Abstract
Fibromyalgia (FM) is thought to involve abnormalities in central pain processing. Recent studies involving small samples have suggested alterations in gray matter volume (GMV) in brains of FM patients. Our objective was to verify these findings in a somewhat larger sample using voxel-based morphometry (VBM), while controlling for presence of affective disorders (AD). T1-weighted magnetic resonance image (MRI) brain scans were obtained on 29 FM patients with AD, 29 FM patients without AD, and 29 age-matched healthy controls (HC) using a 3T scanner. Segmentation, spatial normalization, and volumetric modulation were performed using an automated protocol within SPM5. Smoothed gray matter segments were entered into a voxel-wise one-way ANOVA, and a search for significant clusters was performed using thresholding methods published in previous studies (whole-brain threshold of p<.05 correcting for multiple comparisons; region-of-interest (ROI) threshold of p≤.001 uncorrected, or p<.05 small-volume corrected). The whole-brain analysis did not reveal any significant clusters. ROI-based analysis revealed a significant difference in left anterior insula GMV among the three groups (xyz={−28, 21, 9}; p=.026, corrected). However, on post-hoc testing, FM patients without AD did not differ significantly from HC with respect to mean GMV extracted from this cluster. A significant negative correlation was found between mean cluster GMV and scores of trait anxiety (State-Trait Personality Inventory, Trait Anxiety scale; rho=−.470, p<.001). No other significant clusters were found on ROI-based analysis. Our results emphasize the importance of correcting for AD when carrying out VBM studies in chronic pain.
Keywords: Fibromyalgia, voxel-based morphometry, MRI, gray matter, depression, pain
Fibromyalgia (FM) affects 0.5–4% of the population in developed countries [14,24], and is defined as chronic widespread pain and tenderness in at least 11 of 18 tender points [26]. Individuals with FM are more likely than the general population to also meet criteria for chronic fatigue syndrome, irritable bowel syndrome, temporomandibular joint disorder, vulvodynia, and migraine [11]. Mechanisms of central augmentation of pain and sensory processing are thought to account for the pathophysiology of FM and related conditions [15].
Evidence of abnormal CNS processing in FM can be found in a number of functional neuroimaging studies, including altered resting and stimulus-evoked regional cerebral blood flow in pain and emotional processing regions such as the thalamus, somatosensory cortex, insula, and anterior cingulate cortex [25]. In contrast to functional neuroimaging, structural neuroimaging approaches—such as voxel-based morphometry (VBM)—use differences in gray matter volume (GMV) or density to support hypotheses regarding CNS function, and may reflect trait rather than state characteristics of the brain. With recent improvements in computer processing speed, the automated process of VBM has allowed for fast, reliable calculations of GMV in large samples of subjects [2].
In recent years, VBM has been used to study differences in GMV associated with various pain conditions, including migraine [23], tension headache [17], chronic back pain [1,16], and FM [12,18]. The two studies published to date in FM patients reported global and/or regional GMV differences between patients and controls. However, the sample sizes were modest (≤ 20 in the FM group), and there were no common regions of increased or decreased GMV between the two studies. Furthermore, while both studies addressed depression as a potential confounding variable, one study did not account for less-severe depressive disorders such as dysthymia [12], and the other study did not find any significant GMV differences at the whole-brain level after controlling for depression [18].
In the present study, we applied VBM methodology to a sample of 58 FM patients and 29 age-matched healthy controls, to look for regions of increased or decreased GMV associated with FM, and attempt to replicate previously-published findings. We used statistical thresholds identical to those published in previous studies [12,18]. We then tested whether the results changed when controlling for AD. We hypothesized that one or more regional GMV changes previously reported to be associated with FM would be replicated in this study. We also hypothesized that, even when controlling for AD, FM patients would still exhibit differences in global and/or regional GMV within pain-related brain regions relative to controls.
