Abstract
Hemoglobin (Hb) is crucial to the function of the red blood cell. However, when it is released during intravascular hemolysis from the cell into blood plasma, it produces a state of NO depletion, oxidant stress, and vascular dysfunction, including hypertension. In their study reported in this issue of the JCI, Boretti and colleagues used canine and guinea pig models to demonstrate that pharmacological doses of glucocorticoid can increase the plasma levels of haptoglobin (Hp), the principal plasma-binding protein for free Hb (see the related article beginning on page 2271). Hp prevented Hb-induced hypertension and the generation of oxidant damage to the kidney. Neutralization of free Hb appears to be part of the downstream antiinflammatory properties of glucocorticoid.
Hemoglobin (Hb) plays a critical role in vascular function — it carries oxygen and mediates adaptive vasodilation via NO signaling, most likely via nitrite reductase activity, under hypoxic conditions (1). When red blood cells lyse during intravascular hemolysis, Hb crosses the red blood cell membrane into plasma. Once decompartmentalized from red blood cells, free Hb potently induces oxidative stress and scavenges NO, reducing the bioavailability of this critical antioxidant and master regulator of vascular homeostasis, wreaking havoc on vascular health (2). In this issue of the JCI, Boretti et al. show, in canine and guinea pig models, that pharmacological activation of the glucocorticoid pathway induces expression of the Hb-scavenging protein haptoglobin (Hp), which sequesters cell-free plasma Hb and neutralizes much of its vasculotoxic oxidative stress, protecting against systemic hypertension and other vasculopathic outcomes (3).
Hemolysis, NO scavenging, oxidative stress, and clinical outcomes
For decades, it has been known that Hb is a highly efficient scavenger of NO in vitro. However, only in recent years has it become more widely appreciated that cell-free Hb scavenges NO in vivo in human disease and produces clinical manifestations (Figure 1) (2). The depletion of NO, a crucial endogenous antioxidant, is compounded by the oxidative stress induced by decomposition of Hb into heme and elemental iron, highly oxidative co-conspirators against vascular health. Indeed, not only does a canine model of intravascular hemolysis acutely demonstrate the induction of systemic and pulmonary hypertension but also impaired creatinine clearance, presumably due to decreased renal blood flow (4). Overlapping syndromes have been observed in other human examples of acute hemoglobinemia (excess Hb in the blood plasma), such as the acute hemolytic transfusion reaction (abrupt hemolysis of transfused red blood cell), or following infusion of the first generation of cell-free blood substitutes (2). The acute experimental canine vasculopathy is partially reversed by administration of NO (4) or alternatively, as described in this issue by Boretti et al., by glucocorticoid-induced production of endogenous Hp (3).
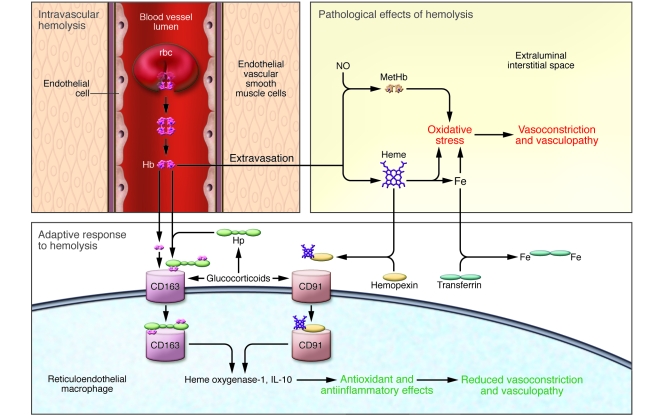
Figure 1. Model of pathologic and adaptive responses to intravascular hemolysis.
