Abstract
The cytotoxic activity of lymphocytes is crucial for immune surveillance and homeostasis. Several independent, naturally occurring genetic models characterized by defects in granule trafficking or exocytosis have helped to decipher the multiple steps and molecules that regulate the cytotoxic process. The study by Rüder and colleagues in this issue of the JCI shows that an engineered absence of EBAG9, previously reported as a tumor-associated antigen, enhances cytotoxic activity of CTLs but not NK cells, likely acting on the endosomal-lysosomal trafficking of the cytotoxic effectors (see the related article beginning on page 2184). This finding adds a new piece to the puzzle of complex mechanisms that tightly regulate the capacity of the cytotoxic response and suggests a new target to negatively modulate CTL responsiveness.
CTLs and NK cells (collectively known as “cytotoxic lymphocytes”) are major players in the body’s defense against viral infection and cancer via their ability to seek out and kill infected or tumorigenic cells. Although CTLs are activated by specific antigen recognition, the cytotoxic activity of NK cells is initiated by specific activating receptors or combinations thereof and is inhibited by self MHC class I recognition. Once cytotoxic cells have recognized their targets and formed a conjugate, the trafficking of granule components, including perforin and granzymes, to the immunological synapse (IS) between the cell and its target, leads to the delivery of perforin and granzymes into the target cells and subsequent target destruction through apoptosis (1, 2).
Studies of natural or engineered mutants involving cytotoxic function tell us a great deal about the in vivo function of this pathway. So far, only genetic diseases leading to the loss of cytotoxic function have been reported (3). These studies have revealed the tremendous importance of this cytotoxic pathway in immune homeostasis. Congenital defects that lead to either impaired perforin function or its dysregulated release result in the specific severe condition hemophagocytic lymphohistiocytosis (HLH) syndrome. This syndrome stems from the inability of activated cytotoxic cells to clear antigen-presenting targets. The failure to clear antigen causes unremitting polyclonal CD8+ T cell expansion, activation, and infiltration of visceral organs associated with macrophage activation, with the deleterious release of multiple inflammatory cytokines including IFN-γ (3, 4).
In addition, the studies of these natural mutants both in humans and in mice have contributed to the characterization of critical effectors of the cytotoxic machinery and their function. They also provided evidence of the exquisite regulation of this process, which occurs in successive steps following cell activation. For instance, studies of Griscelli syndrome type 2, a rare condition characterized by partial albinism and occurrence of an HLH syndrome (5), and familial HLH type 3 (FHL3) (6) have shown the critical role of two proteins, the small GTPase Rab27a and the priming factor Munc13-4, at a late step of the secretory pathway. Rab27a regulates the tethering of mature cytotoxic granules that have polarized at the IS, whereas Munc13-4 primes the docked cytotoxic granules before their fusion with the plasma membrane at the IS. Other proteins, for which their defect impairs lymphocyte cytotoxic activity, likely regulate an upstream step in the cytotoxic pathway. They include adapter protein 3 (AP3), which is deficient in Hermansky-Pudlak syndrome type 2 (HPS2) (7, 8), and the lysosomal trafficking regulator (LYST) protein, which is deficient in Chediak-Higashi syndrome (9, 10). Their respective defects include missorting of transmembrane proteins to lysosomes and an increase in the size of cytotoxic granules. Although the precise functions of these effectors in the cytotoxic pathway remain poorly understood, both proteins likely regulate lysosomal biogenesis and membrane fusion along the cytotoxic pathway.