METHODS
Participants
All subjects with FM who were enrolled in two ongoing non-pharmacological clinical trials were considered for the present analyses. Healthy controls were obtained from the same studies, and also from a previous cross-sectional study performed at our center. At the time of data collection, all FM patients had met 1990 American College of Rheumatology criteria for FM [26], with mean pain duration of 12.8 years (SD = 8.3). No healthy controls had met these criteria, nor did they meet criteria for chronic regional pain (i.e., tension headaches, chronic low back pain, irritable bowel syndrome, or chronic pelvic pain). All subjects were right-handed women between ages 18 and 65. All subjects gave written informed consent, the study protocol was approved by the University of Michigan Institutional Review Board, and all procedures performed in compliance with the Helsinki Declaration.
Affective disorder (AD) was defined as current major depressive episode, bipolar disorder, dysthymia, or general anxiety disorder (according to Diagnostic and Statistical Manual of the American Psychiatric Association IV criteria) as determined upon subject enrollment using a structured interview [19]. Patients with a history of clinical depression according to medical records; and/or patient-reported use of antidepressants to treat depression or anxiety, were also considered to have AD. Individuals with AD were excluded from the healthy control group. Individuals with severe psychiatric illness (current schizophrenia, major depression with suicidal ideation, substance abuse within two years), were excluded from all groups.
A total of 29 FM patients with AD (FM+AD), 29 FM patients without AD (FM−AD), and 29 healthy controls were included in the analysis. The groups were individually matched by age, with an overall difference of no more than three years across each matched trio.
Neuroimaging and analysis
Image acquisition
High-resolution anatomical magnetic resonance image (MRI) scans were obtained on all subjects using the same 3-Tesla scanner (Signa LX, General Electric, Milwaukee, USA). Images were acquired by using spoiled gradient-recalled acquisition in steady state (SPGR) imaging (repetition time, 10.5 ms; echo time, 3.4 ms; flip angle, 20°; field of view 24 cm, number of contiguous images, 106; in-plane resolution .9375 × .9375mm, slice thickness, 1.5 mm). The resulting voxel dimension was of sufficiently high resolution to permit accurate gray matter segmentation [2].
VBM protocol
Data pre-processing and analysis were performed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK) on the Matlab version 6.5 (MathWorks, Natick, MA) platform. Each image was inspected for reconstruction artifacts, and individually corrected for signal inhomogeneity using an automated Matlab protocol developed by G. Glover and K. Christoff (http://rsl.stanford.edu/glover/). Spatial normalization, segmentation, and volumetric modulation were performed using the automated VBM5 toolbox (C. Gaser, Structural Brain Imaging Group, Department of Psychiatry, University of Jena; http://dbm.neuro.unijena.de/vbm/) within the SPM5 environment. The toolbox employs a Hidden Markov Random Field Model in the procedure to segment each image into gray matter, white matter, and cerebrospinal fluid. The toolbox then normalizes the gray matter segment of each image to the International Consortium for Brain Mapping (ICBM) 152 template (Montreal Neurological Institute; MNI), and performs a modulation step to scale each voxel value according to the subject’s total intracranial volume (TIV), as well as the regional gray matter volume (GMV) expansion/contraction that occurs during nonlinear transformation. TIV and global GMV were obtained for each image, using the “Calculate raw volumes” feature of VBM5. Gray matter image segments were inspected for segmentation artifacts, then smoothed using an isotropic Gaussian kernel of 10mm full-width half-maximum (FWHM), to accommodate individual differences in sulcal and gyral anatomy, and to meet the distributional assumptions of the general linear models necessary for statistical analysis. This is the same smoothing kernel as was used in previous studies ([12,18]; a comparison of methods used in the two previous studies and the present study is shown in Table 1).
Table 1.
