Abstract
Aim
To examine environmental sociodemographic risks of high IL-6, a marker of chronic inflammation in older adults.
Methods
We spatially linked 1990 USA Census tract demographic and economic measures to a prospective cohort study of representative community residents aged 71 years and older, from over 1700 of whom a cytokine protein and biological marker of chronic inflammation. IL-6, was collected. Using generalized interactive mixed models, we modeled 1989–1990 individual and census level risk factors for the dependent variable high IL-6 between 1992–1993 (high IL-6 = upper quartile: >2.96 pg/ml).
Results
After individual health risk adjustment, IL-6 remained elevated in communities with greater densities of poor older adults (odd ratio [OR]: 1.25 per 10% increase in exposure. 95% confidence interval [CI]: 1.05, 1.48) and in racially segregated communities (OR: 1.14 per 10% increase in exposure. 95% CI: 1.04, 1.25).
Conclusions
Socially disadvantaged environments may influence IL-6, a biomarker of age-associated inflammation.
Keywords: elderly, environmental impact, health risk, IL-6, Inflammatory marker, longitudinal study
The United States Environmental Protection Agency (EPA) and the National Institute for Environmental Health Sciences (NIEHS) recently outlined the unique health vulnerabilities of older adults and highlighted a need for research on environmental risks to this vulnerable segment of the population [1–3,101]. Gaps identified are especially pressing, given the growing demographic force of the population aged 65 and older and the looming magnitude of potential health risks and economic burden. Challenges highlighted include the observation that few studies are available to identify elders at high and low environmental risk, or to identify biological mechanisms by which environments differentially impact vulnerable elders. Work is also needed to spatially locate environmental exposures that might contribute to, interact with, or enhance existing individual predisposition for adverse environmental effects [1–3, 101]
Data sets linking environmental exposures to physiological biomarkers of exposure and health outcome in older adults are also sorely needed, especially data sources that allow control for a wide range of potential confounding influences [1,4]. Such biomarkers have been frequently used for monitoring health in other vulnerable populations. In children, for example, public health standards and surveillance systems exist for evidence-based monitoring of blood-lead levels [5,6]. Given currently projected demographic trends in the population aged 65 years and older, the absence of information about environmental health risks and biological impact on older adults is at a critical juncture, which has led to a number of recent federally supported initiatives to promote work on markers of environmental health in this fast-growing segment of the population [101].
To begin addressing these issues, we spatially linked geographically-based measures of community level socioeconomic and sociodemographic exposures from the 1990 USA Census to a large, federally supported longitudinal cohort study of older adult health – the Duke Established Populations for Epidemiologic Studies of the Elderly (EPESE) [7,8]. Biological markers of age-associated physiological dysregulation were assayed from blood samples taken from over 1700 older persons in the Duke EPESE data set. IL-6, a key age-associated cytokine biomarker of chronic inflammation and health status [9], was assayed and is reported here.
Importance of inflammation in older adult health & senescence
Our research team published some of the earliest work on biomarkers of chronic systemic and vascular inflammation in seniors, showing that blood markers of inflammation, such as plasma D-dimer (fibrin degradation) product. TGF-β and IL-6, are elevated in older adults, associated with impairments and functional limitations, and predictive of future adverse events including death [9–12]. Since the late 1990s. It has been widely recognized in the aging literature that biomarkers of inflammation are good indicators of current and future older adult health and health events [13–22]. In fact, chronic: inflammation is now purported to be a key mechanism of senescence, playing a significant causal as well as consequential role in age-associated impairments, chronic conditions and functional decline [19–21]. According to this view, causal inflammatory ‘stress’ can be of any origin (viral, bacterial, chemical, psychosociological and so on), each having signaling pathways and mechanisms that trigger the body’s defensive inflammatory responses. According to one theory of inflammatory senescence, a ‘two-hit’ model explains how the body responds in the short- and long-term to such stressful and antigenic stimuli. In one type of ‘hit’ to the aging physiological system, different individuals may have an increased level of susceptibility and response due to heterogeneity in genetic background [19–21]. In another type of ‘hit’, an additional ‘inflammatory background’ is partly determined by accumulating chronic conditions, injuries, insults and defense mechanisms that deteriorate with age, which together serve to additionally increase physiologic vulnerability to exposure.
