Abstract
The objective of the present study was to construct epidermal growth factor receptor (EGFR) targeting cetuximab immunoliposomes (ILs) for targeted delivery of boron compounds to EGFR(+) glioma cells for neutron capture therapy. The ILs were synthesized by using a novel cholesterol-based membrane anchor, maleimido-PEG-cholesterol (Mal-PEG-Chol), to incorporate cetuximab into liposomes by either surface conjugation or a post-insertion method. For post-insertion, transfer efficiency of MAb conjugates from micelles to liposome was examined at varying temperatures, mPEG2000-DSPE ratios, and micelle-to-liposome lipid ratios. Following this, the cetuximab-ILs were evaluated for targeted delivery of the encapsulated boron anion, dodecahydro-closo-dodecaborate (2−) (B12H122−), to human EGFR gene transfected F98EGFR glioma cells as potential delivery agents for boron neutron capture therapy (BNCT). In addition, cellular uptake of cetuximab-ILs, encapsulating a fluorescence dye, was analyzed by confocal fluorescence microscopy and flow cytometry, and boron content was quantified by ICP-MS. Much greater (~ 8-fold) cellular uptake of boron was obtained using cetuximab-ILs in EGFR(+) F98EGFR compared with non-targeted human IgG-ILs. Based on these observations, we have concluded that cholesterol can serve as an effective anchor for MAb in liposomes and cetuximab-ILs are potentially useful delivery vehicles for BNCT of gliomas.
Keywords: immunoliposomes, cholesterol, cetuximab, BNCT, post-insertion
INTRODUCTION
Boron neutron capture therapy (BNCT) is a binary therapeutic modality based on tumor-selective delivery of boron-10 followed by external radiation of low energy (e.g., ~ 0.025 keV) thermal neutron. The resulting neutron capture and fission reactions produces high linear energy transfer (LET) α-particles and recoiling 7Li nuclei (10B + 1n → [11*B] → 4He (α) + 7Li + 2.39 MeV), which are highly lethal to both proliferating and non-proliferating cells. Currently, BNCT has primarily been used to treat high grade brain tumors, such as glioblastoma multiforme (GBM) and anaplastic astrocytomas (AA) (1–3). The efficacy of BNCT is highly dependent upon the selective delivery of a sufficient amount of 10B (~ 20 µg/g tumor) to tumor cells to sustain a lethal 10B (n, α)7Li capture reaction (4, 5).
Liposomes, spherical vehicles formed by phospholipid bilayer, are attractive drug carriers due to their biocompatibility and capacity to carry both hydrophobic and hydrophilic drugs (6, 7). Encapsulation of drugs into liposomes significantly alters their pharmacokinetics and biodistribution, which may result in reduced toxicity and an expanded therapeutic window. Non-targeted liposomes rely on “passive-targeting” to accumulate in solid tumors due to their porous vascular endothelium, reduced lymphatic drainage, and the resulting enhanced permeability and retention (EPR) effect (8, 9). In order to increase tumor specific delivery, liposomes can be linked to tumor targeting ligands (10) such as antibodies to produce immunoliposomes (ILs) (11, 12), receptor ligands such as folate, transferrin, and epidermal growth factor (EGF)-liposomes (13, 14), as well as peptides such as RGD-liposomes (15, 16).
The EGF receptor (EGFR) frequently is overexpressed in GBM and AA, but is undetectable or weakly expressed in the normal brain (17, 18). Therefore, EGFR is an attractive molecular target for the specific delivery of therapeutic agents to high grade gliomas. Delivery of 10B containing agents via liposomes conjugated to the anti-EGFR monoclonal antibody (MAb) cetuximab (C225) and L8A4, which is directly against EGFRvIII is an attractive delivery approach for BNCT, because of their high payload capacity (4, 19). The use of EGFR-targeting boron containing bioconjugates as delivery agents for BNCT of brain tumors has been a major focus of our laboratories (4, 19–22). A highly boronated polyamidoamine (PAMAM) dendrimer has been linked to either EGF, cetuximab or L8A4 for targeted delivery of 10B to gliomas (23). In order to bypass the blood-brain-barrier, the bioconjugates have been administered by either direct intratumoral (i.t.) injection (19) or by convection enhanced delivery (CED) (21, 24). These studies, carried out in rats bearing either EGFR or EGFRvIII gene transfected rat gliomas, designated F98EGFR or F98EGFRvIII respectively, have resulted in a doubling or tripling of the mean survival times (MST) compared to untreated control animals, thereby establishing proof-of-principle for the use of these agents.
To construct ILs, antibodies can be linked to the liposome surface via non-covalent or covalent coupling (10). Non-covalent coupling usually is accomplished by using biotinylated antibodies, which are bound to avidin-derivatized liposomes (25, 26). Covalent coupling utilizes a variety of bioconjugation techniques, such as the forming of thioether, disulfide or amide bonds between lipids and the antibody molecules (27). In general, antibodies can be directly linked to liposomes through covalent conjugation to functional groups on the liposome surface (28) or they can be post-inserted into preformed liposomes via micelles of an antibody-lipid conjugate (29, 30).
Liposomes typically consist of phospholipids and cholesterol, both of which are potentially suitable for anchoring receptor targeting ligands to its lipid bilayer. For example, both phospholipid and cholesterol anchored folate (folate-PEG-DSPE, folate-PEG-Chol) have been used in the preparation of folate receptor targeted liposomes (31, 32). In this report, we have evaluated a new cholesterol derivative, maleimido-PEG-cholesterol (Mal-PEG-Chol), for the anchoring of cetuximab (C225) in ILs. Furthermore, the EGFR-targeting efficiency and subsequent internalization of the C225-ILs by the EGFR overexpressing F98EGFR cells have been determined both by fluorescence microscopy and by flow cytometry. Our results, described in detail in the following report, have demonstrated that ILs potentially are useful delivery vehicles for BNCT of gliomas.
EXPERIMENTAL PROCEDURES
Materials
Hydrogenated soy phosphatidylcholine (HSPC), methoxy-polyethyleneglycol2000- phosphatidylethanolamine (mPEG2000-DSPE), were purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol (Chol), cholesteryl chloroformate, sheep IgG, 2-iminothiolane (Traut’s Reagent), Mercaptoethylamine•HCl (MEA), ninhydrin, PEG3350-bis-amine, 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES), 8-hydroxypyrenetrisulfonic acid trisodium salt (HPTS), Sepharose CL-4B resin, triethylamine (TEA), were obtained from Sigma-Aldrich Co. (St. Louis, MO). Octadecylrhodamine B (R18) was obtained from Molecular Probes (Eugene, OR). Dilithiumdodecahydro-closo-dodecaborate (Li2B12H12) was obtained as a gift from Callery Chemical Company (Pittsburgh, PA). DMEM, fetal bovine serum (FBS) and antibiotics were purchased from Life Technologies (Grand Island, NY). Sephadex LH20 resin and PD-10 desalting columns were purchased from GE Healthcare (Piscataway, NY). Bradford protein assay kit and N-[β-Maleimidopropyloxy]succinimide ester (BMPS) were purchased from Peirce Chemical Co. (Rockford, IL). Polycarbonate membranes were obtained from Avestin Inc. (Ottawa, ON, Canada). Cetuximab (C225) was generously provided by Dr. Daniel Hicklin at ImClone Systems Inc. (New York, NY). All other chemicals were of reagent grade.