The proteins mentioned above are all required for proper cytotoxic activity of lymphocytes. In contrast, Rüder and colleagues report in this issue of the JCI that estrogen receptor–binding fragment–associated antigen 9 (EBAG9) acts as a negative regulator of cytotoxic function (11). Through the generation of Ebag9-deficient mice, they show that loss of EBAG9 enhances the release of cytotoxic granules in vitro as well as the cytotoxic capacity of CTLs from deficient mice in vivo. The increase in the sorting of cytotoxic effector molecules to cytotoxic granules associated with EBAG9 deficiency, together with the demonstration that EBAG9 interacts with the γ2-adaptin adapter protein, indicate that EBAG9 plays a pivotal role in the sorting of endosomal-lysosomal proteins. Therefore, the work presented by Rüder and colleagues introduces a new player involved in the tuning of the cytotoxic function of lymphocytes. However, it remains to be determined what the precise molecular mechanism is that underlies the observed phenotype; there is no evidence of immunopathogenesis in EBAG9-deficient mice. In the future, it may be worth studying the consequences of lymphocytic choriomeningitis virus (LCMV) infection in EBAG9-deficient mice, as in this setting mice impaired in cytotoxic lymphocyte function demonstrate a clear immune disease, i.e., HLH (12). An “opposite” phenotype, with limitation of the immune response to pathogen, might be seen, as we discuss below. The trafficking of lysosomal proteins in other cells, such as mast cells, may also be increased in EBAG9-deficient mice, potentially exacerbating an anaphylactic reaction.
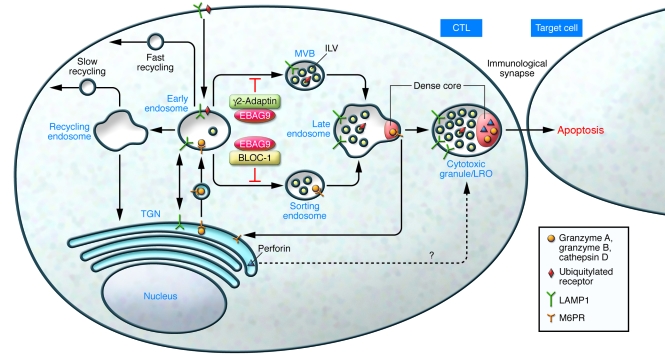
Biogenesis of cytotoxic granules
Mature cytotoxic granules are lysosome-related organelles (LROs) that act as dual-function organelles, carrying out both degradative and secretory functions (2, 13). Cytotoxic granules exhibit an electron-dense core that contains most of the cytotoxic proteins, including perforin and granzymes, similar to secretory organelles. The core is surrounded by a multivesicular/lamellar region typical of lysosomes. The cytotoxic granules have a low lumenal pH and contain lysosomal acid hydrolases such as cathepsin D and receptors such as a member of the lysosomal-associated membrane protein (LAMP) family. The biogenesis of these cytotoxic organelles is not well characterized, but, as in the case of other LROs, they likely use the cooperation of ubiquitous trafficking machineries with cell type–specific cargoes (14) (Figure 1). Granzymes, similar to other soluble lysosomal proteins, are modified during biosynthesis by the addition of a mannose-6-phosphate moiety, which is recognized by mannose-6-phosphate receptors. These transmembrane receptors cycle among the trans-Golgi network and early and late endosomes, carrying soluble proteins to the lysosome structures. However, the pathway used by perforin to reach the lysosomes is still unknown (Figure 1). LAMPs do not require sorting receptors, since they have a tyrosine-based motif that directly interacts with multimeric cytosolic AP1, AP2, and AP3. Biogenesis of LROs in different cell types also depends on the function of the biogenesis of lysosome-related organelles complex-1 (BLOC-1), -2, and -3 protein complexes (15). These protein complexes have been largely defined from the studies of the various genes leading to the phenotype of HPS or a similar disorder in mice (14). Of note, the AP3 complex was shown to interact with BLOC-1 on early endosomes to facilitate the trafficking of LAMP to the lysosome (16). In addition, cytotoxic cells, similar to all cell types, may use the endosomal sorting complex required for transport (ESCRT) (17). Ubiquitylation targets proteins to this pathway. ESCRT complexes recognize ubiquitylated proteins on early endosomes, which are subsequently invaginated into newly forming multivesicular bodies. Ubiquitylated proteins accumulate in these intralumenal vesicles of endosomes and then are sorted to late endosomes and lysosomes. So far, none of the CTL-restricted lysosomal proteins have been found to follow this ESCRT sorting pathway to be incorporated into the vesicular structure of the granules.