Evolving evidence supports the idea that proinflammatory genetic predisposition plays a role in aging [20], and may also vary by race [23–26]. This makes the idea of inflammatory biomarkers of environmental effects doubly interesting given [26–28]:
The substantial health disparities that exist in the USA
The fact that minorities are more likely to live in adverse environments and bear greater toxic burden
There has been little focus on whether and how the environment contributes to these known health disparities
In the current work, we used USA Census-based descriptors of environmental disadvantage to test the hypothesis that the places where senior citizens live may also function as a type of inflammatory ‘hit’ during the aging process. We propose that biomarkers of inflammation such as IL-6 may be excellent indicators of adverse environmental exposure and future health risk, and that exposure risks that segregate to different geographical communities may contribute to age-associated chronic inflammation. We show that a key cytokine biomarker of inflammation and older adult health, IL-6, is differentially elevated in communities with greater densities of poor older adults and in racially segregated communities, effects which are independent of the most important individual level risk factors known to contribute to chronic inflammation in older adults. This work is also important because it demonstrates how existing data sets can easily be merged to meet some of the needs recently highlighted by special panels at the EPA and the NIEHS [1–3, 101].
Materials & methods
Participants
The EPESE at Duke University is a 10-year prospective cohort study of the health and health services use of a representative sample of community residents 65 years of age and over [7,8]. This population was selected using a four-stage sampling design, which yielded a probability sample of household residents 65 years or older in a five-county urban/rural Piedmont area of North Carolina. USA (Durham. Franklin, Granville. Vance and Warren counties). African–Americans were oversampled to increase statistical precision for this group. Of the 5221 persons selected, 4162 (80%) were successfully interviewed (54% African–American, 45% White, 26 [<1%] of other race/ethnicity, 35% male and 56% urban residents). Participants were evaluated in person at home using structured questionnaires at baseline (1986–1987), 3, 6 and 10 years later (1989–1990, 1992–1993 and 1996–1997), and by telephone, 1, 2, 4 and 5 years after baseline. This study was approved by the institutional Review Board of Duke University Medical Center. Signed informed consent was obtained from all sample members.
Primary outcome: serum IL-6, a biomarker of chronic inflammation
In 1992–1993, 6 years after baseline, blood samples were requested from surviving participants who were able to give consent. These blood samples were used for routine hematologic and blood chemistry determination, and to ascertain levels of coagulation and inflammatory factors, including IL-6. Since baseline, 993 (24%) of the original 4162-person cohort had died and 606 sample members (15%) had refused to continue or were otherwise lost to follow-up, leaving 2553 survivors still enrolled. Of 2207 participants who remained in the target area, blood was successfully drawn from 1705 participants and partial blood draws were obtained on an additional 52 people, for a total of 79.6% with blood assays. Of the 450 participants who did not provide blood, 221 were unable to personally give consent because of cognitive difficulties, while 229 refused the blood draw, were unavailable or blood could not be drawn for technical reasons.
Standard ELISA methods were used to measure plasma IL-6 levels (pg/ml) for the current study. Details on measurement of IL-6 in this sample have been reported previously [10], with high reliability and reproducibility over a period of 36 days (intraclass correlation coefficient: 0.87) [29]. IL-6 information was available on 1726 of the 1757 participants who gave blood. Of the donors, 65% were female, 53% Black, 54% urban (46% rural), 40% reported fair or poor health and approximately 43% reported one or more functional limitations. Comparison of those from whom blood was drawn with those from whom blood was not drawn (because of inability to give consent), and with refusers, indicated that donors were younger, included a larger proportion of males and Caucasians, had lower levels of impairment in activities of daily living (ADL), and had better self-rated health and cognitive status [10]. There were no significant differences between the three groups, however, in areas of residence or years of education.
Values on IL-6 were skewed, ranging from minimally perceptible (assays <0.6 pg/ml were coded as 0 pg/ml as in previous studies) to 201 pg/ml, with a median value of 1.70 pg/ml. For this analysis we dichotomized IL-6 at the top quartile (>2.96 pg/ml) as has been done in several recent analyses of this outcome. We then modeled the probability of an individual having an IL-6 level in the highest quartile in this geographically representative cohort of older adults.
Census tract information
Data on geographical areas were obtained from the publicly available 1990 USA Decennial Census data [30] and linked to the Duke EPESE data set by home address. Descriptors were obtained for USA-defined census tracts, which are variable in geographic area but have a mean population size of approximately 4000 people. Based on a review of the literature and inspection of crude associations with measures of IL-6, we selected five sociodemographic domains of heterogeneity in census tract built environments that are similar (to those previously identified to be most important at the census tract level, and which have also previously been linked to health outcomes in different population groups [31].
