a series of biphenol-based phosphoramidites have been prepared by the reaction of R2NPCl2 with the requisite biphenol in the presence of a tertiary amine.4c The procedure described here follows the former protocol with minor revision starting with a high-yielding synthesis of the phosphoryl chloride from the diol, neat phosphorus trichloride and catalytic amount of 1-methyl-2-pyrrolidinone,2a,c followed by the use of a lithium amide to effect the P-N bond formation. A number of other phosphoramidites, including several new ones, were prepared by this general procedure. These, along with the yields (starting from the diphenol) are shown in Figure 1. Use of one of these ligands for asymmetric hydrovinylation is illustrated in the following Organic Syntheses procedure.18e
Figure 1.
Structures and yields (from the corresponding diphenol) of phosphoramidites synthesized by the two-step procedure (shown in brackets are the best reported yields from literature)
Biographies
 T. V. (Babu) RajanBabu received his undergraduate education in India (Kerala University and IIT, Madras). He obtained a Ph.D. degree from The Ohio State University in 1978 under the direction of Professor Harold Shechter, and was a postdoctoral fellow at Harvard University with the late Professor R. B. Woodward. He then joined the Research Staff of Dupont Central Research, becoming a Research Fellow in 1993. He returned to OSU as a Professor of Chemistry in 1995. His research interests are in new practical methods for stereoselective synthesis focusing on enantioselective catalysis, free radical chemistry, and applications in natural product synthesis.
T. V. (Babu) RajanBabu received his undergraduate education in India (Kerala University and IIT, Madras). He obtained a Ph.D. degree from The Ohio State University in 1978 under the direction of Professor Harold Shechter, and was a postdoctoral fellow at Harvard University with the late Professor R. B. Woodward. He then joined the Research Staff of Dupont Central Research, becoming a Research Fellow in 1993. He returned to OSU as a Professor of Chemistry in 1995. His research interests are in new practical methods for stereoselective synthesis focusing on enantioselective catalysis, free radical chemistry, and applications in natural product synthesis.
 Craig R. Smith was born in 1983 in Columbus, OH, USA. He graduated with his B.S. in Chemistry in 2003 and M.S. in Chemistry in 2005 from Youngstown State University under the direction of Professor Peter Norris. The same year, he started his Ph.D. studies at The Ohio State University under the supervision of Professor T. V. RajanBabu. His current interests are the development of asymmetric carbon-carbon, carbon-nitrogen and carbon-sulfur bond forming reactions, and the synthesis of biologically active heterocyclic natural products and analogs thereof.
Craig R. Smith was born in 1983 in Columbus, OH, USA. He graduated with his B.S. in Chemistry in 2003 and M.S. in Chemistry in 2005 from Youngstown State University under the direction of Professor Peter Norris. The same year, he started his Ph.D. studies at The Ohio State University under the supervision of Professor T. V. RajanBabu. His current interests are the development of asymmetric carbon-carbon, carbon-nitrogen and carbon-sulfur bond forming reactions, and the synthesis of biologically active heterocyclic natural products and analogs thereof.
 Dan Mans was born in St. Louis, MO and got his undergraduate education at St. Louis University in 2002. He joined Department of Chemistry at the Ohio State University for his Ph.D., which was completed in 2008 under the supervision of Professor RajanBabu. In his graduate work he was involved with the development of the hydrovinylation reaction and its applications for the synthesis of pseudopterosins and helioporins. His research interests are in development and optimization of organic reactions of broad applicability. In his current position in the pharmaceutical industry, he plans to use his synthetic skills in the area of medicinal chemistry.
Dan Mans was born in St. Louis, MO and got his undergraduate education at St. Louis University in 2002. He joined Department of Chemistry at the Ohio State University for his Ph.D., which was completed in 2008 under the supervision of Professor RajanBabu. In his graduate work he was involved with the development of the hydrovinylation reaction and its applications for the synthesis of pseudopterosins and helioporins. His research interests are in development and optimization of organic reactions of broad applicability. In his current position in the pharmaceutical industry, he plans to use his synthetic skills in the area of medicinal chemistry.
 Son T. Nguyen was born in 1975 in Namdinh, Vietnam. He received his M.S. degree in chemistry from Hanoi University in 1999 with Professor Ngo Thi Thuan. He then joined the laboratory of Professor Ronald Caple at the University of Minnesota-Duluth where he obtained a M.S. degree in 2001. He moved to the University of Minnesota-Twin Cities campus to continue graduate study with Professor Craig J. Forsyth working on the total synthesis of azaspiracids. He graduated with a Ph.D. degree in 2006. He is currently doing postdoctoral research in the laboratory of Professor Scott E. Denmark developing a catalytic allylation process for carbonyl compounds.