Cell Culture
Parental (wild-type) F98WT glioma cell line (33) and human EGFR gene transfected F98EGFR glioma cell lines, expressing 105 receptor sites per cell (19). were cultured as a mononlayer in DMEM media supplemented with 100 units/mL penicillin, 100 µM/mL streptomycin, 200 mg/mL of G418 and 10% FBS in a humidified atmosphere containing 5% CO2 at 37 °C.
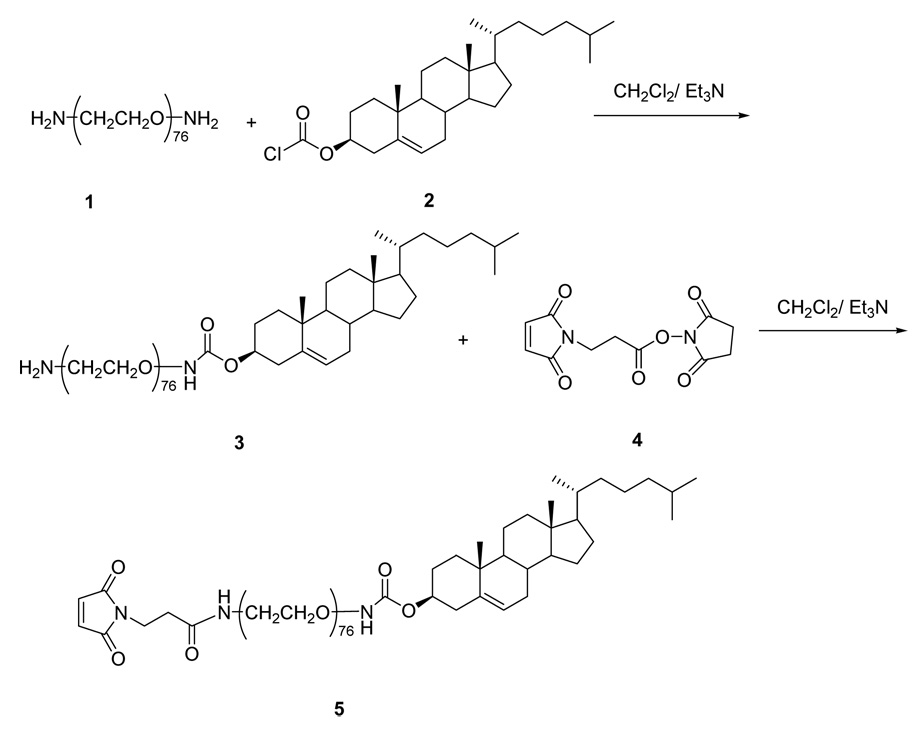
Synthesis of Maleimido-PEG-Cholesterol (Mal-PEG-Chol) [5]
The synthetic scheme for of Mal-PEG-Chol (5) is shown in Figure 1. PEG3350-bis-amine (1) (335 mg, 0.1 mmol) was dissolved in anhydrous CH2Cl2 (30 mL) and TEA (14 µL, 0.1 mmol). Then, cholesteryl chloroformate (2) (49 mg, 0.11 mmol, 1.1 equiv), also dissolved in anhydrous CH2Cl2 (2 mL), was slowly added. After 4 hr reaction at room temperature, thin layer chromatography (TLC) analysis (mobile phase CHCl3: MeOH: H2O at 3:1:0.2, visualized with ninhydrin and KMnO4 staining) indicated the formation of dicholesteryl (Chol-PEG-Chol) (Rf = 0.65), monocholesteryl (Chol-PEG-NH2) (3) (Rf = 0.47), and unreacted PEG-bis-amine (Rf = 0.12). The reaction mixtures were separated by silica gel column chromatography using a step-gradient of methanol (0–20%) in chloroform. The fractions containing monocholesteryl PEG (Chol-PEG-NH2) (3) were pooled and evaporated to dryness under vacuum to yield 56% product. The MALDI mass spectrum of (3) showed a series of 44 Da-spaced lines, corresponding to the mass of a single ethylene oxide unit, that were centered around 3,819. 1H NMR (CDCl3, 300 MHz) δ (ppm): 5.31 (d, 1H), 4.68 (m, 1H), 3.70-3.50 (br, m, PEG protons, ~320 H), 2.46 (d, 2H), 2.04~1.05 (m, Chol protons, ~26H), 1.00 (s, 3H), 0.91 (d, 3H), 0.86 (d, 6H), 0.683 (s, 3H).
Figure 1. Synthetic scheme for Mal-PEG-Chol.
Monocholesteryl PEG (3) (30 mg, 7.9 µmol) and BMPS (4) (3.2 mg, 11.8 µmol, 1.5 equiv.) were dissolved in anhydrous CH2Cl2 (20mL) under N2 and under darkness. After 1 hr, ninhydrin assay showed disappearance of free primary amine group. The reaction was quenched by ethylenediamine; and the reaction mixture was concentrated on a rotary evaporator and then passed through a Sephadex LH20 column, using CH2Cl2/MeOH (1:1 v/v) as the mobile phase. Fractions containing product Mal-PEG-Chol (5) (identified by KMnO4 staining) were pooled and dried by rotary evaporation. The yield was 93%. Analytical data for (5): MALDI showed cluster of peaks centered around 3,992. 1H NMR (CDCl3, 300 MHz) δ (ppm): 6.70 (s, 2H), 5.31 (d, 1H), 4.68 (m, 1H), 3.80-3.50 (br, m, PEG protons, ~312 H), 2.46 (d, 2H), 2.04~1.00 (m, Chol protons, ~26H), 0.99 (s, 3H), 0.90 (d, 3H), 0.85 (d, 6H), 0.663 (s, 3H).
Preparation of Immunoliposomes
Liposome Preparation
Unilamellar liposomes were prepared by the polycarbonate membrane extrusion method (34). A chloroform solution of lipids consisting of HSPC/Chol/mPEG2000-DSPE (60:40:1–4 mole%), with or without Mal-PEG-Chol (0.5 mol%), was dried into a thin film in a round-bottom flask on a rotary evaporator, and then further dried for two hrs under vacuum. The resulting thin lipid film was hydrated in 1 mL of solution of fluorescent dye HPTS (25 mM, in PBS, pH 7.4). The resulting suspension was subjected to 5 cycles of freezing and thawing, and extruded 5 times through a track-etched polycarbonate membrane with a pore size of 100 nm using a Lipex™ Extruder (Northern Lipids, Vancouver, Canada). Unentrapped HPTS was separated from liposomes by gel filtration on a 10 mL PD-10 desalting column equilibrated in PBS (pH 7.4). Liposome size was determined by dynamic light scattering on a Nicomp Particle Sizer Model 370. The mean liposomes diameter obtained by this method was 106.1 ± 35.6 nm by volume weighted averaging. The phospholipid content was measured using a colorimetric assay (35). The liposomes were stored at 4 °C until use.
