Abstract
Superoxide dismutase 1 (SOD1) is an important antioxidant previously shown to impact life span in Drosophila. We examined the consequences of manipulating Sod1 expression throughout the body or in the nervous system or musculature on life span and age- related locomotor impairment (ARLI) in Drosophila. Ubiquitous overexpression of SOD1 extended life span but did not substantially forestall ARLI, whereas ubiquitous knock-down of Sod1 shortened life span and accelerated ARLI. Interestingly, neither overexpression of Sod1 nor expression of Sod1 RNAi in the nervous system or muscle altered life span or ARLI. Our studies suggest that the control of reactive oxygen species by SOD1 in tissues other than the nervous system and musculature support life span and ARLI in Drosophila.
Keywords: oxidative stress, life span, Gal4, RNA interference
1. Introduction
Oxidative damage to macromolecules occurs largely via the action of reactive oxygen species (ROS) [1]. Cells contain a variety of antioxidants that convert ROS into less harmful molecules. Nevertheless, oxidative damage to proteins, nucleic acids and lipids occurs under both pathophysiological and normal physiological conditions [2]. Oxidative damage to macromolecules is consequently thought to contribute to numerous disease states as well as aging [3,4].
Studies in Drosophila show that manipulating antioxidant defenses impacts life span [5]. Conditional overexpression of superoxide dismutase 1 (SOD1, localized predominantly in cytosol) increases life span in flies [6,7]. Interestingly, overexpression of SOD1 in motor neurons is also reported to extend life span in flies [8]. Conversely, complete or partial loss of SOD1 activity in flies results in several deleterious phenotypes including sensitivity to oxidative stress and early-onset mortality [8–10]. Studies in other species including C. elegans [11]and mice [12,13] also suggest that SOD activity influences life span. While these and other studies connect SOD1 activity to life span, it remains unclear whether SOD1-induced changes in survival are associated with alteration of other age-related features. Additionally, previous studies on SOD1 activity and life span do not address whether expression of the enzyme in selected tissues impacts the survival and function of organisms as a whole. Here, we addressed these issues by examining the consequences of augmenting and knocking-down SOD1 activity on life span and age-related locomotor impairments (ARLI) in Drosophila.
2. Materials and methods
2.1 Fly stocks and husbandry
Flies were grown and housed at 25°C/55% relative humidity under a 12 hour light-dark cycle. Flies were fed a sugar : yeast : cornmeal : agar medium (10% : 2% : 3.3% : 1% w/v) supplemented with 0.2% Tegosept (Sigma Chemical Co., St. Louis, MO) and active yeast. We used the Gal4/UAS system [14] to express a human Sod1 transgene (UAS-hSod1) provided by Gabrielle Boulianne (Hospital for Sick Children) [8]. Sod1 was knocked down using Gal4 to express a Sod1 inverted repeat (UAS-Sod1-IR) transgene (obtained from the Vienna Drosophila RNAi Center (Vienna, Austria)). Gal4 lines were obtained from the following: daughterless-Gal4 (da-Gal4), John Phillips (University of Guelph); Mef2-Gal4, Sunita Gupta Kramer (Rutgers); Appl-Gal4; Lawrence Goldstein (University of California, San Diego); D42-Gal4, Gabrielle Boulianne (University of Toronto); actin5C–Gal4, 24B–Gal4, repo-Gal4 and elav-Gal4, Drosophila Stock Center (Bloomington, IN); 91Y–Gal4 and 188Y–Gal4, J. Douglas Armstrong (University of Edinburgh).
2.2 SOD activity
3 groups of 25 males (1–3 days old) per genotype were collected via CO2 anesthesia and homogenized in chilled 50 mM potassium phosphate/0.1 mM EDTA/2% Triton-X-100 buffer (pH 7.8). Samples were sonicated for 20 sec, incubated at 4°C for 45 min and then centrifuged at 16,000 x g for 15 min at 4°C. Equal amounts of total protein from the supernatants (determined with DC Protein Assay, Bio-Rad) were electrophoresed by Native PAGE (4% stacking gel (pH 6.8), 20% resolving gel (pH 8.8)). SOD activity was assessed colorimetrically using an “in-gel” assay as previously described [15]. SOD activity was quantified by densitometry using Alpha Imager software (Alpha Innotech Corp., San Leandro, CA).
2.3 Life span analysis
Adult males (1–3-days old, 150–200 flies/genotype) were collected under brief CO2 anesthesia and placed in fresh food vials, 25 flies/vial. Every 2–4 days, flies were transferred to fresh food vials and the number of dead and surviving flies counted.