Differences among VBM studies of FM patients vs. healthy controls
| Study | N (FM/HC) | Handedness | Mean age (FM/HC) | Mean duration of pain, FM subjects | Screening tool for affective disorder |
|---|---|---|---|---|---|
| Kuchinad et al. [12] | 10/10 | Not reported | 52/45a | 6.85 yrs (time since diagnosis) | DSM-IV criteria (MDD) |
| Schmidt-Wilcke et al. [18] | 20/22 | Not reported | 53.6/50.7 | 14.4 yrs(widespread pain) | Beck Depression Inventory |
| present study | 58/29 | All right-handed | 42.1/42.2 | 12.8 yrs (regional or widespread pain) | DSM-IV criteria(MDD, dysthymia, and GAD) |
| Study | Magnet strength | Homogeneity correction? | Smoothing kernel(FWHM) | Significance threshold, WB | Significance threshold, ROI |
|
| |||||
| Kuchinad et al. | 1.5 T | Yes | 10mm | p < .05b | p < .001c |
| Schmidt-Wilcke et al. | 1.5 T | Yes | 10mm | p < .05b | p < .05d |
| present study | 3 T | Yes | 10mm | p < .05b | p < .001c and p < .05d |
Age was entered as a covariate in the model [12].
Corrected for multiple comparisons.
Uncorrected.
Corrected for multiple comparisons and size of search volume.
Abbreviations: FM = fibromyalgia; HC = healthy controls; DSM IV = Diagnostic and Statistical Manual of the American Psychiatric Association IV; MDD = major depressive disorder; GAD = general anxiety disorder; T = tesla; FWHM = full-width half-maximum; WB = whole-brain search; ROI = ROI-based search.
Voxel-wise comparison of GMV between FM patients and healthy controls
The normalized, modulated, and smoothed gray matter image segments in each group were entered into a voxel-wise one-way ANOVA in SPM5, with a null hypothesis of no GMV difference among the three groups (FM+AD, FM−AD, and HC). Because the modulation step reintroduces information about the subject’s TIV prior to normalization, TIV was included as a covariate. An absolute threshold mask of 0.20 was used (identical to the threshold used in a previous study [18]), to avoid possible edge effects around the border between gray and white matter. Due to the occasional presence of susceptibility artifacts at the base of the brain in our sample, an explicit mask was also used to exclude all voxels inferior to z = −22 (Talairach space).
A whole brain search for significant clusters was performed using the previously-published voxel-wise threshold of p < .05 corrected for multiple comparisons [12,18]. In addition, an ROI-based search using all a priori anatomically-defined ROIs from the two previously published studies (Table 2) was conducted. For the ROI-based search, all clusters with either a voxel-level p < .05 (small-volume and family-wise-error corrected using masks from the MarsBar ROI toolbox [6], accessible at http://marsbar.sourceforge.net/) or an uncorrected voxel-level p < .001 were considered significant. Since post-hoc tests were not available for SPM5 at the time of these analyses, post-hoc analysis was performed using between-group contrasts on the F map to determine the peak significance value of any voxels within the cluster, and a Bonferroni-corrected p < .05/3 or .0167 was used as the threshold of significance. MNI coordinates for significant clusters were transformed into Talairach coordinates using a nonlinear transformation proposed by the Cognition and Brain Sciences unit of the Medical Research Council of the United Kingdom (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach). Mean voxel values for any significant clusters, as well as for the entire smoothed gray-matter image segment, was extracted from each subject for use in further analyses.
Table 2.
Regions of interest used in VBM studies of fibromyalgia patients vs. healthy controls, defined a priori.
| Study | Regions of Interest |
|---|---|
| Kuchinad et al. | cingulate cortex (anterior, mid, and posterior), insular cortex, dorsolateral prefrontal cortex, medial prefrontal cortex, parahippocampul gyrus |
| Schmidt-Wilcke et al. | striatum (lentiform and caudate nuclei), thalamus |
| present study | All of the above |
Continuous measures of pain and affective symptoms
Pain duration
We chose pain duration, rather than momentary pain level, as a representation of clinical pain in this study due to the highly fluctuating nature of momentary pain in FM [9], and the notion that long-term changes in brain structure would more likely be affected by the time since onset of pain, rather than the momentary magnitude of pain. Pain duration was defined as the number of years between onset of pain and MRI scan. Because FM patients usually present with a preceding history of chronic regional pain [5], onset of pain from regional pain conditions (such as tension headaches, temporomandibular joint disorder, irritable bowel syndrome, and low back pain) was used when reported.