First, we included a measure of population density; that is, number or persons/km2. Second, we examined three markets of area diversity (in terms of race, age and gender). Racial segregation was determined by examining the percent nonLatino-Black in each census tract (original sampling showed that White people in the sample and source population were also primarily non-Latino at the time of this study). The effects of age and gender segregation were examined by including the percent of elderly residents within the census tract, and the percent of female-headed households.
Third, following previous work identifying economic variables critical to the study of area-based effects [31], the following census level poverty-related indicators were selected:
Median family income
Percent of residents of all ages living in poverty, according to the poverty line established by the USA Census Bureau based on year 1990 data
Percent of residents aged 65 years and over and in poverty
The fourth domain was occupational class. We examined occupational class effects by characterizing the ‘percent of blue-collar workers within a census tract area’. This was a summary variable, comprised of the sum of the proportion of residents in a census tract whose primary occupation was classified by the USA Census Bureau as ‘sales’, ‘clerical’, ‘services’, ‘farming’, ‘craft’, ‘operative’ or ‘laborer’.
Our fifth and final domain of investigation examined aspects of housing quality and/or desirability in census tracts. As measures of housing desirability we evaluated the percent of all housing units that were vacant or owner-occupied and we included median housing value.
With the exception of housing value and income, all census tract variables were recorded in percentage units and were analyzed in units of 10% Increment. The odds ratio (OR) estimates for these variables can be interpreted as the amount of increase in the odds of having high IL-6 for each 10% change in exposure. ORs for median family income and median housing value are reported per US$1000 increment.
Individual level information
The individual level characteristics selected for study were either known covariates of IL-6, or characteristics hypothesized to be associated in a potentially causal way with systemic inflammation in older adults. For example, older age has repeatedly been found to be associated with increased IL-6 levels, as have selected health conditions, functional limitations and depression; although in uncontrolled cross-sectional analysis race, gender and urban (versus rural) residence have not [9–11]. Low-grade inflammation, and hence a likely association with IL-6, has been associated with cognitive decline, obesity and smoking [32]. While information on these characteristics has been included largely for covariate control, we point out that most of the previous study designs have been cross-sectional; this study is unique in its investigation of these individual level variables as prospective indicators of future inflammation and in its simultaneous adjustment for area-based effects.
We focused on individual health status in 1989–1990, which was coincident with 1990 census data, and prior to determination of IL-6 level (1992–1993). Information was obtained on individual demographic characteristics (age and education), race (Black and non-Black), gender and income (adjusted to year 2006 standards using the most recently published data from the USA Bureau of the Census [33]). Health status measures included the number of prescription drugs taken in the previous 2 weeks, a four-point self-rated assessment of health (excellent: 1. poor: 4) and trichotomized BMI: BMI less than 25 kg/m2 (normal: reference group), 25–30 kg/m2 (overweight), or greater than 30 kg/m2 (obese) [34]. Functional status was measured by the aggregated sum of 13 ADL tasks [35], comprised of five basic ADL tasks [36], five instrumental ADL items [37] and three mobility tasks [38] Depressive symptomatology was ascertained by a modified Center for Epidemiologic Studies Depression (CES-D) scale with Score dichotomized to indicate severe depressive symptomatology [8, 39], and cognitive status by number of errors on the ten-item Short Portable Mental Status Questionnaire [40]. Health behaviors included current smoking and alcohol intake at baseline, and whether any vitamins, or minerals were being taken (an indicator of preventive health behavior). In addition, the number of self-reported negative life events experienced in the previous year was used as an indicator of recent stress (e.g., occurrence of severe illness or injury to the individual or a close family member, death of a close family member, relocation or financial challenges). Finally, information was obtained from each person on duration of residence at the current address and adequacy of current housing (absence of any one of indoor toilet, electricity, running water or adequate heat indicated inadequacy).
Statistical analysis
For continuous variables, descriptive characteristics of the sample were determined using means, standard deviations and ranges. Frequencies are reported for categorical variables. As the majority of variables had no more than 4% missing data, the Biomedical Data Processing (BMDP) regression estimation procedure for missing data was the general method of choice used to impute regression-predicted scores for most missing variables [41].