2.4 Negative geotaxis
Groups of 25 adult males (1–3 days old) were collected under brief CO2 anesthesia and left to recover at least 18 hours at 25°C and 55% relative humidity prior to assessing negative geotaxis (bang-induced climbing) in Rapid Iterative Negative Geotaxis (RING) assays as previously described [16]. Five vials per genotype were tested to generate N=5. Following testing, flies were placed in fresh food vials for housing until the next RING test.
2.5 Statistical analyses
One- and two-way ANOVA, Tukey’s HSD multiple comparison tests, and log-rank tests were performed with JMP 5.01 (SAS Institute, Cary, NC, USA). P values greater than 0.05 were considered not significant (n.s.). Error bars represent S.E.M.
3. Results and Discussion
3.1 Ubiquitous over expression of hSod1 extends life span but minimally affects ARLI
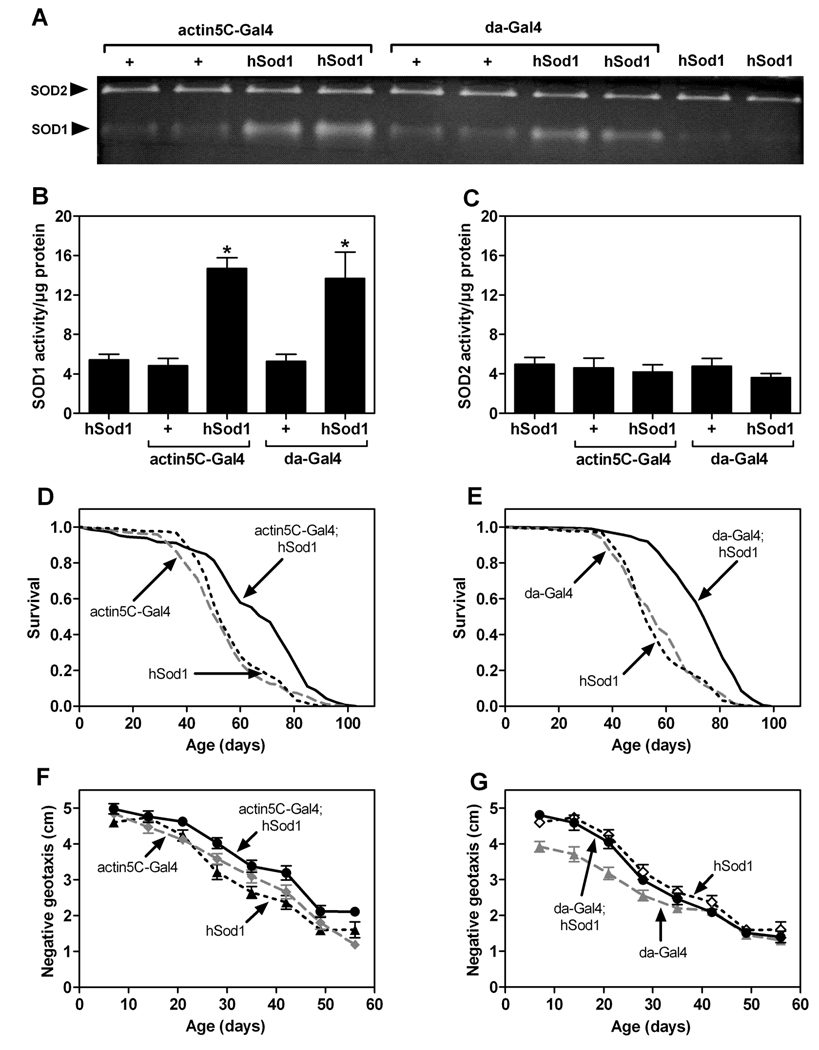
Ubiquitous overexpression of human Sod1 (hSod1) via either actin5C–Gal4 or da-Gal4 increased SOD1 activity by approximately 3-fold (Figure 1A and 1B) without altering SOD2 activity (Figure 1A and 1C). Consistent with a previous study using a conditional expression strategy [6], we found that ubiquitous expression of hSod1 driven by actin5C–Gal4 or da-Gal4 extended mean life span by 15–30% (Figure 1D and 1E). The increased life span in flies with ubiquitous hSod1 overexpression suggested that other manifestations of aging might be delayed in these animals. To address this issue, we assessed ARLI (defined as the age-related impairment in negative geotaxis, a locomotor behavior [17]). Ubiquitous expression of hSod1 via actin5C–Gal4 modestly delayed ARLI (Figure 1F), whereas hSod1 expression driven by da-Gal4 did not (Figure 1G).