Son T. Nguyen was born in 1975 in Namdinh, Vietnam. He received his M.S. degree in chemistry from Hanoi University in 1999 with Professor Ngo Thi Thuan. He then joined the laboratory of Professor Ronald Caple at the University of Minnesota-Duluth where he obtained a M.S. degree in 2001. He moved to the University of Minnesota-Twin Cities campus to continue graduate study with Professor Craig J. Forsyth working on the total synthesis of azaspiracids. He graduated with a Ph.D. degree in 2006. He is currently doing postdoctoral research in the laboratory of Professor Scott E. Denmark developing a catalytic allylation process for carbonyl compounds.
Appendix Chemical Abstracts Nomenclature; (Registry Number)
(R)-(+)-1,1′-bi(2-naphthol): (R)-Binol; (18531-94-7)
Phosphorus trichloride (7719-12-2)
1-Methyl-2-pyrrolidinone (872-50-4)
(−)-Bis[(S)-1-phenylethyl]amine (56210-72-1)
(−)-Bis[(S)-1-phenylethyl]amine hydrochloride (40648-92-8)
n-Butyllithium (109-72-8)
(R)-(1,1′-Binaphthalene-2,2′-dioxy)chlorophosphine: (R)-Binol-P-Cl; (155613-52-8)
(R)-2,2-Binaphthoyl-(S,S)-di(1-phenylethyl)aminoylphosphine; (11bR)-4-[N,N-bis[(1S)-1-Phenylethyl]amino]-dinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin (415918-91-1)
References
- 1.Department of Chemistry, The Ohio State University, 100 W. 18th Avenue, Columbus, OH 43210.
- 2.(a) Lucas HJ, Mitchell FW, Jr, Scully CN. J Am Chem Soc. 1950;72:5491–5497. [Google Scholar]; (b) Green N, Kee TP. Synth Commun. 1993;23:1651–1657. [Google Scholar]; (c) Cramer N, Laschat S, Baro A. Organometallics. 2006;25:2284–2291. [Google Scholar]
- 3.Arnold LA, Imbos R, Mandoli A, de Vries AHM, Naasz R, Feringa BL. Tetrahedron. 2000;56:2865–2878. [Google Scholar]
- 4.Feringa BL, Pineschi M, Arnold LA, Imbos R, de Vries A. Angew Chem, Int Ed Engl. 1997;36:2620–2623.Feringa B. Acc Chem Res. 2000;33:346–353. doi: 10.1021/ar990084k.For the use of structurally related biphenol-derived phosphoramidites, see: Alexakis A, Polet D, Rosset S, March S. J Org Chem. 2004;69:5660–5667. doi: 10.1021/jo049359m.See also: Franciò G, Faraone F, Leitner W. Angew Chem, Int Ed. 2000;39:1428–1430. doi: 10.1002/(sici)1521-3773(20000417)39:8<1428::aid-anie1428>3.0.co;2-k.Huttenloch O, Spieler J, Waldmann H. Chem Eur J. 2001;7:671–675. doi: 10.1002/1521-3765(20010202)7:3<671::aid-chem671>3.0.co;2-m.
- 5.Imbos R, Minnaard J, Feringa BL. J Am Chem Soc. 2002;124:184–185. doi: 10.1021/ja017200a. [DOI] [PubMed] [Google Scholar]
- 6.Tissot-Croset K, Polet D, Alexakis A. Angew Chem Int Ed. 2004;43:2426–2428. doi: 10.1002/anie.200353744. [DOI] [PubMed] [Google Scholar]
- 7.(a) d’Augustin M, Palais L, Alexakis A. Angew Chem Int Ed. 2005;44:1376–1378. doi: 10.1002/anie.200462137. [DOI] [PubMed] [Google Scholar]; (b) Alexakis A, Albrow V, Biswas K, Augustin M, Prieto O, Woodward S. Chem Commun. 2005;2843–:2845. doi: 10.1039/b503074a. [DOI] [PubMed] [Google Scholar]
- 8.Biswas K, Prieto O, Goldsmith PJ, Woodward S. Angew Chem Int Ed. 2005;44:2232–2234. doi: 10.1002/anie.200462569. [DOI] [PubMed] [Google Scholar]