For boron uptake studies, dodecahydro-closo-dodecaborate (2−) (B12H122−) (50mM) was encapsulated in liposomes by the extrusion method described above. The boronated liposomes were sterilized by passing through a 0.22 µm filter, and stored at 4 °C.
Construction of C225-ILs
ILs were constructed by conjugation with either an intact MAb or an Fab’ fragment. MAb (IgG, as non-targeting control MAb and C225, as the targeting MAb) was thiolated for 1 hr at room temperature by reacting with 5-fold excess of Traut’s reagent in degassed HEPES buffer (20 mM HEPES, 140 mM NaCl, 2 mM EDTA, pH 8.0). Unreacted Traut’s reagent was removed by PD-10 column, eluted with degassed HEPES buffer (pH 6.5). The thiolated MAb was store at 4 °C and used within 1 hr of preparation.
For direct coupling of MAb to liposomes, thiolated MAb (or Fab’ fragment) was added to preformed liposomes (HSPC/Chol/mPEG2000-DSPE/Mal-PEG-Chol at 60:40:2:0.5 mole ratio) at 40 µg MAb per µmole of lipids, and then gently shaken at room temperature for 4 hrs under N2. Excess maleimide groups were quenched by incubation with MEA (1 mM) for 30 min at room temperature. Unbound MAb and MEA were then removed by Sepharose CL-4B gel filtration. A Bradford protein assay was performed to determine the coupling efficiency (36).
Fab’ fragments of C225 were produced as described by Mamot et al. (37). Briefly, intact C225 was digested with pepsin (at C225 to pepsin weight ratio of 1:20) in 0.1 M sodium citrate buffer (pH 4.5) at 37 °C for 4 hrs, followed by dialysis against HEPES buffer (pH 6.5). The resulting C225-F(ab)2 fragment was reduced by MEA (15 mM) under N2 for 15 min and then purified by PD-10 gel filtration.
ILs also were constructed by the post-insertion method. First, liposomes composed of HSPC/Chol (60:40, mole ratio) and varying amounts of mPEG2000-DSPE were prepared by extrusion, as described above. To synthesize MAb-lipid micelle conjugate, Mal-PEG-Chol was dissolved in chloroform and dried into a thin film in a round-bottom flask on a rotary evaporator and then further dried overnight under vacuum. The lipid film was then rehydrated with degassed HEPES Buffer (pH 6.5) at a concentration above the critical micelle concentration (CMC) of Mal-PEG-Chol with gentle vortexing followed by incubation at 60 °C. Mal-PEG-Chol micelles were mixed with thiolated MAb (or Fab’ fragment) at 40 µg MAb (or 80 µg Fab’) per µg lipid and gently shaken for 4 hrs at room temperature. This was followed by quenching of free thiols by MEA (1 mM) and PD-10 gel filtration. The resulting MAb-PEG-Chol micelles were evaluated for efficiency of MAb transfer into preformed liposomes under varying conditions, including temperatures (37 °C and 60 °C), percentages of mPEG2000-DSPE (1, 2, 3, and 4 mole%); and micelle-to-liposome lipid ratios (up to 1 to 10). Unincorporated MAb-PEG-Chol micelles were separated from ILs by Sepharose CL-4B gel filtration. Bradford protein assays was performed to determine the antibody content of ILs which enabled calculation of incorporation efficiency.
Binding and Internalization of Liposomes
F98EGFR and F98WT cells cultured as monolayers in T75 flasks were harvested by treatment with PBS containing 5mM EDTA. The detached cells were pelleted by centrifugation at 1000 rpm for 5 min, resuspended in serum supplemented media at a density of 2.5 × 105 cell/mL, and then aliquoted into 1.5-mL microcentrifuge tubes. Cells were incubated for 2 hrs with either targeted or non-targeted ILs (at 5 µM phospholipid concentration) encapsulating HPTS at 37 °C under gentle shaking. After 3 times washing with PBS (pH 7.4), the cells were kept on ice and subjected to flow cytometric analysis on a BD FACS Calibur analyzer and visualization by laser scanning confocal microscopy on a Zeiss 510 META microscope.
Kinetic analysis of cell surface bound and endocytosed ILs was performed as described previously (12, 38). F98EGFR glioma cells were incubated with HPTS-loaded C225-ILs for 2 hrs at 37 °C, washed with ice-cold PBS, and then harvested with PBS-EDTA. Due to the pH-sensitivity of HPTS and the relatively low internal pH of endosomes (pH 4–5), surface-binding and internalization of ILs were quantified based by measurement of the ratio between fluorescence intensity at excitation wavelength (λex) of 413 nm (isosbestic point) and 454 nm.
For quantitative analysis of boron uptake, F98EGFR glioma cells (106 cells/mL) were incubated with IgG or C225 ILs, prepared by post-insertion method, encapsulating Li2B12H12 for 2 hrs at 37 °C. These liposomes had 144 and 117 µg boron/mL, and mean diameters of 135.6 ± 41.5 and 126.8 ± 39.5 nm, respectively. After washing with PBS (pH 7.4) for 3 times, the cells were subjected to boron content analysis by inductively coupled plasma-mass spectroscopy (ICP-MS) on a Perkin-Elmer Sciex ELAN 6000.
RESULTS
Synthesis of Immunoliposomes (ILs)
Direct coupling
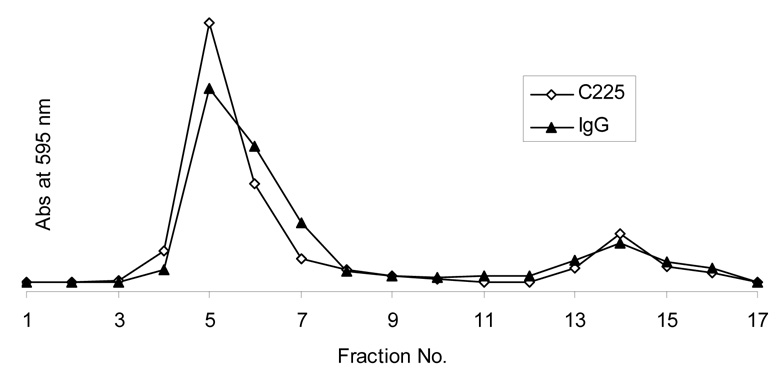
Liposomes, composed of HSPC/Chol/PEG-DSPE/Mal-PEG-Chol (60:35:5:0.5, mole%) were prepared by the lipid hydration and extrusion method, and had a mean diameter of ~ 100 nm. Thiolated antibodies (IgG and C225) were reacted to the maleimide group on the distal termini of PEG chains on these liposomes to form thioether linkages, yielding ~ 25 – 35 µg MAb per µmol of lipid (Figure 2).