We estimated the association between IL-6 and neighborhood level variables, while accounting for individual level measurements from individuals ‘clustered’ within geographical areas (i.e., census tracts). Special statistical models are needed for this type of analysis because individuals who are ‘clustered’ within the same Census tract will tend to be more alike in their responses and outcomes than individuals in different tracts. This has the potential to induce lack of independence and correlated errors in individual measures of predictor and outcome variables if traditional regression models are used. For this reason, we used the generalized linear interactive mixed modeling procedure (GLIMMIX), specified with a logic link, in order to model our binary outcome while at the same time accounting for spatially clustered effects and correlated errors (SAS Statistical Analysis package version 8.0; SAS Institute, Cary, NC, USA). The GLIMMIX application has previously been shown to be particularly useful for analysis of hierarchically and spatially clustered data. From the GLIMMIX model specified, we report ORs and 95% CIs representing the odds of being in the top quartile of IL-6 per each unit change in the predictor variable (or for each 10% change in census tract variables that were recorded as percentages).
Results
Distribution of individual covariates & serum IL-6 levels in the Duke EPESE area
Descriptive characteristics of the 1989–1990 Duke EPESE sample (n = 1726) are shown in Table 1. These data are concurrent with the 1990 USA Census data and were used to adjust for individual level health risks. Figure 1 shows the kurtotic distribution of individual IL-6 values in the Duke EPESE cohort. Assays ranged from 0–201 pg/ml (median: 1.70 pg/ml). As in previous work, high IL-6 was defined as an IL-6 assay value in the upper quartile (>2.96 pg/ml) [9].
Table 1.
Descriptive characteristics of the Duke EPESE sample in 1989–1990.
| Individual level covariates | Mean (SD) or % |
|---|---|
| Demographic characteristics | |
| Age | 74.6 years (5.4) |
| Sex (% female) | 65.0% |
| Race (% Black) | 52.8% |
| Education | 9.0 (4.1) |
| Income* | |
| • ≤US$8414 | 35.5% |
| • US$8415–15,894 | 27.9% |
| • >US$15,894 | 36.6% |
| Health status | |
| Number of prescription drugs | 2.3 (2.2) |
| Self-rated health | |
| •Excellent | 17.4% |
| •Good | 44.4% |
| • Fair | 32.4% |
| • Poor | 7.8% |
| BMI | |
| • <25 | 42.5% |
| • 25–30 | 37.7% |
| • >30 | 19.7% |
| Depression | 7.8% |
| Short Portable Mental Status Questionnaire (mean number of errors [SD]) | 1.2 (1.5) |
| Functional limitations on 13-item scale (mean [SD]) | 1.4 (2.3) |
| • 0 problems | 52.2% |
| • 1–2 problems | 29.2% |
| • 3+ problems | 18.6% |
| Health behaviors | |
| Current smoker | 13.1% |
| Alcohol drunk/month (ounces absolute alcohol) | 0.6 (8.1) |
| • Any | 22.8% |
| • None | 77.2% |
| Current vitamin/mineral use (any) | 17.4% |
| Negative life events (including potential housing exposure) in past year | |
| No negative life events | 53.1% |
| One negative life event | 26.6% |
| Two or more negative life events | 21.3% |
| Years at current address | 23.8 (17.3) |
| Missing indoor toilet, electricity, hot/cold water or enough heat | 8.0% |
| Census tract variables‡ | |
| Population density (persons per sq. kilometer) | 575 (688) |
| % Non-Latino Black | 49.17 (25.94) |
| % Female-headed household | 27.22 (14.89) |
| Median housing value | US$61.956 (22.357) |
| % Blue collar | 62.39 (11.77) |
| % aged 65 years and over | 14.71 (4.73) |
| Median income | US$34.444 (11.641) |
| % Occupied housing | 54.87 (19.11) |
| % Persons in poverty | 17.85 (10.67) |
| % Elderly in poverty | 22.87 (11.37) |
Income adjusted to year 2006 using the most recent data from US Census Bureau [34].
Weighted means reported for Census tract variables represent 1726 individuals in 84 Census tracts.
EPESE: Established Populations for Epidemiologic Studies of the Elderly; SD: Standard deviation.
Figure 1. Distribution of IL-6 values in the Duke EPESE cohort.