A variety of disease conditions can give rise to intravascular hemolysis, including hemolytic anemias such as sickle-cell disease, thalassemia, hereditary spherocytosis, and red blood cell enzymopathies and, to a less-appreciated extent, infection, inflammation, and diabetes mellitus. Extracellular Hb produces a set of pathological effects. It is oxidized to methemoglobin (MetHb) as it scavenges NO directly, depleting this endogenous vasodilator, antioxidant, and master regulator of vascular health. In addition, Hb releases heme and elemental iron (Fe), both of which are intensely oxidative. The net result of extracellular Hb release is oxidative stress, vasoconstriction, and vasculopathy. Glucocorticoids can increase the expression of CD163 and CD91 and, as shown in the study in this issue of the JCI by Boretti et al. (3), also Hp, potentially by either physiologic or pharmacologic stimulation. Hp avidly binds extracellular Hb and suppresses its oxidative consequences, as demonstrated by Boretti et al., largely by preventing its extravasation from the blood vessel lumen to the extraluminal interstitial space, in which some of these oxidative events appear to occur. Hp escorts Hb to the CD163 scavenger receptor on reticuloendothelial macrophages. These cells internalize the receptor-Hp-Hb complex, with consequent induction of heme oxygenase–1 and IL-10, providing antioxidant and antiinflammatory effects. CD163 also is capable, to some extent, of binding free Hb directly. Another adaptive protein, hemopexin, binds free heme and carries it to the macrophage receptor CD91, which also induces heme oxygenase–1. Transferrin transports non-heme iron in plasma, protecting against irons’s oxidative properties.
Sickle-cell disease has provided the most well-documented example of chronic hemolysis and clinical vasculopathy, with markers of hemolysis-induced NO scavenging statistically linked with pulmonary hypertension, leg ulceration, priapism, and cerebrovascular disease (5). Reports also have begun to accumulate for these same exact complications, arising in other non-sickling, acute, and chronic hemolytic disorders, supporting a contributory role for hemolysis in the pathophysiology of this vasculopathy syndrome (5). This growing list includes thalassemia, autoimmune hemolytic anemia, malaria, paroxysmal nocturnal hemoglobinuria, unstable hemoglobinopathy, and hereditary membranopathies (5).
The biological importance of these pathways is emphasized by the redundant and overlapping mechanisms that have evolved to detoxify cell-free plasma Hb. First among these is Hp, a tetrameric plasma glycoprotein that binds cell-free plasma Hb and quickly escorts it to the CD163 protein, in which the Hp-Hb complex is avidly bound and cleared from plasma, depleting plasma Hp in the process (6) (Figure 1). Boretti et al. (3) found that Hp binding to Hb is sufficient to prevent the generation of oxidant species from cell-free Hb that would otherwise mediate hypertension and other adverse vascular outcomes, perhaps in part by sequestering Hb in a high-molecular-weight complex that would not extravasate into the subendothelial space. Interestingly, Boretti et al. also show that Hp-bound Hb has a very high oxygen affinity, which should correspond to increased nitrite reductase activity (1), potentially stimulating the activity of Hp-Hb complexes to produce the endogenous antioxidant NO from nitrite. As part of the teleological evidence of the biological influence of cell-free plasma Hb, additional Hb-binding pathways have evolved, including sequestration of Hb in HDL particles by Hp-related protein and the ability of CD163 scavenger receptor–1 to bind free Hb to some extent, even after Hp has been depleted (7). In addition, the heme-binding protein hemopexin has evolved to mop up the toxic porphyrin heme ring released from decomposing cell-free Hb, and heme-metabolizing enzymes, such as heme oxygenase–1, provide a functional antioxidant effect that is protective to vascular health (8, 9) (Figure 1). Finally, plasma transferrin protein sequesters and safely transports elemental iron released from the heme ring, one of the most oxidative substances in the human body.