Figure 2. Elution profiles of ILs constructed with Mal-PEG-Chol and unbound MAbs.
The reaction mixture was loaded on to a Sepharose CL-4B gel filtration column, and eluted with PBS (pH 7.4). The peak in fraction 4–8 represents the C225 or IgG ILs. Fractions 12–17 are free unbound C225 and IgG. Each fraction contained 0.5 mL. The protein content was measured by Bradford protein assay, which measures absorbance at 595 nm.
Post-insertion
The CMC of Mal-PEG-Chol was evaluated by monitoring changes in turbidity via OD at 254 nm, as previously described (39). Regression analysis indicated that micelles were formed at 2 µM (data not shown). To investigate whether micelles formed by MAb conjugates with cholesterol anchors could be incorporated into preformed liposomes, the transfer efficiency was examined at varying incubation temperatures, mPEG2000-DSPE contents in the liposome, and micelle-to-liposome lipid ratios (Figure 3a). Sepharose CL-4B gel filtration chromatography was used to determine the amount of MAb incorporated into liposomes. Unincorporated micelles were eluted in a broad peak, which was well separated from ILs, which were eluted in the void volume (Figure 3b).
Figure 3. Post-insertion method for ILs construction.
a: Schematic representation of post-insertion method for ILs construction. Antibodies or antibody fragments are coupled to micelles formed by Chol-PEG-Mal, and then incubated with preformed boron-loaded liposomes to form ILs. b: A typical elution profiles of IgG-ILs constructed by post-insertion method at 60 °C for 1 hr. IgG micelles were formed by coupling thiolated IgG with Mal-PEG-Chol micelles. After post-insertion, the mixture was loaded on to a Sepharose CL-4B gel filtration column, and eluted with PBS (pH7.4). The peak of fractions 4–7 represents the IgG-ILs. Fractions 9–14 are IgG-lipid micelles. Each fraction contained 0.5 mL. The protein content was measured by Bradford protein assay, which measures absorbance at 595 nm.
The incorporation of MAb into preformed liposomes was determined at 37°C and at 60°C. The incorporation rate at 60 °C was higher than that at 37°C (Table 1). This was expected since 60°C is above the transition temperature of the bilayer, which would facilitate lipid transfer. Meanwhile, the mean diameters of the particles also were increased at 60°C, possibly due to lipid exchange (Table 1).
Table 1.
Increase in IL size and IgG-PEG-Chol incorporation efficiency at different mPEG2000-DSPE content.
| Mole% of mPEG2000-DSPE in liposomes | Particle Size Increase % | MAb insertion efficiency % | ||
|---|---|---|---|---|
| 60 °C | 37 °C | 60 °C | 37 °C | |
| 1 | 28 | 18 | 75 | 23 |
| 2 | 24 | 9 | 58 | 18 |
| 3 | 19 | 7 | 53 | 8 |
| 4 | 6 | 4 | 35 | 3 |
Unilamellar preformed liposomes composed of HSPC/Chol (60:40, mole ratio) and various mPEG2000-DSPE (1, 2, 3, and 4 mole%) were prepared by thin film hydration-extrusion, as described in the Experimental Procedures section. Micelles were incubated with liposome (1:30, lipid molar ratio) for 1 hr at 37 °C or 60 °C.
The presence of mPEG2000-DSPE, which sterically stabilized the liposomes, adversely affected the incorporation efficiency of the MAb (Table 1). The higher the mPEG2000-DSPE content of the liposomes, the lower the efficiency of MAb insertion into the liposomes. In liposomes containing 4 mole% of mPEG2000-DSPE, the insertion of MAb was significantly inhibited, even at 60 °C. In contrast, MAb insertion efficiency was minimal at 37 °C when mPEG2000-DSPE content exceeded 2 mole%.
Saturation of MAb insertion was observed at high micelle-to-liposome lipid ratios. The percent of MAb incorporation at the ratio of 1:30 was close to that at the ratio of 1:10 (Table 2), which indicated that incorporation had essentially reached a plateau. The micelles of Fab’ also were prepared by the same method, and the incorporation rate was close to that of the whole MAb. Approximately 60% C225-Fab’ was transferred to liposomes containing 2 mole% of mPEG2000-DSPE with micelle-to-liposome lipid ratio of 1:30, following incubation at 60 °C for 1 hr.
Table 2.
The effect of micelle-to-liposome lipid ratio on IgG-PEG-Chol incorporation into preformed liposomes
| Micelle-to-liposome lipid molar ratio | % Mab incorporated |
|---|---|
| 1:100 | 72 |
| 1:30 | 55 |
| 1:15 | 36 |
| 1:10 | 21 |
The liposomes, composed of HSPC/Chol/mPEG2000-DSPE (60:40:2 mole ratio) were incubated with the micelles at 60 °C for 1 hr.