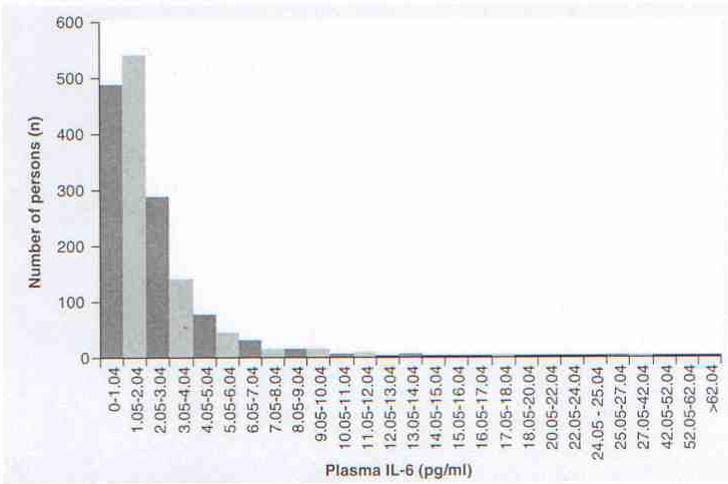
Median = 1.70 pg/ml; 25th percentile = 0.95 pg/ml; 50th percentile = 1.69 pg/ml; 75th percentile = 2.95 pg/ml
Figure 2 presents median plasma IL-6 concentrations for Duke EPESE participants (age 71–105 years) measured in 1992–1993 by quartile of selected census tract characteristics for the year 1990. These data show that median IL-6 increases as person density (per km2), the proportion of blue collar workers and percentage housing vacancy increases, and as median housing value and owner occupied housing decreases. Higher levels of IL-6 were also observed in census tracts with a greater proportion of non-Latino Black people, female-headed households, percentage elderly and overall percentage living in poverty, and in census tracts where median family income was lower. These results are consistent with the hypothesis that poor neighborhood quality may contribute to patterns of chronic inflammation in old age.
Figure 2. Median plasma IL-6 concentrations for Duke EPESE participants.
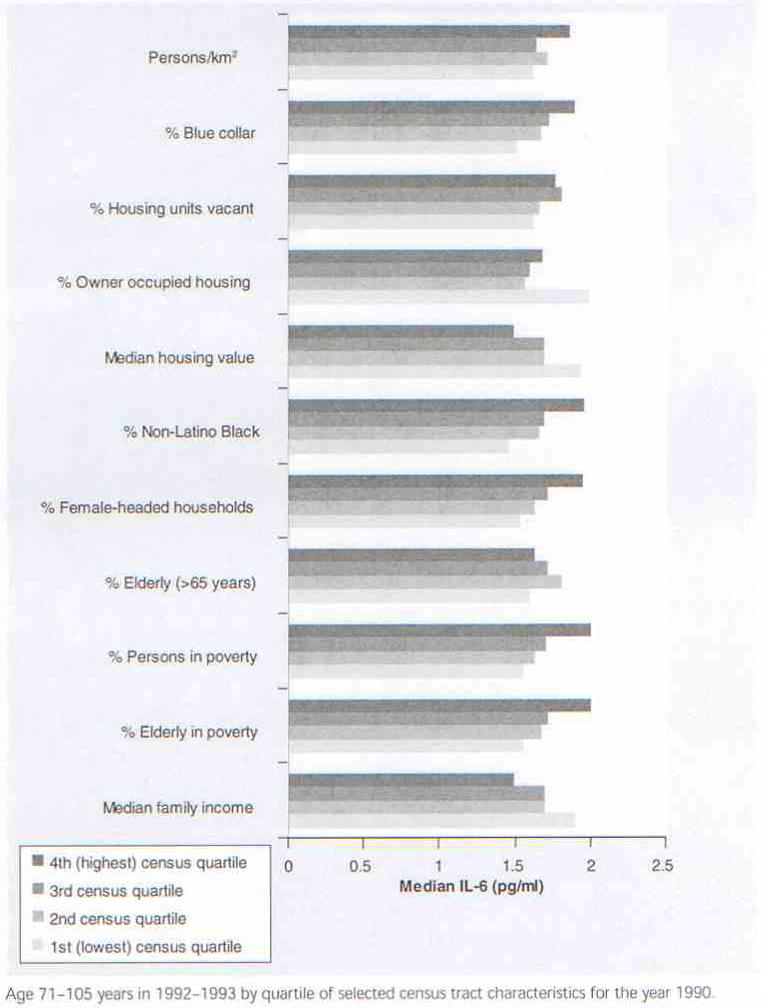
Age 71–105 years in 1992–1993 by quartile of selected census tract characteristics for the year 1990.