Figure 1.
Ubiquitous overexpression of hSod1 extends life span and has minimal effects on ARLI. (A) In-gel SOD2 (upper band) and SOD1 (lower band) activity in replicate protein extracts (45µg/lane)from flies with the indicated genotypes. (B) SOD1 activity (determined by densitometry) was increased in flies expressing hSod1 via actin5C–Gal4 or da-Gal4 compared to controls with single transgenes (one-way ANOVA, p ≤ 0.0045; Tukey’s HSD, * p < 0.05; n = 3). (C) SOD2 activity was not affected by hSod1 overexpression (one-way ANOVA, n.s., n = 3). Survival was altered by expression of hSod1 via actin5C–Gal4 (D) or da-Gal4 (E) relative to controls with single transgenes (log-rank tests, p < 0.0001, n = 200 flies/genotype). (F) Age and genotype affected negative geotaxis (two way ANOVA, p < 0.0001). Flies expressing hSod1 via actin5C–Gal4 performed significantly better than controls (Tukey’s HSD, p < 0.05; n = 9–10 vials of 25 flies/genotype). (G) Although age and genotype affected negative geotaxis (two-way ANOVA, p<0.0001), flies with da-Gal4-driven expression of hSod1 were not different from hSod1 controls (Tukey’s HSD, n.s., n = 9–10 vials of 25 flies/genotype).
Although ubiquitous overexpression of hSod1 extended life span, it did not substantially delay ARLI. Ubiquitous overexpression of hSod1, therefore, does not appear to impact all aspects of aging equally in flies. Interestingly, dietary restriction and a mutant allele of methuselah also extend life span in flies, but neither manipulation significantly forestalls locomotor or olfactory senescence [18,19]. The experimental dissociation between life span and behavioral senescence in flies with genetic or dietary manipulations is consistent with a model in which different aspects of aging are driven by distinct mechanisms.
3.2 Overexpression of hSod1 in the nervous system or muscle has no effect on life span
Due to their high metabolic demands with attendant high rates of ROS generation, neuronal and muscle cells are thought to experience more oxidative damage than other cells [2]. This has led to the hypothesis that these tissues may benefit the most from augmentation of antioxidant defenses. We therefore reasoned that ubiquitous hSod1 overexpression might extend life span as a result of increased expression in the nervous system or musculature.
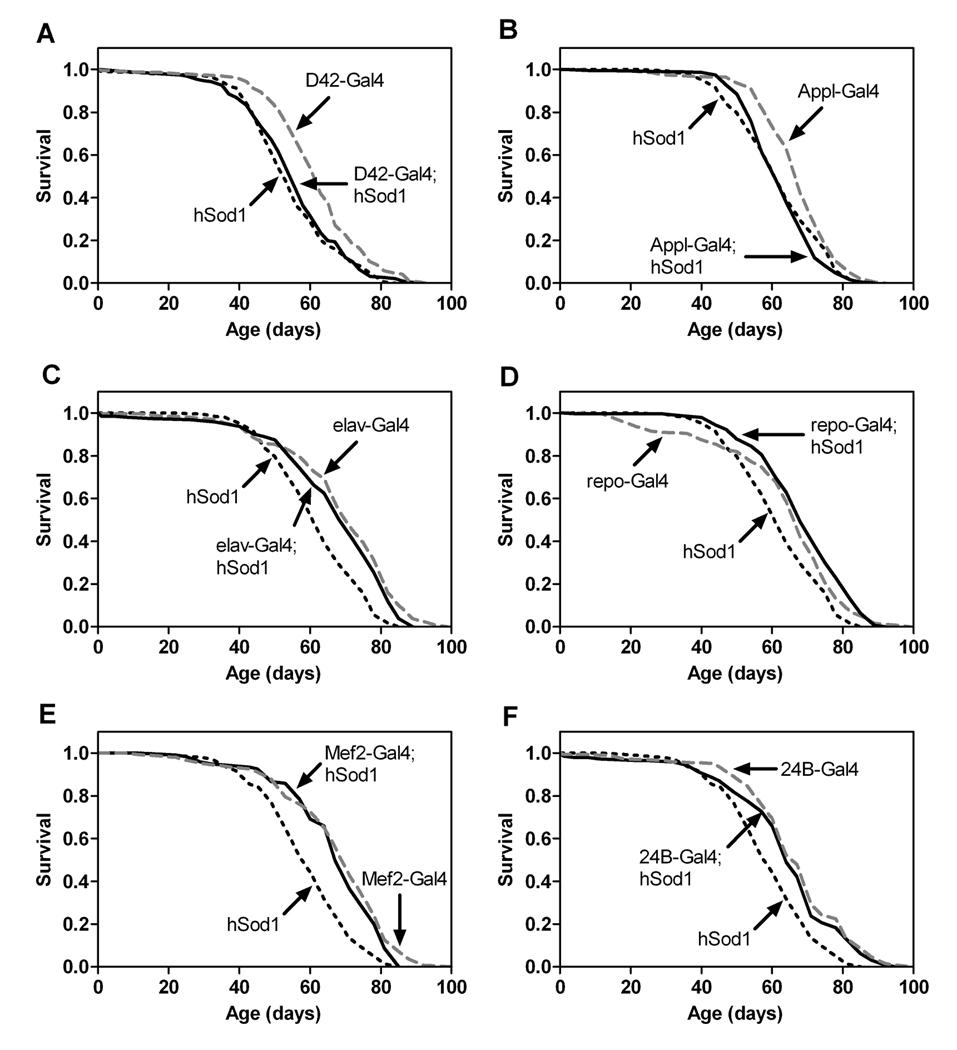