Depressive symptoms
Depressive symptoms were rated using the Center for Epidemiological Studies-Depression Scale (CES-Depression [3]), a 20-item self-report inventory designed to assess depressive mood. Respondents are asked to indicate how frequently they experienced each of a set of symptoms during the past week, ranging from 0 (less than 1 day) to 3 (5–7 days). The total possible score, ranging from 0 to 60, reflects both the number of symptoms and the frequency of their occurrence. The CES-Depression scale was administered within one week of the MRI scan.
Anxiety
Trait anxiety was measured using the 10-item Trait Anxiety scale from the State-Trait Personality Inventory (STPI [21]). Scores range from 10 to 40, with higher value indicating higher anxiety symptoms. This subscale has strong evidence of concurrent validity with other validated measures of anxiety, and has shown good test-retest stability [20]. This inventory was administered prior to any study-specific therapeutic intervention.
Statistical analysis
Aside from statistical parametric mapping, all statistical analyses were performed using SPSS, version 14.0 (SPSS Inc., Chicago, IL). Variables were examined for normality, and means and standard errors of the mean were calculated separately for each group. Between-group differences were tested using a one-way ANOVA, with a significance threshold of p < .05. For any significant clusters, the standardized residuals from a linear regression between mean voxel value (dependent) and TIV (independent) were calculated. Nonparametric bivariate correlations between cluster-specific GMV and other variables were performed using these standardized residuals, and Spearman correlation coefficients were calculated. A Bonferroni correction for multiple comparisons (4 variables: cluster-specific GMV, pain duration, depressive symptoms, and trait anxiety) was used, requiring a p value of < .05/4 or .0125.
RESULTS
Demographic and global morphometric comparisons
The FM+AD, FM−AD, and healthy control groups were closely matched with respect to age (mean age 41.7, 42.6, and 42.2 respectively; p = .94; Table 3), gender (all female), and handedness (all right-handed). The three groups did not differ significantly with respect to TIV (1531, 1556, and 1521 ml; p = .70) or global GMV (617, 637, and 635 ml; p = .30). There was also no significant difference in pain duration between FM+AD and FM-AD groups (p = .31).
Table 3.
Mean age and morphometric characteristics in FM subjects and age-matched healthy controlsa
| FM+AD (N=29) | FM−AD (N=29) | HC (N=29) | p value | |
|---|---|---|---|---|
| Age | 41.7 ± 3.8 | 42.6 ± 3.7 | 42.2 ± 3.8 | .94 (NS) |
| TIV (ml) | 1531 ± 53 | 1556 ± 64 | 1521 ± 66 | .70 (NS) |
| Global GMV (ml) | 617 ± 20 | 637 ± 21 | 635 ± 20 | .30 (NS) |
| Duration of pain (yrs) | 12.0 ± 3.5 | 13.6 ± 2.9 | – | .31 (NS) |
All values are presented as 95% confidence intervals. Abbreviations: FM+AD = fibromyalgia subjects with affective disorder; FM−AD = fibromyalgia subjects without affective disorder; HC = healthy controls; NS = not significant; TIV = total intracranial volume; GMV = gray matter volume.
Voxel-wise group comparisons in GMV using one-way ANOVA
Using the whole-brain search approach, the main effect of group revealed no clusters of significantly different GMV among the three groups, when correcting for multiple comparisons. The ROI-based search yielded a cluster in the ventral portion of the left anterior insula, in which the main effect of group on GMV was significant (xyz = {−28, 21, −9}; F(2, 83) = 10.88; p = .026 corrected; Table 4; Figure 1). Post-hoc analysis revealed a significant difference between healthy controls and FM+AD (p = .0033 corrected), but no significant difference between healthy controls and FM−AD (p = .65 corrected), and no significant difference between FM+AD and FM−AD (p = .32 corrected; Table 4; Figure 2)
Table 4.