Hp, glucocorticoids, and the inflammatory response
Hp has long been known as one of the acute phase reactants — a set of proteins considered to be biomarkers of the inflammatory response but likely also to play a direct role in mediating or regulating the inflammatory response. With the evidence reported in the current study by Boretti et al. (3), it appears that Hp induction might occur as part of the glucocorticoid-induced compensatory response to inflammation, besides induction of CD163 and CD91, which has already been shown (10). This begs the question: why would Hb scavenging be an evolved adaption to inflammation? It might mean that hemolysis is part of the inflammatory process. Indeed, shortened red blood cell survival, a low-grade form of hemolysis, is part of the classical syndrome of anemia of chronic disease, more accurately termed the anemia of inflammation (11). Hp induction during inflammation might speculatively be an evolutionary response to attenuate oxidant stress resulting from hemolysis, a well-characterized feature of serious bacterial infections and malaria (12).
This suspicious link between inflammation, hemolysis, Hp, and vascular disease extends beyond the anemia of inflammation. Levy’s group has demonstrated that an Hp genetic variant that clears cell-free plasma Hb less efficiently than wild-type Hp is linked to accelerated atherosclerosis in patients with diabetes mellitus (13, 14). Provocatively, in diabetes, glycation of the red blood cell surface protein CD59 weakens its ability to withstand complement attack on the membrane, resulting in continuous, low-grade intravascular hemolysis (15), and glycosylation of Hb impairs Hp activity (16). It is an intriguing possibility that part of diabetic vasculopathy might be caused by this largely unappreciated hemolysis, with its attendant NO scavenging and potent oxidative stress.
Finally, the data reported by Boretti et al. (3) raise another very interesting potential clinical link between pharmacological glucocorticoids and relief from the potential toxicity of hemolysis. As mentioned earlier, chronic hemolysis in sickle-cell disease is associated with persistently diminished NO bioavailability and vasculopathy, especially pulmonary hypertension (5). However, this chronic hemolysis has been noted by multiple groups to accelerate during the characteristic sickle-cell vaso-occlusive pain crisis (VOC; typically bone pain occurring due to sickle cells blocking vessel blood flow) and its pulmonary counterpart, the acute chest syndrome (ACS) (17). Intriguingly, glucocorticoids have been demonstrated in double-blind, randomized, placebo-controlled trials to initially reduce the severity of VOC (18) and ACS (19). Could it be that the salutary effect of glucocorticoids in this setting is mediated partly by induction of Hp production, boosting its capacity to neutralize and clear vasculotoxic extracellular Hb? Sickle-cell vaso-occlusion sometimes can rebound in severity when the steroids are stopped or the dose is tapered (18–20). Could this be occurring because Hp levels then fall while hemolysis is still brisk, promoting once more the accumulation of plasma Hb and its consequent NO scavenging, oxidative toxicity, and acute vasculopathy? These are testable questions.
Conclusions
This formulation also suggests a potential benefit from infusion of Hp during acute sickle-cell vaso-occlusion, supporting published anecdotal observations of clinical benefit from plasma exchange in severe sickle-cell disease complications (21). For that matter, Hp effects might also explain some of the life-saving effects of glucocorticoids and aggressive plasma exchange transfusion therapy in thrombotic thrombocytopenic purpura, a disease very well known for its profound intravascular hemolysis (22). The identification of Hp induction as a therapeutic response to pharmacological glucocorticoid administration should stimulate a search for additional drugs that can readily stimulate Hp production and perhaps revive the search for a therapeutic use for purified Hp infusions. Such new research could potentially supplement nature’s armamentarium for detoxifying extracellular Hb.
Acknowledgments
The author thanks Mark Gladwin for providing a constructive review of this Commentary.
Footnotes
Conflict of interest: The author has received research support in the form of a Collaborative Research and Development Agreement between the National Institutes of Health and Ikaria–INO Therapeutics.
Nonstandard abbreviations used: Hb, hemoglobin; Hp, haptoglobin.
Citation for this article: J. Clin. Invest. 119:`–2142 (2009). doi:10.1172/JCI40258
See the related article beginning on page 2271.