Consistent with the nervous system component of this hypothesis, expression of hSod1 in motor neurons using D42-Gal4 was previously reported to extend life span in Drosophila [8]. To further investigate the effects of augmenting nervous system antioxidant defenses on life span, we assessed survival in flies expressing hSod1 in motor neurons using D42-Gal4, in all neurons using Appl-Gal4 and elav-Gal4, and throughout the glia using repo-Gal4. Surprisingly, we found that overexpression of hSod1 in motor neurons did not extend life span (Figure 2A). Furthermore, neither pan-neuronal (Figure 2B and C) nor pan-glial (Figure 2D) overexpression of hSod1 extended life span. Our results suggest that augmentation of antioxidant defenses selectively within the nervous system via overexpression of hSod1 does not have substantive effects on life span in flies. We note, however, that there could be complex effects of genotype or environment that permit the observed life span effects of nervous system hSod1 expression in some laboratories [8] while they preclude such effects in other experimental settings. For example, it would be interesting to determine whether the genetic background used in the previous studies [8] gives rise to elevated levels of oxidative stress as compared to the genetic background in our studies and whether this difference in oxidative stress might explain the divergent effects of hSod1 overexpression on life span. Nevertheless, overexpression of hSod1 in the nervous system does not appear to consistently extend life span in flies.
Figure 2.
Overexpression of hSod1 in the nervous system or muscle has no effect on life span. Flies expressing hSod1 via D42-Gal4 (A), Appl-Gal4 (B), elav-Gal4 (C), repo-Gal4 (D), Mef2-Gal4 (E) or 24B–Gal4 (F) were not longer-lived than controls with single transgenes (log-rank tests, n.s., n = 150–200 flies/genotype).
To determine whether increasing antioxidant defenses in muscle influences life span, we assessed survival of flies that expressed hSod1 in this tissue via Mef2-Gal4 or 24B–Gal4. Overexpression of hSod1 in muscle did not alter life span (Figure 2E and 2F). Thus, overexpression of hSod1 in the musculature is not sufficient to extend life span in Drosophila.
Our data suggest that the life span extension found in flies with ubiquitous hSod1 expression is not due solely to increased SOD1 activity in the nervous system or musculature. It is possible, therefore, that augmentation of antioxidant defenses in all tissues via ubiquitous hSod1 expression is required to extend life span in flies. An alternative possibility is that overexpression of hSod1 in a key, unidentified tissue outside of the nervous system and musculature is required for life span extension in Drosophila.