Figure 1. Sorting proteins to the cytotoxic granules/secretory lysosomes.
The newly synthesized soluble proteins granzymes A and B acquire the mannose-6-phosphate signal in the Golgi complex, enabling their association with the mannose-6-phosphate receptor (M6PR) in the trans-Golgi network (TGN) and transport to early endosomes before they are sorted to late endosomes. LAMP1 is sorted by AP complexes (not shown) and transported from the plasma membrane to the endosomes, preferentially to the perimeter membrane of the multivesicular bodies (MVBs) in the sorting endosome and then to the late endosome and cytotoxic granule/LRO. Early endosomes sort cargoes to different destinations. Cargoes can be recycled back to the plasma membrane via fast or slow pathways, delivered to the lysosomes through sorting endosomes and multivesicular bodies, or sorted to the trans-Golgi network. BLOC-1 is involved in the exit of cargoes from an early endosome subdomain toward intermediate/sorting endosomes. γ2-Adaptin has been reported (19) to associate with the ubiquitin machinery, which directs cargo incorporation into intraluminal vesicles (ILVs) of multivesicular bodies by the ESCRT machinery. The study by Rüder et al. in this issue of the JCI (11) reports that EBAG9 associates with and inhibits γ2-adaptin and associates with two BLOC-1 subunits. Loss of EBAG9 enhances the cytotoxic activity of T lymphocytes, likely acting on the endosomal-lysosomal trafficking of the cytotoxic effectors. Although the detailed mechanism accounting for the transport of perforin from the trans-Golgi network to late endosomes is still poorly understood, the findings of Rüder et al. (11) suggest a connection among granzymes and perforin trafficking, the BLOC-1 components, and the ESCRT machinery.
Thus different machineries may participate along the endosomal pathway in the sorting of cargoes involved in LRO biogenesis. The new data reported by Rüder et al. (11) provide some evidence of a link among EBAG9, the BLOC-1 components, the ESCRT machinery, and the biogenesis of cytotoxic granules (Figure 1).
Which step of the cytotoxic pathway is regulated by EBAG9?
Rüder and colleagues used various approaches to determine which step of cytotoxic granule biogenesis is regulated by EBAG9 expression (11). Their findings provide compelling evidence that EBAG9 does not directly regulate the secretion of secretory lysosomes, but rather acts upstream of the exocytic process, in the trafficking of the cytotoxic components along the endosomal-lysosomal pathway. They observed that the loss of EBAG9 enhanced the sorting of granzyme B and of the acid hydrolase cathepsin D to the secretory lysosomes. In contrast, the sorting of lysosomal membrane marker LAMP1, which uses an AP-dependent pathway, was reduced in EBAG9-deficient cells. Interestingly, they report (although this data is not shown) that the sorting of perforin was also enhanced, suggesting that the sorting of perforin may occur via the same trafficking pathway as the sorting of granzymes.
A role for EBAG9 in the trafficking between endosomal and lysosomal compartments is further supported by the finding that EBAG9 interacts with different proteins involved in the sorting of proteins at an early step along this route (11). By yeast two-hybrid analysis, Rüder et al. identified EBAG9 as interacting with two subunits of BLOC-1, snapin and BLOS2, although the authors failed to confirm this interaction by coimmunoprecipitation of the proteins expressed at the endogenous level (11). BLOC-1 mainly localizes to a subdomain of early endosomes and is involved in the exit of selective cargoes from this domain to maturing LROs (15). Although loss of BLOC-1 components has never been shown before to be required for cytotoxic function (18), the possibility that EBAG9 regulates a BLOC-1–dependent cytotoxic granule maturation, as suggested by these data, remains an attractive hypothesis. Another role of snapin in membrane fusion, through its interaction with the SNARE proteins (SNAP-25 and -23), has also been reported (15). This interaction may also participate in cargo exit or travel by regulating membrane fusion events.