Results of one-way ANOVA, showing significant main effect of group on GMV.
| Search strategy | Region | MNI coordinates of peak |
F | contrast | pa | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| WB | No significant clusters found | ||||||
| ROI | Left anterior insula | −28 | 21 | −9 | 10.88 | omnibus | .026 |
| HC > FM+AD | .0033* | ||||||
| HC > FM-AD | .65 | ||||||
| FM-AD > FM+AD | .32 | ||||||
Corrected for small search volume and multiple voxel-wise comparisons using family-wise error.
Significant after Bonferroni correction for post-hoc analysis.
Abbreviations: GMV = gray matter volume; MNI = Montreal Neurological Institute; WB = whole-brain search; ROI = region-of-interest search; HC = healthy controls; FM+AD = fibromyalgia patients with affective disorder; FM−AD = fibromyalgia patients without affective disorder.
Figure 1.
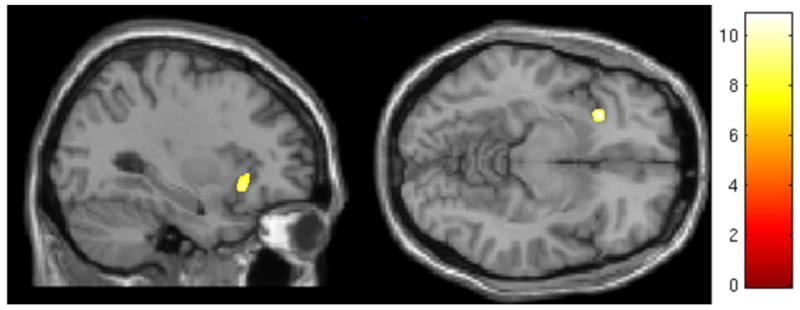
Cluster showing significant main effect of group on gray matter volume, using region-of-interest search, located within the left anterior insula. Color scale is for F statistic.
Figure 2.
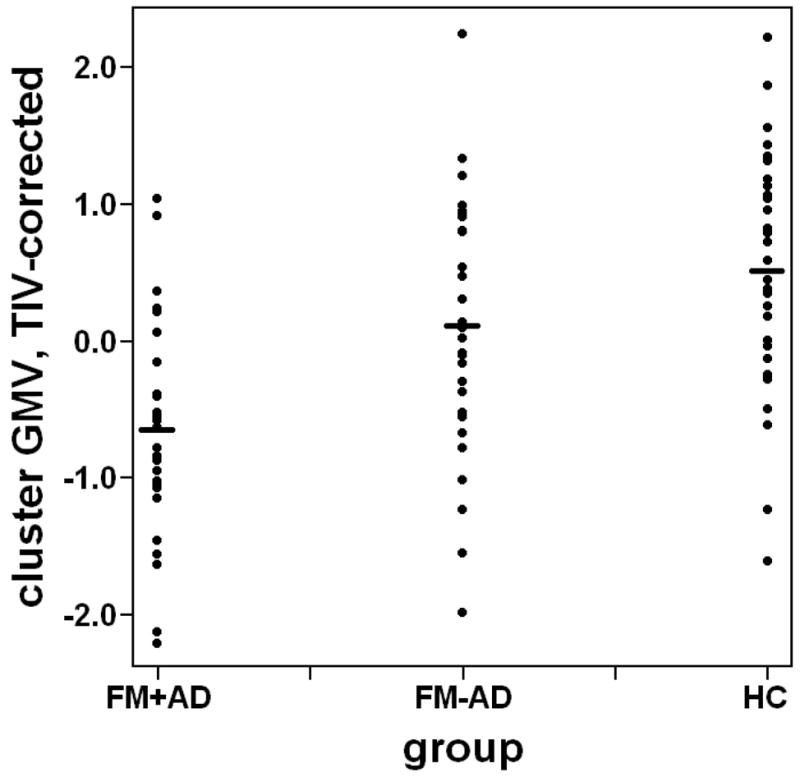
Scatter plot of gray matter volume (GMV) within the left anterior insula cluster, corrected for total intracranial volume (TIV), by group. Abbreviations: FM+AD = fibromyalgia subjects with affective disorder; FM−AD = fibromyalgia subjects without affective disorder; HC = healthy controls.