3.3 Ubiquitous knock-down of Sod1 shortens life span and accelerates ARLI
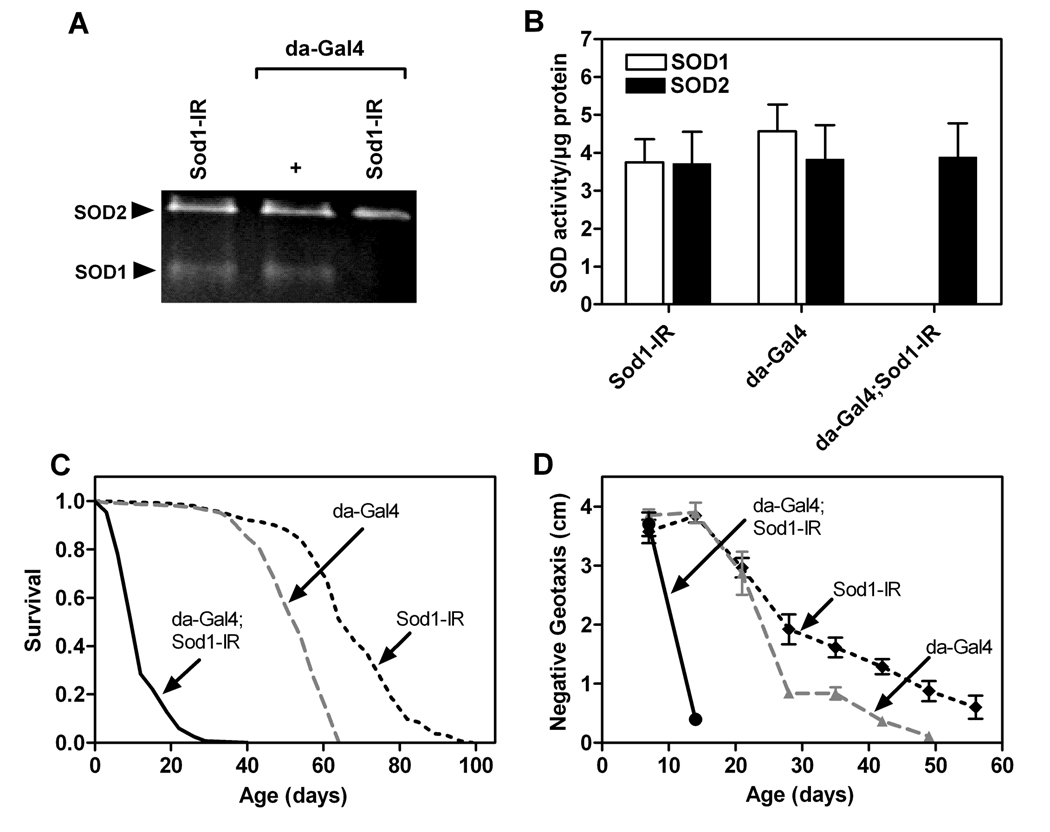
Sod1 loss-of-function mutations and knock-down of Sod1 function through RNA interference (RNAi) dramatically reduce life span in flies [20,21]. Toward further investigating the normal protective effects of Sod1, we knocked-down Sod1 function via RNAi through Gal4-driven expression of a UAS-Sod1-inverted repeat (Sod1-IR) transgene. We confirmed that ubiquitous expression of Sod1-IR driven by da-Gal4 substantially decreased SOD1 activity without altering SOD2 activity (Figure 3A and 3B). We also confirmed that ubiquitous expression of Sod1-IR substantially shortened life span (Figure 3C) and further found that it dramatically accelerated ARLI (Figure 3D).
Figure 3.
Ubiquitous knock-down of SOD1 activity decreases life span and accelerates ARLI. (A) SOD1 and SOD2 activity in protein extracts (45 µg/lane) from the indicated genotypes. (B) SOD1 activity was reduced to below detectable levels in flies expressing Sod1-IR via da-Gal4 (one-way ANOVA, p < 0.0001, n = 3) while SOD2 activity was unaffected (one-way ANOVA, n.s., n = 3). (C) Ubiquitous knock-down of Sod1 significantly shortened life span relative to controls (log-rank tests, p < 0.0001, n = 150 flies/genotype). (D) ARLI was accelerated by ubiquitous Sod1 knock-down relative to controls (one-way ANOVA, p < 0.0001; Tukey’s HSD, p <0.05; n = 5 vials of 25 flies/genotype).