In addition, Rüder et al. show that EBAG9 not only associates with BLOC-1 components but also interacts with the adapter protein γ2-adaptin (11). γ2-Adaptin is a unit of the AP complexes, but unlike the other members of the adapter protein family, γ2-adaptin has a ubiquitin-binding ability and can recruit the ubiquitination machinery (19). Thus, by interacting with γ2-adaptin, EBAG9 may participate in the endosomal/multivesicular body sorting and trafficking system within the ESCRT machinery. This function again localizes EBAG9 at the exit of cargo from the early endosome. Of note, depletion of γ2-adaptin results in an enlargement of endosomal vesicles and the accumulation in these vesicles of proteins destined to lysosomal degradation (19). In contrast loss of EBAG9 enhances proteolytic processing of the lysosomal protein cathepsin D and decreases cytotoxic granule size. This opposite phenotype supports a role of EBAG9 as a negative regulator of γ2-adaptin.
Although our present knowledge of the molecular mechanisms that finely regulate the intracellular trafficking of proteins in CTLs precludes further assumption on the precise role of EBAG9 in these cells, some points should be underscored. First, the interaction of EBAG9 with two BLOC-1 components and with γ2-adaptin, if functionally relevant, indicate that EBAG9 regulates sorting of proteins from early endosomes, potentially through two different sorting pathways. Second, it is intriguing to note that the association of an increase in cytotoxic component trafficking, an enhanced cytotoxic function, and a reduced size of cytotoxic granules, which all result from loss of function of EBAG9, inversely mirror the phenotypes evoked by functional loss of LYST in Chediak-Higashi syndrome and of AP3 in HPS2 (8, 20, 21). Both of these proteins are known to participate in the sorting of lysosomal proteins (9, 15). LYST was also recently reported to interact with HRS and LIP5, two ESCRT-associated proteins, and aberrant enlarged vacuolar structures are found when HRS is deficient (17, 22). These observations suggest opposite functions of EBAG9 (negative) and LYST and AP3 (positive) in the regulatory sorting of endosomal proteins into lysosomes.
Together with previous observations that AP3 is able to interact with BLOC-1 and that both HRS and snapin were shown to interact with SNAP-25 in neuroendocrine cells (15, 22), the work of Rüder et al. (11) further suggests that the formation of LROs, among them cytotoxic granules, is more complicated than that of modified lysosomes. Their work further suggests that these granules mature by the addition of cargoes sorted through different pathways and that molecular connections between distinct sorting machineries may potentially exist.
Together with the previous work of Rüder and colleagues, which shows that overexpression of EBAG9 in neuroendocrine cells leads to a decrease in high K+–induced granule release (23), a role for EBAG9 in the negative regulation of the cytotoxic activity of lymphocytes is therefore most likely.
Does EBAG9 deficiency affect serial killing capacity of CTLs?
CTLs are able to successively kill several target cells in a short time period. The iterative killing ability of these cells depends on the combination of two factors: following target cell recognition, TCR triggering leads to the release of only a fraction of the mature cytotoxic granules from CTLs, and at the same time cytotoxic proteins are synthesized to refill the store of cytotoxic components. Regulation of the quantum of cytotoxic granules secreted at the IS may occur at a very late stage of cytotoxic granule maturation, when these granules are polarized together with endosomal exocytic vesicles at the IS and fuse with these vesicles prior to secretion (24). Thus, a pool of cytotoxic granules ready to release their contents persists in CTLs following killing of a first target. This pool can then be used to successively kill other encountered targets. An interesting question is whether the rate of protein synthesis is sufficient to compensate for the increase in trafficking and therefore release of cytolytic components when EBAG9 is defective.