Bivariate correlations with pain duration and affective symptoms
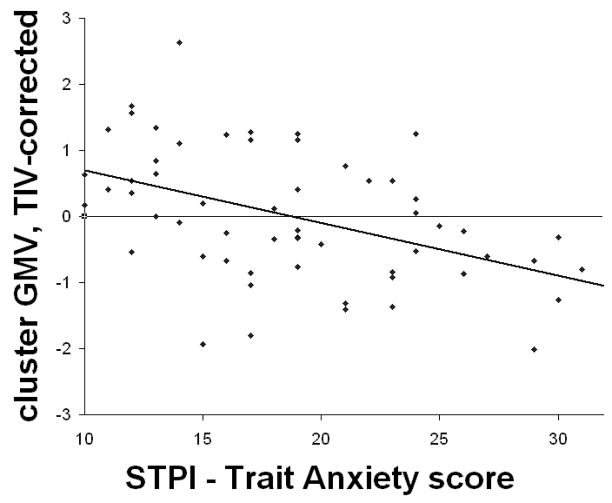
Mean voxel values from the left anterior insula cluster were extracted for all FM subjects and corrected for TIV. There was a significant negative correlation between mean GMV in the left anterior insula cluster and STPI-Trait Anxiety scores (rho = −.470, p = .0002; Figure 3) within FM subjects. This correlation remained significant even when controlling for group (rho = −.345, p = .008). No significant correlations were found between cluster-specific GMV and pain duration or CES-Depression scores.
Figure 3.
Significant negative correlation between gray matter volume (GMV) in the left anterior insula cluster corrected for total intracranial volume (TIV), and trait anxiety scores, within FM patients. Abbreviations: STPI = State-Trait Personality Inventory.
DISCUSSION
This is the largest study to date investigating differences in GMV between FM patients and healthy controls. Our main finding is a reduction in GMV in the left anterior insula in FM patients with AD, compared to healthy controls. However, when comparing FM patients without AD to healthy controls, this difference in GMV disappears. Furthermore, we found that GMV in this region is inversely correlated with trait anxiety. Thus, it appears that the finding of decreased GMV in the left anterior insula can be attributed to affective disturbance.
Previous research has shown the anterior insula to be involved in processing a wide variety of interoceptive stimuli (including pain, heartbeat awareness, thirst, coolness, warmth, gut and bladder distension), and a similarly wide range of emotional feelings (including anger, fear, sadness, disgust, unfairness, maternal and romantic love, happiness, sexual arousal, trust, and sculptural beauty) [7]. Our finding of an inverse relationship between anterior insula GMV and trait anxiety thus appears to be consistent with “burnout” due to overutilization of the emotional-processing function of this region. However, one can also argue that this finding is consistent with atrophy due to under-utilization of positive affective states, especially given evidence that positive emotional processing is lateralized to the left forebrain [7,22].
Despite our somewhat large sample size, we were unable to replicate previously-reported findings of a global GMV difference between patients and controls [12]. We were also unable to replicate differences in regional GMV in most previously-reported ROIs (left parahippocampal gyrus, left and right mid/posterior cingulate, medial frontal cortex [12]; bilateral striatum, and left thalamus [18]). There are several potential reasons for the discrepancies between our present findings and previously-published studies. One explanation may be that our patient population has less severe disease on average, compared to those enrolled in previous studies. Indeed, the mean age of our FM subjects is a decade younger than the mean ages of the FM subjects in previous studies (Table 1). However, the mean duration of pain, which we defined as time since onset of continuous chronic regional or widespread pain, is comparable to that reported in previous studies. Nevertheless, we cannot rule out a synergistic interaction between age and disease duration that may explain previously-published differences in regional GMV that were not replicated in the present study.