3.4 Sod1-IR expression in the nervous system or muscle has modest effects on life span and ARLI
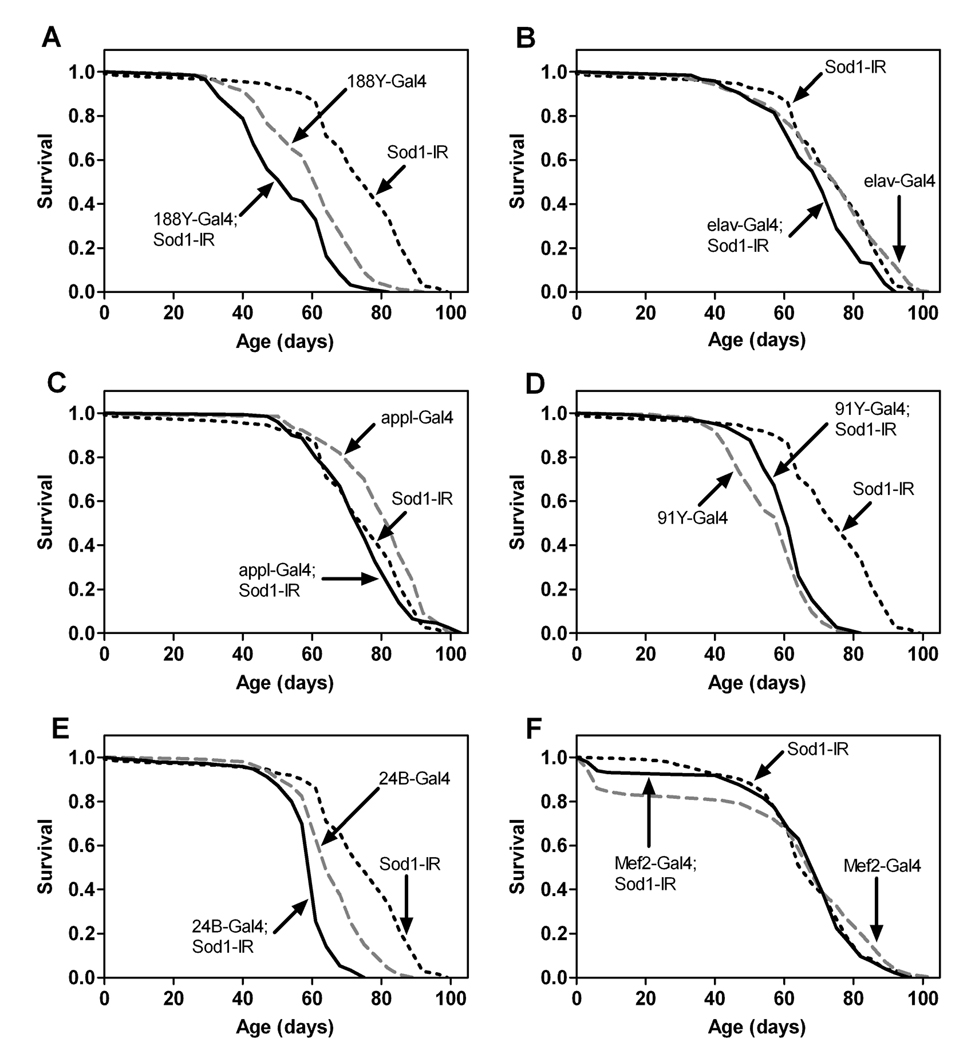
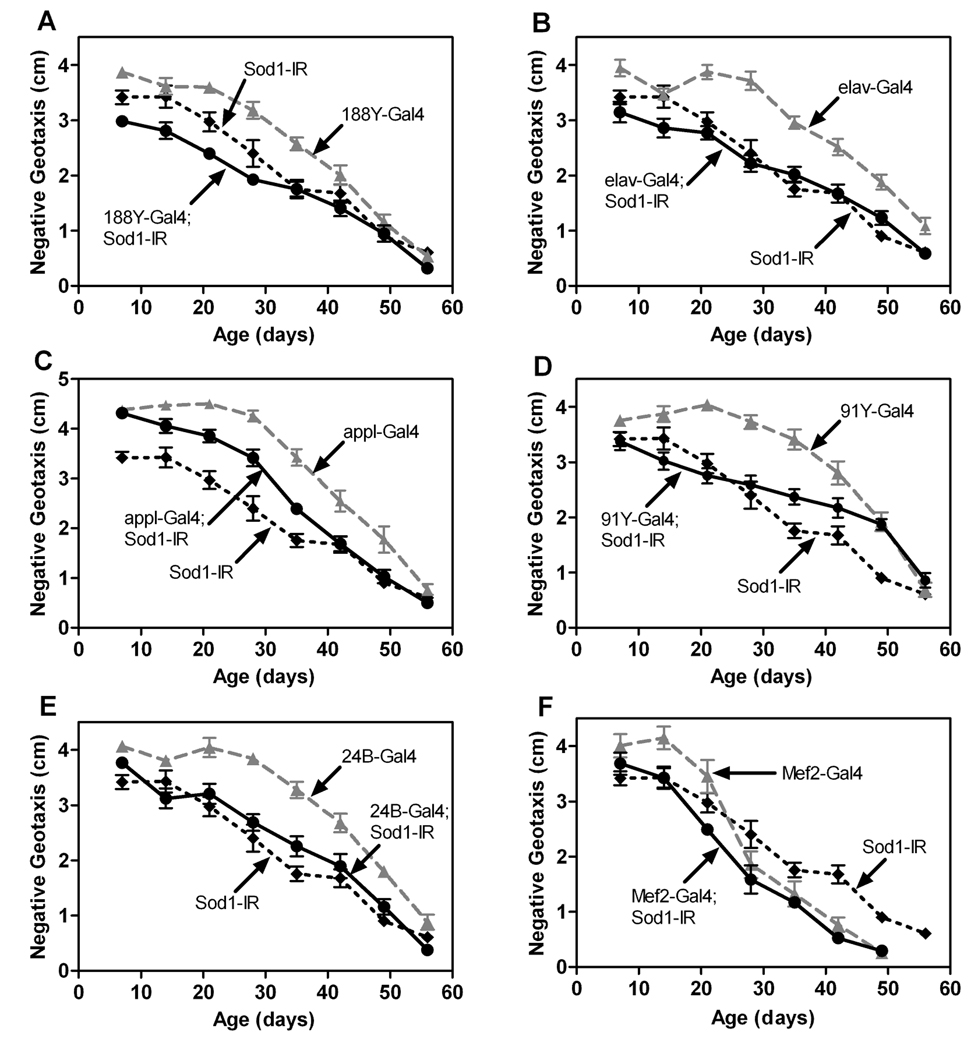
Overexpression of hSod1 in the nervous system or musculature alone had no major effect on life span (Figure 2). Thus, SOD1 activity in these tissues might not be central to aging in flies. If true, then expression of Sod1-IR in the nervous system or muscle should not affect life span or other aspects of aging. Expression of Sod1-IR via the pan-neuronal Gal4 drivers 188Y and elav resulted in small but statistically discernable decreases in life span compared to controls harboring Gal4 or Sod1-IR alone (Figure 4A and 4B). Sod1-IR expression via two other pan-neuronal drivers, Appl-Gal4 or 91Y–Gal4, however, did not significantly affect life span (Figure 4C and 4D). Expression of Sod1-IR in the muscle via 24B–Gal4 also resulted in a statistically significant, albeit small, reduction in life span (Figure 4E). In contrast, Sod1-IR expression in the muscle via Mef2-Gal4 had no significant effect on life span (Figure 4F).
Figure 4.
Expression of Sod1-IR in the nervous system or muscle has limited effects on life span. Pan-neuronal expression of Sod1-IR via 188Y–Gal4 (A) or elav-Gal4 (B) decreased life span relative to UAS-Sod1-IR