Could excessive cytotoxic function of lymphocytes disturb immune homeostasis?
The granule-dependent cytotoxic activity of lymphocytes plays a major role in the elimination of infected cells, simultaneously regulating the magnitude of the immune response. LCMV infection in mice deficient for the cytotoxic activity of lymphocytes reproduces the immunopathology of HLH observed in humans (12, 25). Not all strains of viruses can trigger such immune manifestations in mice deficient for cytotoxic lymphocyte activity. The fact that clearance of LCMV infection is absolutely dependent on perforin-mediated cytolysis may be an important parameter. Thus, in the context of LCMV infection, the balance between cytotoxicity, antigen presentation, and cytokine production determines the outcome of the immune response. Rüder and colleagues report that EBAG9-deficient mice exhibited augmented CTL clearance of antigen when immunized with Listeria monocytogenes (11). It may be worth studying this mouse model to determine whether, during LCMV infection, enhanced viral antigen clearance has any consequences on the magnitude or the quality of the immune response. One may expect that, in this setting, a rapid elimination of antigens would diminish the expansion of antigen-specific effector cells or the associated Th1 cytokine production (e.g., IFN-γ, TNF-α, IL-6) and also ultimately dampen the generation of memory CD8+ T cells and modify the immunodominance hierarchy of the T cell response (i.e., the specificity of the epitopes present in a given antigen that will preferentially elicit the CD8+ T cell response), as previously shown in mice deficient for IFN-γ (26). Alternatively, the increase in the cytotoxic granule release by CTLs may cause toxic bystander effects, leading to destruction of neighboring cells or tissue infiltrated by the effector cytotoxic cells.
To our knowledge, this report (11) describes the second non-receptor protein whose silencing potentiates T lymphocyte cytotoxicity. Janus kinase and microtubule–interacting protein 1 was previously described as able to restrain T cell–mediated cytotoxicity, potentially acting on cytotoxic granule transport along microtubules (27). Thus, EBAG9 appears to be a new player able to downregulate the process of lymphocyte cytotoxic activity. It suggests that CTL activity, as other major effector pathways of the immune response, is controlled at multiple checkpoints. It is not foolish to predict a future description of several of these checkpoints.
Acknowledgments
We thank Alain Fischer for critical reading of this manuscript. The authors are supported by grants from the French National Institute for Health and Medical Research (INSERM), the French National Research Agency (ANR), and the Fondation pour la Recherche Médicale (FRM).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: AP1, adaptor protein 1; BLOC-1, biogenesis of lysosome-related organelles complex 1; EBAG9, estrogen receptor–binding fragment–associated antigen 9; ESCRT, endosomal sorting complex required for transport; HLH, hemophagocytic lymphohistiocytosis; HPS2, Hermansky-Pudlak syndrome type 2; IS, immunological synapse; LAMP, lysosomal-associated membrane protein; LCMV, lymphocytic choriomeningitis virus; LRO, lysosome-related organelle; LYST, lysosomal trafficking regulator.
Citation for this article: J. Clin. Invest. 119:2136–2140 (2009). doi:10.1172/JCI40270
See the related article beginning on page 2184.