Another potential reason for our inability to replicate most of these previously-reported GMV differences between patients and controls is the manner in which we controlled for affective disorder. Unlike Kuchinad et al., we considered the presence of dysthymia and general anxiety disorder, in addition to major depressive disorder, as potential confounders in the relationship between FM diagnosis and changes in GMV. While controlling only for major depressive disorder, Kuchinad et al. still found a reduction in left insula regional GMV in FM patients compared to controls. However, while controlling not only for major depressive disorder in our study, but also for dysthymia and general anxiety disorder, we found no difference in left insula GMV between FM patients and healthy controls. Our finding of a significant relationship between trait anxiety and left insula GMV further underscores the importance of controlling for other types of affective disorder when comparing FM patients to controls.
A final explanation for our inability to replicate most of the previous findings, is that our VBM protocol may have had a lower signal-to-noise ratio than the methods used in previous studies. For example, our voxel dimensions (.9375 × .9375 × 1.5mm) were slightly larger than that reported by Schmidt-Wilcke et al (1 × 1 × 1.08mm), and may have contributed to slightly decreased accuracy in gray-matter segmentation. However, our voxel dimensions were still within the maximum voxel size suggested by the accepted VBM guidelines [2]. Regarding our segmentation, normalization, and modulation algorithm, several other studies have used the same VBM5 toolbox and were able to detect significant GMV changes in illnesses such as major depression [8], bipolar disorder [13], and Alzheimer’s dementia [10], and also in normal childhood development [4]. While these studies are not a substitute for formal testing and comparison of methods, they do provide some evidence of the sensitivity of the VBM5 toolbox in detecting differences in GMV between clinical populations and controls.
There are several potential limitations to the interpretation of the present findings. First, aside from potential differences in clinical severity as mentioned above, the populations from which we drew our sample groups inevitably differ from the populations sampled in previously-published studies, in ways that may have altered our ability to detect differences in global or regional GMV. For example, our sample of healthy controls may have had a distribution of cognitive aptitude worse than the controls used in previous studies, thus biasing our results towards the null. Secondly, the retrospective nature of this study prohibits any conclusion regarding a causal relationship between affective disorder in FM and decreased regional GMV. Furthermore, due to the occasional presence of susceptibility artifacts in the base of the brain in our sample, we excluded all voxels inferior to z = −22, and therefore could not assess for possible differences in regional GMV in the amygdala, brainstem, or cerebellum. Finally, given that our study sample was limited to right-handed women, our findings may not be generalizeable to men or to left-handed individuals.
In conclusion, we found a reduction in gray matter volume in the left anterior insula in FM patients with affective disorder compared to healthy controls, and this difference in GMV appears to be attributable to affective disturbance. Despite a somewhat larger sample size, our study did not replicate previous reports of other regional and global GMV differences between patients and controls, perhaps due to our use of a younger sample population and a broader definition of affective disorder. We recommend that future investigations using VBM in chronic pain populations should control for all of these factors when making group-level comparisons.