Cellular Binding and Internalization
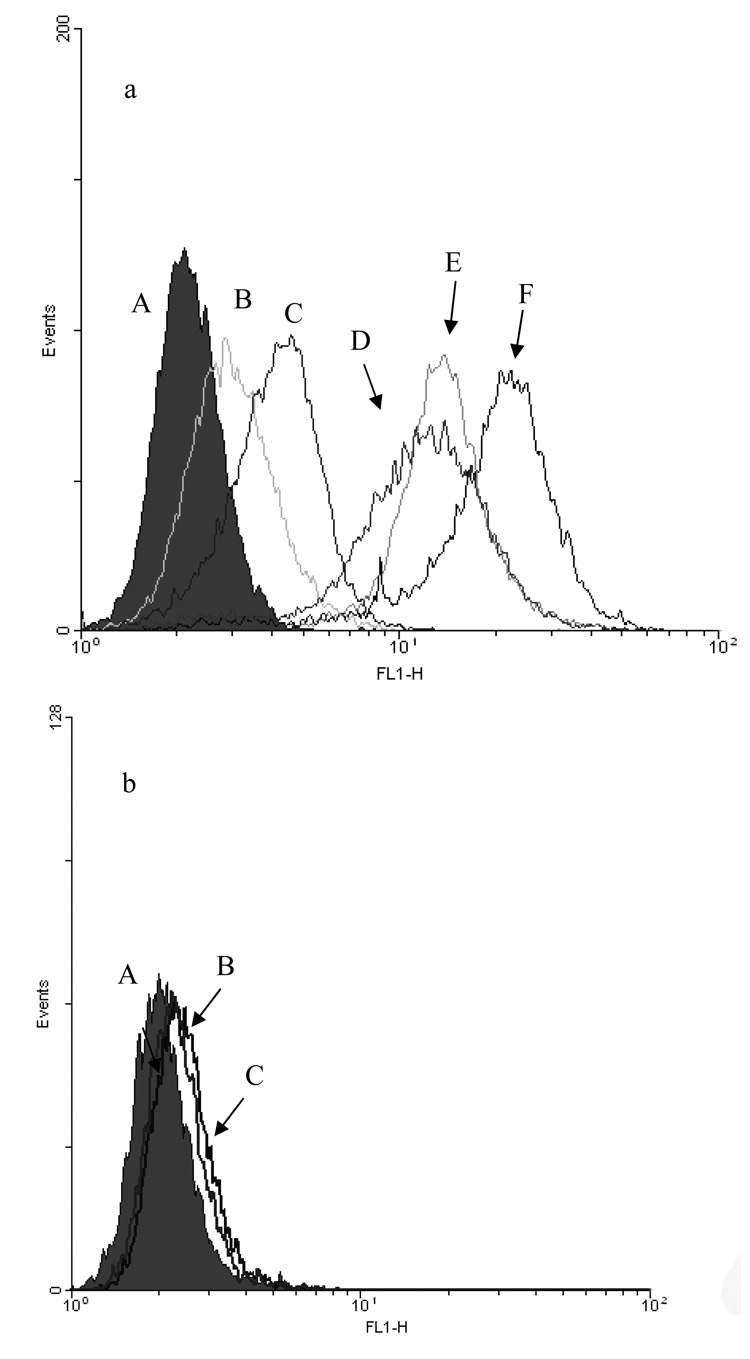
Cellular binding and internalization of ILs were evaluated by flow cytometry in F98WT and F98EGFR rat glioma cells. In this study, IgG-ILs and C225-ILs, constructed by direct-coupling or by post-insertion method, were loaded with fluorescent dye HPTS and incubated with the cells for 2 hrs at 37 °C. The uptake of C225-ILs was 10-fold higher than that of the non-targeted IgG-ILs. In a competitive binding assay, free C225 was added, which significantly reduced binding of C225-ILs by F98EGFR cells (Figure 4a). Both directly coupled and post-inserted C225 in the liposomes appeared to greatly increase liposome binding to EGFR-expressing F98EGFR cells but not to F98WT. Meanwhile, C225-Fab’ liposomes constructed by the post-insertion method were evaluated using F98WT and F98EGFR cells. The C225-Fab’ containing liposomes showed extensive binding to F98EGFR cells, but minimal binding to F98WT cells (Figure 4b). These results clearly demonstrated selective uptake of anti-EGFR ILs between two congenic cell lines based on differing EGFR expression levels.
Figure 4. Binding of ILs to F98EGFR cells evaluated by flowcytometric assay.
a: ILs encapsulating HPTS were incubated with cells for 2 hrs at 37 °C, washed 3 times, and stored on ice until analysis. (A): (A) untreated F98EGFR cells, (B) IgG-ILs, (C) C225-ILs with free C225 as a blocking agent, (D) C225-ILs prepared by post-insertion method with preformed liposomes containing 2 mole% mPEG2000-DSPE, (E) C225-Fab’ ILs constructed by post-insertion with preformed liposomes containing 2 mole% mPEG2000-DSPE, (F) C225-ILs prepared by direct coupling, b: C225-Fab’ ILs with non-EGFR-expressing F98WT cells. (A) untreated F98WT cells, (B) C225-Fab’ ILs plus free C225, (C) C225-Fab’ ILs;
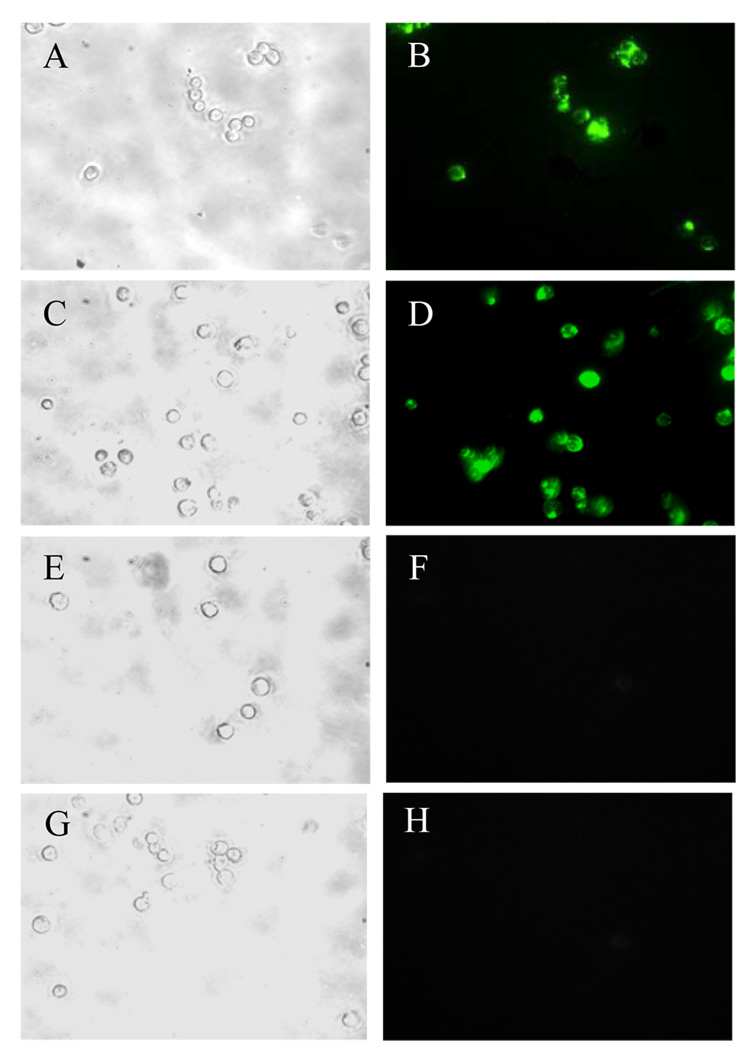
Confocal fluorescence microscope was used to visualize the binding and subsequent intracellular localization of C225-Fab’ ILs by F98 cells. F98EGFR cells incubated with ILs prepared by either direct-coupling or post-insertion method both showed high levels of fluorescence. In contrast, cells exposed to non-targeted IgG-Fab’ ILs showed little fluorescence (Figure 5a, A–F). Finally, the C225-Fab’ ILs did not show significant binding to non-EGFR expressing F98WT cells (Figure 5a, G and H). Overall, the results of the confocal fluorescence microscopy studies were consistent with those of the flow cytometric assays.
Figure 5. Fluorescence micrographs of F98 cells treated with ILs.