Census-based geospatial variables associated with chronic inflammation in the Duke EPESE cohort
Odds ratios for census level and individual level characteristics are shown in Figure 3, reflecting their simultaneously estimated association with elevated levels of IL-6 in the Duke EPESE cohort. After controlling for confounding at the individual and census level, each 10% increase in the proportion of older adults living in poverty was associated with a 25% elevation in the OR for high levels of chronic inflammation (OR: 1.25, 95% CI: 1.05–1.48). Similarly, each 10% increase in the proportion of African–Americans in a census tract was also associated with an increased risk of high IL-6 (OR: 1.14, 95% CI: 1.04–1.25). We could not attenuate these effects despite the most comprehensive adjustment ever conducted for important individual level and census-based risk factors for chronic inflammation. Neither could we attenuate the effect of living in a census tract with a higher proportion of elderly living in poverty by simultaneous adjustment for percent in poverty (regardless of age) or by controlling for the proportion of elderly people living in a given area (without considering poverty). In fact, neither percent in poverty nor percentage elderly had any effect on IL-6 in the fully adjusted models. Similarly, the census level effect of racial segregation was not eliminated even though we controlled for the substantial individual level effect of African–American race on IL-6 (Figure 3).
Figure 3. Census-based geospatial factors associated with chronic inflammation in older adult residents.
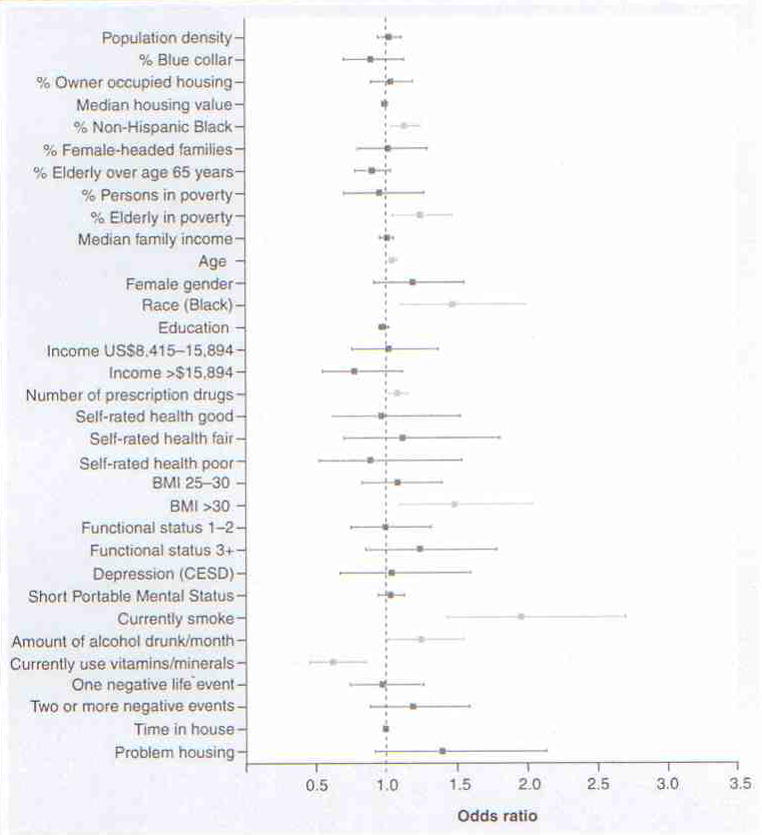
Statistically significant associations are shown in light blue. Housing value and family income are reported per US$1000. Odds ratios for all other census variables are reported per 10% elevation in exposure. For age, elevation in the odds ratio is shown per each 1-year increment in age.
Individual level factors contributing to chronic inflammation in older adults
While the focus of our investigation was on census level effects that remained after controlling for individual health risks, the individual level data also yielded interesting and novel results that may be especially important in this study given the prospective design and the simultaneous control for census level exposures. Being older, African–American, taking more prescription drugs, having a BMI greater than 30, consuming greater levels of alcohol and being a current smoker were all strong and important individual level predictors of elevated IL-6. Vitamin/mineral use, a likely indicator of protective health behavior, substantially reduced the odds of elevated IL-6.
Having two or more negative life events and subjective report of problems with housing were also positively associated with high levels of IL-6, but the effects did not attain statistical significance. The length of time lived in place was also not associated with high IL-6. Nevertheless, we retained these individual level covariates in all final analysis models to maintain optimal control over potential confounding by these characteristics.
Where are the high-risk communities?