and Gal4 controls (log-rank tests, p ≤ 0.0006, n = 150 flies/genotype), whereas flies expressing Sod1-IR via Appl-Gal4 (C) or 91 Y-Gal4 (D) were not shorter-lived than both control groups (log-rank tests, n.s., n = 150 flies/genotype). Expression of Sod1-IR in muscle via 24B–Gal4 (E) shortened life span relative to both control groups (log-rank tests, p < 0.0001, n = 150 flies/genotype), while expression via Mef2-Gal4 (F) did not (log-rank tests, n.s., n = 150 flies/genotype).
Expression of Sod1-IR throughout the nervous system using 188Y–Gal4 subtly but significantly impaired negative geotaxis across age relative to controls carrying either 188Y–Gal4 or Sod1-IR alone (Figure 5A). The locomotor defect in flies with 188Y–driven expression of Sod1-IR was restricted to thefirst 28days of their life, though, indicating that these lies have a generalized decrease in locomotor behavior as opposed to an age-dependent acceleration of ARLI. Sod1-IR expression in the nervous system via elav-Gal4 (Figure 5B), Appl-Gal4 (Figure 5C) or 91Y–Gal4 (Figure 5D) also did not accelerate ARLI. Furthermore, expression of Sod1-IR in the muscle via 24B–Gal4 (Figure 5E) had no effect on ARLI while Mef2-Gal4-driven Sod1-IR expression (Figure 5F) resulted in a subtle but statistically discernable acceleration in ARLI.
Figure 5.