References
- 1.Voskoboinik I., Smyth M.J., Trapani J.A. Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 2006;6:940–952. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 2.Stinchcombe J.C., Griffiths G.M. Secretory mechanisms in cell-mediated cytotoxicity. Annu. Rev. Cell Dev. Biol. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 3.Menasche G., Feldmann J., Fischer A., de Saint Basile G. Primary hemophagocytic syndromes point to a direct link between lymphocyte cytotoxicity and homeostasis. Immunol. Rev. 2005;203:165–179. doi: 10.1111/j.0105-2896.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 4.Fischer A., Latour S., de Saint Basile G. Genetic defects affecting lymphocyte cytotoxicity. Curr. Opin. Immunol. 2007;19:348–353. doi: 10.1016/j.coi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Ménasché G., et al. Mutations in RAB27A cause Griscelli syndrome associated with hemophagocytic syndrome. Nat. Genet. 2000;25:173–176. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 6.Feldmann J., et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. 2003;115:461–473. doi: 10.1016/S0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 7.Dell’Angelica E.C., Shotelersuk V., Aguilar R.C., Gahl W.A., Bonifacino J.S. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol. Cell. 1999;3:11–21. doi: 10.1016/S1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- 8.Clark R.H., et al. Adaptor protein 3-dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nat. Immunol. 2003;4:1111–1120. doi: 10.1038/ni1000. [DOI] [PubMed] [Google Scholar]
- 9.Faigle W., et al. Deficient peptide loading and MHC class II endosomal sorting in a human genetic immunodeficiency disease: the chediak-higashi syndrome. J. Cell Biol. 1998;141:1121–1134. doi: 10.1083/jcb.141.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward D.M., Griffiths G.M., Stinchcombe J.C., Kaplan J. Analysis of the lysosomal storage disease Chediak-Higashi syndrome. Traffic. 2000;1:816–822. doi: 10.1034/j.1600-0854.2000.011102.x. [DOI] [PubMed] [Google Scholar]
- 11.Rüder C., et al. The tumor-associated antigen EBAG9 negatively regulates the cytolytic capacity of mouse CD8+ T cells. . J. Clin. Invest. . 2009;119:2184–2203. doi: 10.1172/JCI37760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan M.B., Hildeman D., Kappler J., Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 13.Bossi G., Griffiths G.M. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin. Immunol. 2005;17:87–94. doi: 10.1016/j.smim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Raposo G., Marks M.S., Cutler D.F. Lysosome-related organelles: driving post-Golgi compartments into specialisation. Curr. Opin. Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setty S.R., et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol. Biol. Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Pietro S.M., et al. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol. Biol. Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams R.L., Urbe S. The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 18.Bossi G., et al. Normal lytic granule secretion by cytotoxic T lymphocytes deficient in BLOC-1, -2 and -3 and myosins Va, VIIa and XV. Traffic. 2005;6:243–251. doi: 10.1111/j.1600-0854.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- 19.Rost M., Doring T., Prange R. gamma2-Adaptin, a ubiquitin-interacting adaptor, is a substrate to coupled ubiquitination by the ubiquitin ligase Nedd4 and functions in the endosomal pathway. J. Biol. Chem. 2008;283:32119–32130. doi: 10.1074/jbc.M802632200. [DOI] [PubMed] [Google Scholar]
- 20.Barbosa M.D.F.S., et al. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature. 1996;382:262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagle D.L., et al. Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat. Genet. 1996;14:307–311. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- 22.Tchernev V.T., et al. The Chediak-Higashi protein interacts with SNARE complex and signal transduction proteins. Mol. Med. 2002;8:56–64. [PMC free article] [PubMed] [Google Scholar]
- 23.Ruder C., et al. EBAG9 adds a new layer of control on large dense-core vesicle exocytosis via interaction with Snapin. Mol. Biol. Cell. 2005;16:1245–1257. doi: 10.1091/mbc.E04-09-0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menager M.M., et al. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat. Immunol. 2007;8:257–267. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- 25.Pachlopnik Schmid J., et al. A Griscelli syndrome type 2 murine model of hemophagocytic lymphohistiocytosis (HLH). Eur. J. Immunol. 2008;38:3219–3225. doi: 10.1002/eji.200838488. [DOI] [PubMed] [Google Scholar]
- 26.Badovinac V.P., Tvinnereim A.R., Harty J.T. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 27.Libri V., et al. Jakmip1 is expressed upon T cell differentiation and has an inhibitory function in cytotoxic T lymphocytes. J. Immunol. 2008;181:5847–5856. doi: 10.4049/jimmunol.181.9.5847. [DOI] [PubMed] [Google Scholar]