County and census tract boundaries for the Study area in North Carolina, USA, as well as locations of major interstates are shown in Figure 4 (top map). Figure 4 also shows gradations in exposure distributions for the two primary Variable of interest (non-Latino Black and age-associated poverty), and for median census tract IL-6. Gradients in levels of census tract exposure and outcome are determined here using Jenks natural breaks [42]. These crude descriptive data spatially complement the more fully adjusted statistical analyses. For the three census tract maps showing percentage non-Latino Black, percentage elderly in poverty and median IL-6, simultaneous overlap in darker-shaded areas are observed in portions of Granville, Franklin, Nash and Halifax counties. In addition, dual elevations in the elderly in poverty and IL-6 are noted in portions of Granville and Orange counties (since first interviewed, some residents had moved to neighboring counties). Interestingly, for some counties, exposure and outcome are elevated only for percentage elderly in poverty, while for other counties percentage non-Latino Black (and not percentage elderly in poverty) appears to be the more relevant exposure. This qualitative finding is consistent with the objective statistical finding that percentage elderly in poverty and percentage non-Latino Black both have independent effects in the fully adjusted models. In combination with the statistical results, these figures illustrate qualitatively how mapping of exposures and biomarkers such as IL-6 might highlight geographic areas for more detailed study.
Figure 4. County and census tract boundaries for the study area in NC, USA.
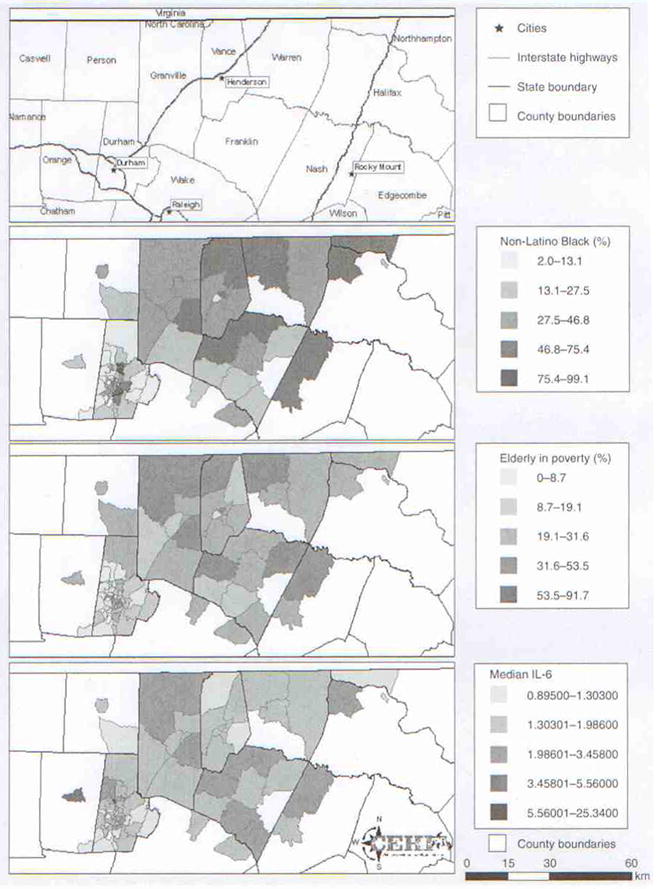
Gradations in exposure distributions for the two primary variables of interest (non-Latino Black and age-associated poverty), and for median census tract IL-6. Graphical maps of descriptive data for census tract variables, census tract boundaries and interstate highway locations were created using ARCGIS software (version 9.1, Environmental Systems Research Institute, Inc., CA, USA).
Discussion
Generating biological & health data to study environmental impacts on seniors
Our group and others have previously reported individual level associations between IL-6 and mortality and morbidity [9–13]. This paper reports the first ever identified associations between a biologically relevant blood biomarker of inflammation in older adults and spatially based descriptors of the environments in which older people live, particularly environments with substantial segregation according to race and old-age poverty. While provocative, these findings are not conclusive but suggest a need to further explore the types of inflammatory exposures older adults experience in different types of geographical areas, interactions between personal and environmental risks and ongoing evaluation of spatially referenced environmental stressors contributing to chronic inflammation in older adults.