Expression of Sod1-IR in the nervous system or muscle has minimal effects on ARLI. Overall, age and genotype affected negative geotaxis in studies with flies expressing Sod1-IR via 188Y–Gal4 (A), elav-Gal4 (B), Appl-Gal4 (C), 91Y–Gal4 (D), 24B–Gal4 (E) or Mef2-Gal4 (F) (individual two-way ANOVAs, p < 0.0001, n = 5–10 vials of 25 flies/genotype). Compared to single transgene controls, negative geotaxis was reduced only in flies with Sod1-IR expression driven by 188Y–Gal4 (A) or Mef2-Gal4 (F) (Tukey HSD, p<0.05).
When viewed together, our studies indicate that expression of Sod1-IR in the nervous system or muscle has very limited effects on life span and ARLI in flies. Given that expression of the Sod1 -IR transgene efficiently knocks-down SOD1 activity in the whole body (Figure 3A) and that the nervous system and muscle Gal4 lines strongly express in their respective tissues (Martin et. al, submitted), the lack of effect of nervous system or muscle expression of Sod1-IR does not appear to be related to poor transgene function. SOD1 activity in either of these tissues alone, therefore, is not required to protect flies from premature mortality and locomotor demise with age. Since ubiquitous knock-down of Sod1 has severe consequences on life span and ARLI, it is possible that the collective effects of Sod1 throughout the body or in a tissue outside of the nervous system and musculature is important for normal life span and locomotor function across age in flies.
3.5 Summary
Our studies indicate that a ubiquitous increase or decrease in Sod1 expression impacts life span and, to a limited extent, ARLI. These effects do not appear to be connected to altered expression of Sod1 in the nervous system or musculature. Our studies also support the hypothesis that life span and ARLI are driven by distinct mechanisms in Drosophila.
Acknowledgements
The authors thank Devin Rhodenizer for expert technical assistance and the individuals listed in Materials and Methods for providing fly stocks. This work was supported in part by a grant to M.G. from the N.I.A. (AG024259).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B, Gutteridge JM. Oxford: Oxford University Press; 1999. Free Radicals in Biology and Medicine. [Google Scholar]
- 3.Martin I, Grotewiel MS. Oxidative damage and age-related functional declines. Mech Ageing Dev. 2006;127:411–423. doi: 10.1016/j.mad.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Cutler RG, Mattson MP. The adversities of aging. Ageing Res Rev. 2006;5:221–238. doi: 10.1016/j.arr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol Cell Biol. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J, Molitor J, Tower J. Effects of simultaneous over-expression of Cu/ZnSOD and MnSOD on Drosophila melanogaster life span. Mech Ageing Dev. 2004;125:341–349. doi: 10.1016/j.mad.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 9.Reveillaud I, Phillips J, Duyf B, Hilliker A, Kongpachith A, Fleming JE. Phenotypic rescue by a bovine transgene in a Cu/Zn superoxide dismutase- null mutant of Drosophila melanogaster. Mol Cell Biol. 1994;14:1302–1307. doi: 10.1128/mcb.14.2.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wicks S, Bain N, Duttaroy A, Hilliker AJ, Phillips JP. Hypoxia rescues early mortality conferred by superoxide dismutase deficiency. Free Radic Biol Med. 2009;46:176–181. doi: 10.1016/j.freeradbiomed.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 11.Melov S, et al. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- 12.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 14.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 15.Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci U S A. 2002;99:16162–16167. doi: 10.1073/pnas.252342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol. 2005;40:386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Res Rev. 2005;4:372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Cook-Wiens E, Grotewiel MS. Dissociation between functional senescence and oxidative stress resistance in Drosophila. Exp Gerontol. 2002;37:1347–1357. doi: 10.1016/s0531-5565(02)00096-7. [DOI] [PubMed] [Google Scholar]
- 19.Bhandari P, Jones MA, Martin I, Grotewiel MS. Dietary restriction alters demographic but not behavioral aging in Drosophila. Aging Cell. 2007;6:631–637. doi: 10.1111/j.1474-9726.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 20.Phillips JP, Campbell SD, Michaud D, Charbonneau M, Hilliker AJ. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989;86:2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahadorani S, Bahadorani P, Phillips JP, Hilliker AJ. The effects of vitamin supplementation on Drosophila life span under normoxia and under oxidative stress. J Gerontol A Biol Sci Med Sci. 2008;63:35–42. doi: 10.1093/gerona/63.1.35. [DOI] [PubMed] [Google Scholar]