References
- 1.Crawford J.H., et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rother R.P., Bell L., Hillmen P., Gladwin M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 3.Boretti F.S., et al. 2009Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J. Clin. Invest 1192271–2280. . 10.1172/JCI39115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minneci P.C., et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J. Clin. Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato G.J., Gladwin M.T., Steinberg M.H. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Reviews. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaer D.J., Alayash A.I., Buehler P.W. Gating the radical hemoglobin to macrophages: the anti-inflammatory role of CD163, a scavenger receptor. Antioxid. Redox Signal. 2007;9:991–999. doi: 10.1089/ars.2007.1576. [DOI] [PubMed] [Google Scholar]
- 7.Schaer D.J., et al. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107:373–380. doi: 10.1182/blood-2005-03-1014. [DOI] [PubMed] [Google Scholar]
- 8.Hvidberg V., et al. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 9.Peterson S.J., Frishman W.H. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol. Rev. 2009;17:99–111. doi: 10.1097/CRD.0b013e31819d813a. [DOI] [PubMed] [Google Scholar]
- 10.Maniecki M.B., Moller H.J., Moestrup S.K., Moller B.K. CD163 positive subsets of blood dendritic cells: the scavenging macrophage receptors CD163 and CD91 are coexpressed on human dendritic cells and monocytes. Immunobiology. 2006;211:407–417. doi: 10.1016/j.imbio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Cartwright G.E. The anemia of chronic disorders. Semin. Hematol. 1966;3:351–375. [PubMed] [Google Scholar]
- 12.Means R.T., Jr. The anaemia of infection. Baillieres Best Pract. Res. Clin. Haematol. 2000;13:151–162. doi: 10.1053/beha.1999.0065. [DOI] [PubMed] [Google Scholar]
- 13.Levy A.P., et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J. Am. Coll. Cardiol. 2002;40:1984–1990. doi: 10.1016/S0735-1097(02)02534-2. [DOI] [PubMed] [Google Scholar]
- 14.Asleh R., Guetta J., Kalet-Litman S., Miller-Lotan R., Levy A.P. Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ. Res. 2005;96:435–441. doi: 10.1161/01.RES.0000156653.05853.b9. [DOI] [PubMed] [Google Scholar]
- 15.Acosta J., et al. Molecular basis for a link between complement and the vascular complications of diabetes. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5450–5455. doi: 10.1073/pnas.97.10.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asleh R., et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ. Res. 2003;92:1193–1200. doi: 10.1161/01.RES.0000076889.23082.F1. [DOI] [PubMed] [Google Scholar]
- 17.Ballas S.K., Marcolina M.J. Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia. Transfusion. 2006;46:105–110. doi: 10.1111/j.1537-2995.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 18.Griffin T.C., McIntire D., Buchanan G.R. High-dose intravenous methylprednisolone therapy for pain in children and adolescents with sickle cell disease. N. Engl. J. Med. 1994;330:733–737. doi: 10.1056/NEJM199403173301101. [DOI] [PubMed] [Google Scholar]
- 19.Bernini J.C., et al. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 1998;92:3082–3089. [PubMed] [Google Scholar]
- 20.Strouse J.J., Takemoto C.M., Keefer J.R., Kato G.J., Casella J.F. Corticosteroids and increased risk of readmission after acute chest syndrome in children with sickle cell disease. Pediatr. Blood Cancer. 2008;50:1006–1012. doi: 10.1002/pbc.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geigel E.J., Francis C.W. Reversal of multiorgan system dysfunction in sickle cell disease with plasma exchange. Acta Anaesthesiologica Scandinavica. 1997;41:647–650. doi: 10.1111/j.1399-6576.1997.tb04759.x. [DOI] [PubMed] [Google Scholar]
- 22.Sadler J.E. Thrombotic thrombocytopenic purpura: a moving target. Hematology Am. Soc. Hematol. Educ. Program. 2006;2006:415–420. doi: 10.1182/asheducation-2006.1.415. [DOI] [PubMed] [Google Scholar]