Acknowledgments
We acknowledge Rupal Patel, Linda Skalski, Laura Mayo-Bond, Emily Thorpe, and Rosie Iordanova for their invaluable assistance with data acquisition and management. Our research was supported in part by the National Institutes of Health (NIH T-32 HD007422-17, 5-R01-AR050044, and K01 AT01111-01), Department of Defense (DAMD 17-00-2-0018, W81XWH-07-2-0050), and University of Michigan General Clinical Research Center (National Center for Research Resources, UL1RR024986). There are no conflicts of interest regarding this study, for any of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004 Nov 17;24(46):10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 3.Barlow JH, Wright CC. Dimensions of the Center of Epidemiological Studies-Depression Scale for people with arthritis from the UK. Psychol Rep. 1998;83:915–9. doi: 10.2466/pr0.1998.83.3.915. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner T, Speck D, Wettstein D, Masnari O, Beeli G, Jäncke L. Feeling present in arousing virtual reality worlds: prefrontal brain regions differentially orchestrate presence experience in adults and children. Front Hum Neurosci. 2008;2:8. doi: 10.3389/neuro.09.008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman S, Herrstrom P, Jacobsson LT, Petersson IF. Chronic widespread pain: a three year followup of pain distribution and risk factors. J Rheumatol. 2002;29:818–25. [PubMed] [Google Scholar]
- 6.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract] NeuroImage. 2002;16:S497. [Google Scholar]
- 7.Craig AD. How do you feel – now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 8.Frodl T, Koutsouleris N, Bottlender R, Born C, Jäger M, Mörgenthaler M, Scheuerecker J, Zill P, Baghai T, Schüle C, Rupprecht R, Bondy B, Reiser M, Möller HJ, Meisenzahl EM. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Mol Psychiatry. doi: 10.1038/mp.2008.62. published online 1 July 2008. [DOI] [PubMed] [Google Scholar]
- 9.Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, Gracely RH, Clauw DJ. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis Rheum. 2005;52:3670–4. doi: 10.1002/art.21407. [DOI] [PubMed] [Google Scholar]
- 10.Honea RA, Thomas G, Harsha A, Cronk B, Donnelly J, Brooks WM, Burns JM. O1-02-02: Physical fitness is associated with preservation of brain volume in Alzheimer’s disease. Alzheimers Dement. 2008;4:T109. [Google Scholar]
- 11.Kato K, Sullivan PF, Evengård B, Pedersen NL. Chronic widespread pain and its comorbidities: a population-based study. Arch Intern Med. 2006;166:1649–54. doi: 10.1001/archinte.166.15.1649. [DOI] [PubMed] [Google Scholar]
- 12.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004–7. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladouceur CD, Almeida JR, Birmaher B, Axelson DA, Nau S, Kalas C, Monk K, Kupfer DJ, Phillips ML. Subcortical gray matter volume abnormalities in healthy bipolar offspring: potential neuroanatomical risk marker for bipolar disorder? J Am Acad Child Adolesc Psychiatry. 2008;47:532–9. doi: 10.1097/CHI.0b013e318167656e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindell L, Bergman S, Petersson IF, Jacobsson LT, Herrström P. Prevalence of fibromyalgia and chronic widespread pain. Scand J Prim Health Care. 2000;18:149–53. doi: 10.1080/028134300453340. [DOI] [PubMed] [Google Scholar]
- 15.Mease P, Arnold LM, Bennett R, Boonen A, Buskila D, Carville S, Chappell A, Choy E, Clauw D, Dadabhoy D, Gendreau M, Goldenberg D, Littlejohn G, Martin S, Perera P, Russell IJ, Simon L, Spaeth M, Williams D, Crofford L. Fibromyalgia syndrome. J Rheumatol. 2007;34:1415–25. [PubMed] [Google Scholar]
- 16.Schmidt-Wilcke T, Leinisch E, Gänssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Wilcke T, Leinisch E, Straube A, Kämpfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–6. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt-Wilcke T, Luerding R, Weigand T, Jürgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007;132:S109–16. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–23. [PubMed] [Google Scholar]
- 20.Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) (self-evaluation questionnaire) Palo Alto, CA: Consulting Psychologists Press; 1979. [Google Scholar]
- 21.Spielberger CD. Preliminary manual for the State-Trait Personality Inventory. Tampa, FL: Human Resources Institute, University of South Florida; 1979. [Google Scholar]
- 22.Tucker DM. Lateral brain function, emotion, and conceptualization. Psychol Bull. 1981;89:19–46. [PubMed] [Google Scholar]
- 23.Valfrè W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48:109–17. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 24.White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: the prevalence of fibromyalgia syndrome in London, Ontario. J Rheumatol. 1999;26:1570–6. [PubMed] [Google Scholar]
- 25.Williams DA, Gracely RH. Biology and therapy of fibromyalgia. Functional magnetic resonance imaging findings in fibromyalgia. Arthritis Res Ther. 2006;8:224. doi: 10.1186/ar2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]