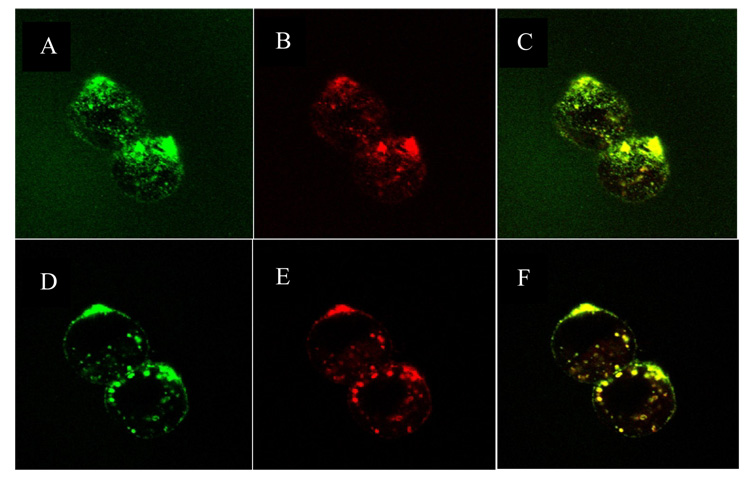
a. Internalization of C225-Fab’ ILs and non-targeted IgG-Fab’ ILs in EGFR-expressing F98EGFR cells. F98EGFR cells were incubated with C225-Fab’ or IgG-Fab’ ILs, containing HPTS fluorescent dye for 2 h at 37 °C. Left panels show cells visualized in the phase contract mode; right panels show cells visualized in the fluorescence mode. (A, B) F98EGFR cells treated with C225-Fab’ ILs constructed by directed coupling; (C, D) F98EGFR cells treated with C225-Fab’ ILs prepared by post-insertion method; (E, F) F98EGFR cells treated with non-targeted IgG-Fab’ ILs; (G, H) F98WT cells with C225-Fab’ ILs. b: Confocal micrographs of F98EGFR cells incubated with C225-Fab’ ILs constructed by post-insertion method with preformed liposomes containing 2 mole% mPEG2000-DSPE. The ILs were loaded with HPTS and labeled with R18. Left column: green HPTS fluoresence; central column: red rhodamine fluorescence; right column, superimposed images. (A–C), top view image showed cell surface-bound liposomes; (D-F), central stack image showed liposomes accumulated in the endosomal compartments.
C225-Fab’ liposomes containing 2 mole% PEG2000-DSPE prepared by post-insertion method were loaded with pH-sensitive fluorophore HPTS in the aqueous core and labeled with octadecyl-rhodamine B (R18) (0.5 mole%) in the lipid bilayer. These liposomes were incubated with F98EGFR cells for 2 hrs at 37 °C. Fluorescence was distributed throughout the cell, including cell plasma membrane, intracellular vesicles, and the cytosol (Figure 5b). Superimposition of fluorescence images based on HPTS and R18 indicated that the fluorescence was not due to HPTS leakage or R18 lipid exchange.
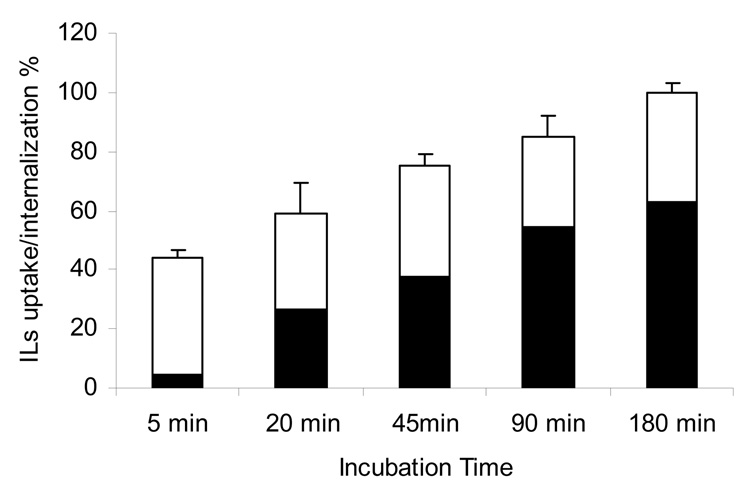
The pH sensitivity of HPTS absorption spectrum allows for the simultaneous analyses of both surface-bound (neutral pH) and endosome-localized (low pH) liposomes based on the I445/413 nm excitation ratios. C225-Fab’ liposomes bound to F98EGFR cells were detected within 5 min of incubation. Meanwhile, intracellular accumulation was observed within 20 min (Figure 6). The results clearly showed increasing lysosomal uptake of the liposomes over a 3-hr incubation period.
Figure 6. Kinetics of C225-Fab’ IL internalization in the EGFR overexpressing F98EGFR cells.
C225-Fab’ ILs were constructed by post-insertion method with preformed liposomes containing 2 mole% mPEG2000-DSPE, and loaded with pH-sensitive dye HPTS and incubated with F98EGFR cells at 37 °C. At various time points, the sample was analyzed by measuring the fluorescence intensity at excitation wavelengths of 454 and 413 nm. Surface-bound fraction ▭ Internalized fraction ▬. The 180 min time point was set at 100%, n = 3.
For quantitative analysis of boron uptake, boron-loaded C225-ILs delivered ~ 8 times more boron (509.75 ± 148.85 µg B/109 F98 cells) to F98EGFR cells than non-targeted IgG-ILs (61.00 ± 1.76 µg B/109 F98 Cells), greatly exceeding the required level of boron (~ 20 µg/g tissue) for NCT (1).
DISCUSSION
A novel cholesterol-based anchor (Mal-PEG-Chol [5]) has been synthesized for the attachment of receptor targeting ligands to the lipid bilayer of liposomes. Factors affecting post insertion efficiency, in vitro targeting, internalization of IgG-ILs, anti-EGFR C225-ILs, C225-Fab’-ILs and their drug carrying capacity were evaluated in EGFR(–) F98wt and EGFR overexpressing F98EGFR cells. Functionalization of liposomes with receptor targeting ligands previously had been carried out by using phosphatidylethanolamine (PE) derivatives as anchors to the lipid bilayer. These have included Mal-PEG-DSPE, Hz-PEG-DSPE and PDP-PEG-DSPE (40). Cholesterol, which is less expensive than PE based lipids, is a major component of the biological membranes. PEG-Chol derivatives have been used for the preparation of sterically stabilized liposomes with long circulating time (41). We previously have prepared a cholesterol based folate conjugate, folate-PEG-Chol, which was successfully used for the construction of folate receptor targeted liposomes (32). Carrion et al. have used cholesterol derivatized with SPDP for the preparation of ILs (42). The present data have clearly demonstrated that cholesterol is an attractive alternative to PE as an effective lipid anchor for the construction of ILs. The strategy for the synthesis of Mal-PEG-Chol (5) from readily available starting materials is simple and efficient. The maleimide group in the cholesterol derivative proved to be especially useful for conjugation to Fab’ fragments, which contain a free thiol group. Moreover, MAbs-PEG-Chol in micelles was found to be efficiently incorporated into preformed liposomes during post-insertion, which is a convenient method for the construction of targeted liposomes.