- 9.(a) van den Berg M, Minnaard AJ, Schudde EP, van Esch J, de Vries AHM, de Vries JG, Feringa BL. J Am Chem Soc. 2000;122:11539–11540. [Google Scholar]; (b) Pena D, Minnaard AJ, de Vries JG, Feringa BL. J Am Chem Soc. 2002;124:14552–14553. doi: 10.1021/ja028235t. [DOI] [PubMed] [Google Scholar]; (c) Bernsmann H, van den Berg M, Hoen R, Minnaard AJ, Mehler G, Reetz MT, de Vries JG, Feringa BL. J Org Chem. 2005;70:943–951. doi: 10.1021/jo048374o. [DOI] [PubMed] [Google Scholar]; (d) Liu Y, Ding K. J Am Chem Soc. 2005;127:10488–10489. doi: 10.1021/ja052749l. [DOI] [PubMed] [Google Scholar]
- 10.Giacomina F, Meetsma A, Panella L, Lefort L, de Vries AHM, de Vries JG. Angew Chem Int Ed. 2007;46:1497–1500. doi: 10.1002/anie.200603930. [DOI] [PubMed] [Google Scholar]
- 11.(a) Bartels B, Helmchen G. Chem Commun. 1999;741–:742. [Google Scholar]; (b) Lipowsky G, Miller N, Helmchen G. Angew Chem Int Ed. 2004;43:4595–4597. doi: 10.1002/anie.200460016. [DOI] [PubMed] [Google Scholar]; (c) Streiff S, Welter C, Schelwies M, Lipowsky G, Miller N, Helmchen G. Chem Commun. 2005;2957–:2959. doi: 10.1039/b503713a. [DOI] [PubMed] [Google Scholar]
- 12.(a) Ohmura T, Hartwig JF. J Am Chem Soc. 2002;124:15164–15165. doi: 10.1021/ja028614m. [DOI] [PubMed] [Google Scholar]; (b) Weihofen R, Dahnz A, Tverskoy O, Helmchen G. Chem Commun. 2005;2957:3541–3543. doi: 10.1039/b505197e. [DOI] [PubMed] [Google Scholar]; (c) Singh OV, Han H. J Am Chem Soc. 2007;129:774–775. doi: 10.1021/ja067966g. [DOI] [PubMed] [Google Scholar]
- 13.(a) Lopez F, Ohmura T, Hartwig JF. J Am Chem Soc. 2003;125:3426–3427. doi: 10.1021/ja029790y. [DOI] [PubMed] [Google Scholar]; (b) Fischer C, Defieber C, Suzuki T, Carreira E. J Am Chem Soc. 2004;126:1628–1629. doi: 10.1021/ja0390707. [DOI] [PubMed] [Google Scholar]
- 14.Boiteau JG, Minnaard AJ, Feringa BL. J Org Chem. 2003;68:9481–9484. doi: 10.1021/jo035155e. [DOI] [PubMed] [Google Scholar]
- 15.(a) Jagt RBC, Toullec PY, Geerdink D, de Vries JG, Feringa BL, Minnaard AJ. Angew Chem Int Ed. 2006;45:2789–2791. doi: 10.1002/anie.200504309. [DOI] [PubMed] [Google Scholar]; (b) Marelli C, Monti C, Gennari C, Piarulli U. Synlett. 2007;2213–:2216. [Google Scholar]
- 16.Du H, Yuan W, Zhao B, Shi Y. J Am Chem Soc. 2007;129:11688–11689. doi: 10.1021/ja074698t. [DOI] [PubMed] [Google Scholar]
- 17.Yu R, Rovis T. J Am Chem Soc. 2006;128:12370–12371. doi: 10.1021/ja064868m. [DOI] [PubMed] [Google Scholar]
- 18.Franció G, Faraone F, Leitner W. J Am Chem Soc. 2002;124:736–737. doi: 10.1021/ja012099v.Park H, Kumareswaran R, RajanBabu TV. Tetrahedron. 2005;61:6352–6367.Zhang A, RajanBabu TV. J Am Chem Soc. 2006;128:5620–5621. doi: 10.1021/ja060999b.Smith CR, RajanBabu TV. Org Lett. 2008;10:1657–1659. doi: 10.1021/ol800395m.Smith C, Zhang A, Mans D, RajanBabu TV. Org Synth. 2008;85:248–266. doi: 10.15227/orgsyn.085.0248.For the use of a different type of phosphoramidite, see: Shi WJ, Zhang Q, Xie JH, Zhu SF, Hou GH, Zhou QL. J Am Chem Soc. 2006;128:2780–2781. doi: 10.1021/ja057654y.
- 19.Kumareswaran R, Nandi N, RajanBabu TV. Org Lett. 2003;5:4345–4348. doi: 10.1021/ol0356284. [DOI] [PubMed] [Google Scholar]
- 20.(a) Zhang A, RajanBabu TV. J Am Chem Soc. 2006;128:54–55. doi: 10.1021/ja0561338. [DOI] [PubMed] [Google Scholar]; (b) Saha B, Smith CR, RajanBabu TV. J Am Chem Soc. 2008;130:xxxx–xxxx . doi: 10.1021/ja711475f. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phosphoramidites can also be synthesized by treatment of the diol with hexamethylphosphorus triamide. Hulst R, de Vries NK, Feringa BL. Tetrahedron: Asymmetry. 1994;5:699–708.