The use of C225-ILs as 10B delivery vehicles for BNCT of GBM is particularly attractive due to potential synergy between the high LET radiation produced in the 10B (n, α)7Li neutron capture and the potential signal transduction cascade that ILs upon multivalent binding of the ILs to the cellular EGFRs. Therefore, C225-ILs also were evaluated in vitro as boron delivery vehicles with EGFR expressing F98EGFR glioma cells. The excellent cell-targeting capabilities of anti-EGFR ILs was demonstrated by fluorescence microscopy and flow cytometry. Non-targeted IgG-ILs showed only minimal fluorescence, while C225-ILs showed preferential binding and internalization by F98EGFR cells, which was indicative of EGFR-specific binding and subsequent endocytosis. Another important feature of ILs as boron delivery vehicles was their ability to selectively target only those cells overexpressing EGFR, which was demonstrated by the low binding and uptake by F98WT cells. In the present study, the boron-rich compound, dodecaborate anion (B12H122−) was used. This previously has been encapsulated in RGD-liposomes for the delivery of 10B to human umbilical vein endothelial cells (HUVEC) (16). In the present study, boronated C225-ILs delivered 509.75 ± 148 µg 10B per 109 (~ 1 gram) F98EGFR cells, which was ~ 8 times greater than the amount delivered by non-targeted IgG-liposomes. This exceeded the therapeutically required amount of 10B (~20 µg/g tissue).
In conclusion, our studies have demonstrated that both MAbs and Fab’ fragments could be efficiently linked to Mal-PEG-Chol. The resulting MAb and Fab’-PEG-Chol bioconjugates could be efficiently incorporated into preformed liposome to yield ILs by the post-insertion method. C225-ILs were internalized by EGFR-overexpressing F98EGFR cells in vitro, presumably by receptor-mediated endocytosis, resulting in the selective and efficient intracellular delivery of boron. This approach could also be applied to the delivery of other therapeutic agents to EGFR positive tumor cells. In vivo studies are planned to further explore the tumor targeting potential of anti-EGFR ILs for potential use for BNCT and stereotactic synchrotron radiotherapy (43, 44).
Acknowledgements
This work was supported in part by NSF grant EEC-0425626 and NIH grants I RO1-CA098945 (to R.F. Barth) and P30CA16058.
Reference
- 1.Barth RF, Coderre JA, Vicente MG, Blue TE. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- 2.Barth RF. A critical assessment of boron neutron capture therapy: an overview. J Neurooncol. 2003;62:1–210. doi: 10.1007/BF02699929. [DOI] [PubMed] [Google Scholar]
- 3.Zamenhof RG, Coderre JA, Rivard MJ, Patel H. Topics in Neutron Capture Therapy. Proceedings of the Eleventh World Congress on Neutron Capture Therapy. Applied Radiation and Isotopes. 2004;61:731–1130. [Google Scholar]
- 4.Wu G, Barth RF, Yang W, Lee RJ, Tjarks W, Backer MV, Backer JM. Boron containing macromolecules and nanovehicles as delivery agents for neutron capture therapy. Anticancer Agents Med Chem. 2006;6:167–184. doi: 10.2174/187152006776119153. [DOI] [PubMed] [Google Scholar]
- 5.Vicente MGH. Boron in medicinal chemistry. Anticancer Agents Med Chem. 2006;6:73–181. doi: 10.2174/187152006776119135. [DOI] [PubMed] [Google Scholar]
- 6.Pan XQ, Wang H, Shukla S, Sekido M, Adams DM, Tjarks W, Barth RF, Lee RJ. Boron-containing folate receptor-targeted liposomes as potential delivery agents for neutron capture therapy. Bioconjug Chem. 2002;13:435–442. doi: 10.1021/bc015557y. [DOI] [PubMed] [Google Scholar]
- 7.Sudimack JJ, Adams D, Rotaru J, Shukla S, Yan J, Sekido M, Barth RF, Tjarks W, Lee RJ. Folate receptor-mediated liposomal delivery of a lipophilic boron agent to tumor cells in vitro for neutron capture therapy. Pharm Res. 2002;19:1502–1508. doi: 10.1023/a:1020408716807. [DOI] [PubMed] [Google Scholar]
- 8.Jain RK. Delivery of molecular medicine to solid tumors: lessons from in vivo imaging of gene expression and function. J Control Release. 2001;74:7–25. doi: 10.1016/s0168-3659(01)00306-6. [DOI] [PubMed] [Google Scholar]
- 9.Pan X, Lee RJ. Tumour-selective drug delivery via folate receptor-targeted liposomes. Expert Opin Drug Deliv. 2004;1:7–17. doi: 10.1517/17425247.1.1.7. [DOI] [PubMed] [Google Scholar]
- 10.Sapra P, Allen TM. Ligand-targeted liposomal anticancer drugs. Prog Lipid Res. 2003;42:439–462. doi: 10.1016/s0163-7827(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 11.Leserman LD, Machy P, Barbet J. Cell-Specific Drug Transfer from Liposomes Bearing Monoclonal- Antibodies. Nature. 1981;293:226–228. doi: 10.1038/293226a0. [DOI] [PubMed] [Google Scholar]
- 12.Kirpotin D, Park JW, Hong K, Zalipsky S, Li WL, Carter P, Benz CC, Papahadjopoulos D. Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry. 1997;36:66–75. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- 13.Xu L, Pirollo KF, Chang EH. Transferrin-liposome-mediated p53 sensitization of squamous cell carcinoma of the head and neck to radiation in vitro. Hum Gene Ther. 1997;8:467–475. doi: 10.1089/hum.1997.8.4-467. [DOI] [PubMed] [Google Scholar]
- 14.Bohl Kullberg E, Bergstrand N, Carlsson J, Edwards K, Johnsson M, Sjoberg S, Gedda L. Development of EGF-conjugated liposomes for targeted delivery of boronated DNA-binding agents. Bioconjug Chem. 2002;13:737–743. doi: 10.1021/bc0100713. [DOI] [PubMed] [Google Scholar]
- 15.Janssen AP, Schiffelers RM, ten Hagen TL, Koning GA, Schraa AJ, Kok RJ, Storm G, Molema G. Peptide-targeted PEG-liposomes in anti-angiogenic therapy. Int J Pharm. 2003;254:55–58. doi: 10.1016/s0378-5173(02)00682-8. [DOI] [PubMed] [Google Scholar]
- 16.Koning GA, Fretz MM, Woroniecka U, Storm G, Krijger GC. Targeting liposomes to tumor endothelial cells for neutron capture therapy. Appl Radiat Isot. 2004;61:963–967. doi: 10.1016/j.apradiso.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Sauter G, Maeda T, Waldman FM, Davis RL, Feuerstein BG. Patterns of epidermal growth factor receptor amplification in malignant gliomas. Am J Pathol. 1996;148:1047–1053. [PMC free article] [PubMed] [Google Scholar]
- 18.Schwechheimer K, Huang S, Cavenee WK. GFR gene amplification--rearrangement in human glioblastomas. Int J Cancer. 1995;62:145–148. doi: 10.1002/ijc.2910620206. [DOI] [PubMed] [Google Scholar]
- 19.Barth RF, Yang W, Adams DM, Rotaru JH, Shukla S, Sekido M, Tjarks W, Fenstermaker RA, Ciesielski M, Nawrocky MM, Coderre JA. Molecular targeting of the epidermal growth factor receptor for neutron capture therapy of gliomas. Cancer Res. 2002;62:3159–3166. [PubMed] [Google Scholar]
- 20.Wu G, Barth RF, Yang W, Chatterjee M, Tjarks W, Ciesielski MJ, Fenstermaker RA. Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjug Chem. 2004;15:185–194. doi: 10.1021/bc0341674. [DOI] [PubMed] [Google Scholar]
- 21.Yang W, Barth RF, Wu G, Ciesielski MJ, Fenstermaker RA, Moffat BA, Ross BD, Wikstrand CJ. Development of a syngeneic rat brain tumor model expressing EGFRvIII and its use for molecular targeting studies with monoclonal antibody L8A4. Clin Cancer Res. 2005;11:341–350. [PubMed] [Google Scholar]
- 22.Barth RF, Wu G, Yang W, Binns PJ, Riley KJ, Patel H, Coderre JA, Tjarks W, Bandyopadhyaya AK, Thirumamagal BT, Ciesielski MJ, Fenstermaker RA. Neutron capture therapy of epidermal growth factor (+) gliomas using boronated cetuximab (IMC-C225) as a delivery agent. Appl Radiat Isot. 2004;61:899–903. doi: 10.1016/j.apradiso.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Yang W, Barth RF, Wu G, Kawabata S, Sferra TJ, Bandyopadhyaya AK, Tjarks W, Ferketich AK, Moeschberger ML, Binns PJ, Riley KJ, Coderre JA, Ciesielski MJ, Fenstermaker RA, Wikstrand CJ. Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin Cancer Res. 2006;12:3792–3802. doi: 10.1158/1078-0432.CCR-06-0141. [DOI] [PubMed] [Google Scholar]
- 24.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips NC, Tsoukas C. Immunoliposome targeting to CD4+ cells in human blood. Cancer Detect Prev. 1990;14:383–390. [PubMed] [Google Scholar]
- 26.Schnyder A, Krahenbuhl S, Torok M, Drewe J, Huwyler J. Targeting of skeletal muscle in vitro using biotinylated immunoliposomes. Biochem J. 2004;377:61–67. doi: 10.1042/BJ20031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JW, Hong K, Kirpotin DB, Papahadjopoulos D, Benz CC. Immunoliposomes for cancer treatment. Adv Pharmacol. 1997;40:399–435. doi: 10.1016/s1054-3589(08)60146-5. [DOI] [PubMed] [Google Scholar]
- 28.Zalipsky S, Hansen CB, Lopes de Menezes DE, Allen TM. Long-circulating, polyethylene glycol-grafted immunoliposomes. Journal of Controlled Release. 1996;39:153–161. [Google Scholar]
- 29.Ishida T, Iden DL, Allen TM. A combinatorial approach to producing sterically stabilized (Stealth) immunoliposomal drugs. FEBS Lett. 1999;460:129–133. doi: 10.1016/s0014-5793(99)01320-4. [DOI] [PubMed] [Google Scholar]
- 30.Iden DL, Allen TM. In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion approach. Biochim Biophys Acta. 2001;1513:207–216. doi: 10.1016/s0005-2736(01)00357-1. [DOI] [PubMed] [Google Scholar]
- 31.Lee RJ, Low PS. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J Biol Chem. 1994;269:3198–3204. [PubMed] [Google Scholar]
- 32.Guo WJ, Lee T, Sudimack J, Lee RJ. Receptor-specific delivery of liposomes via folate-PEG-Chol. Journal of Liposome Research. 2000;10:179–195. [Google Scholar]
- 33.Barth RF. Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol. 1998;36:91–102. doi: 10.1023/a:1005805203044. [DOI] [PubMed] [Google Scholar]
- 34.Olson F, Hunt CA, Szoka FC, Vail WJ, Papahadjopulos D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophs Acta. 1979;557:9–23. doi: 10.1016/0005-2736(79)90085-3. [DOI] [PubMed] [Google Scholar]
- 35.Chen PS, Toribara TY, Warner H. Microdetermination of phosphorus. Analyt. Chemistry. 1956;28:1756–1758. [Google Scholar]
- 36.Bradford MM. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1974;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 37.Mamot C, Drummond DC, Greiser U, Hong K, Kirpotin DB, Marks JD, Park JW. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003;63:3154–3161. [PubMed] [Google Scholar]
- 38.Straubinger RM, Papahadjopoulos D, Hong KL. Endocytosis and intracellular fate of liposomes using pyranine as a probe. Biochemistry. 1990;29:4929–4939. doi: 10.1021/bi00472a025. [DOI] [PubMed] [Google Scholar]
- 39.Bhadra D, Bhadra S, Jain NK. Pegylated lysine based copolymeric dendritic micelles for solubilization and delivery of artemether. J Pharm Pharm Sci. 2005;8:467–482. [PubMed] [Google Scholar]
- 40.Nobs L, Buchegger F, Gurny R, Allemann E. Current methods for attaching targeting ligands to liposomes and nanoparticles. J Pharm Sci. 2004;93:1980–1992. doi: 10.1002/jps.20098. [DOI] [PubMed] [Google Scholar]
- 41.Beugin S, Edwards K, Karlsson G, Ollivon M, Lesieur S. New sterically stabilized vesicles based on nonionic surfactant, cholesterol, and poly(ethylene glycol)-cholesterol conjugates. Biophys J. 1998;74:3198–3210. doi: 10.1016/S0006-3495(98)78026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrion C, Domingo JC, de Madariaga MA. Preparation of long-circulating immunoliposomes using PEG-cholesterol conjugates: effect of the spacer arm between PEG and cholesterol on liposomal characteristics. Chem Phys Lipids. 2001;113:97–110. doi: 10.1016/s0009-3084(01)00178-5. [DOI] [PubMed] [Google Scholar]
- 43.Biston MC, Joubert A, Adam JF, Elleaume H, Bohic S, Charvet AM, Esteve F, Foray N, Balosso J. Cure of Fisher rats bearing radioresistant F98 glioma treated with cis-platinum and irradiated with monochromatic synchrotron X-rays. Cancer Res. 2004;64:2317–2323. doi: 10.1158/0008-5472.can-03-3600. [DOI] [PubMed] [Google Scholar]
- 44.Adam JF, Joubert A, Biston MC, Charvet AM, Peoc'h M, Le Bas JF, Balosso J, Esteve F, Elleaume H. Prolonged survival of Fischer rats bearing F98 glioma after iodine-enhanced synchrotron stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:603–611. doi: 10.1016/j.ijrobp.2005.09.004. [DOI] [PubMed] [Google Scholar]