Another highly important outcome of this study is its demonstration that merging existing geographical and health-related databases can create unique opportunities to evaluate the health impacts of environments on vulnerable individuals, fast-growing senior segments of the population. Comparable analysis in similar merged data sets might quickly address several major gaps in our knowledge of older vulnerable populations that have recently been highlighted by both the EPA and NIEHS. Our group has further plans to use a similar methodological approach to the one used in this study to investigate census level effects on mortality and morbidity, as well as health utilization and access.
Environmental contributions to health disparities?
We found that racial differences in IL-6 were present both for the individual level race variable and for census tract measures of racial segregation. These results were somewhat unanticipated given our previous report in the EPESE cohort that race was not associated with IL-6 [9]. However, in contrast with the previously reported findings, which were only minimally adjusted for statistical confounding, the present study is comprehensively adjusted for a number of known social and biological risk factors for inflammation. Most notably novel compared with the previous report, in this study we were able to control for confounding factors by a wide range of census-based (including racial segregation) effects on inflammation, which was not possible in the previous investigation of this cohort (geocoding and linkage to census information had not yet been completed at the time of the previous report). Importantly, we find that the individual level effects of race do not attain statistical significance without the additional control of census level effects, even with extensive individual level control of confounding factors.
The finding of enhanced inflammation in African–Americans and in areas with a greater proportion of African–American resident is consistent with previous work on health disparities in minority communities, and highlights the possibility of environmental contributions to those health disparities via inflammatory mechanisms. We note that racial differences in allele frequencies for proinflammatory cytokine genes such as IL-6 have been identified [23] and documented in samples of North Carolina residents [24], and speculate whether such differences may put African–Americans at greater risk if they are also preferentially exposed to environments with higher levels of social stressors or greater pathogenic burden. Ongoing work in this area could help to determine whether this type of gene–environment interaction may be important in a number of health outcomes known to differ among Caucasians and African–Americans in North Carolina, including cardiovascular disease, cancer, HIV, stroke, diabetes and preterm birth [43].
The combination of environmental risk & individual age-associated vulnerability
We would expect older adults living in environments with large antigen burden to be at greater risk than those living in healthier environments. In addition to a genetic predisposition to increased inflammation that may be present for some individuals [21], increased age additionally predisposes individuals to more total (i.e., life cumulative) antigenic burden, hence greater immune challenge and potential compromise in the body’s normal defenses, with consequent enhanced inflammation. We examined whether the effects of age-specific poverty that we identified in census tracts represent Straightforward compositional effects of individuals within areas rather than environmental effects of census tract areas as hypothesized. For example, we wondered whether IL-6 might be elevated within tracts simply because of the presence of more old individuals (who are known to have higher IL-6) or to more global poverty (irrespective of age). Our results show that this is not the case. Merely living in areas with large numbers of older adults or in areas with higher global levels of poverty are not the key age-associated area-based risks for older adults (Figure 3). Rather, the greatest census level risk came from higher proportions of people who were vulnerable owing to both age and poverty – a type of ‘double jeopardy’.
This finding is novel and relevant to current discussions of the interaction between environmental risk and individual vulnerability. We note that the longest-lived older adults in recent studies of longevity have been shown to have genetic predisposition to ‘damping down’ inflammatory responses to environmental anti-genie stimuli [19], which might confer additional advantages if such individuals live in safer environments. Future work should continue to investigate whether the converse may also be true: living in adverse environments may confer the greatest harm to the most vulnerable segments of the older adult population (i.e., poor elders and certain minority groups), who are already at higher risks for chronic inflammation and poor health.
Limitations
One of the principal limitations of this study is a lack of repeated measures of IL-6. IL-6 has been criticized by some as unstable because of its relatively short half-life (~2 h) compared with other biomarkers of inflammation such as C-reactive protein [44]. However, a recently published extensive review of the literature and meta-analysis of the effects of IL-6 on coronary disease outcomes accounted for within-person variability owing to fluctuations over time, and showed that after accounting for this variance, associations with IL-6 were actually greater than previously reported in the literature [43]. This finding is of special concern with respect to the current report if our findings similarly underestimate the potential magnitude of associations with IL-6. Ongoing investigations of environmental associations with chronic inflammation are needed in which repeated measurements of both IL-6 and the environment are available.
An additional concern in any observational study is the possibility of residual confounding. In response to one reviewer, we conducted sensitivity analysis of potential confounding by seasonality effects and diabetes on the estimates of association with IL-6 reported here. No differences in the results were obtained and we were unable to attenuate the results reported. Overall, while we acknowledge that unmeasured confounding is always a threat to the validity of observational studies, the control of measured potential confounders in this study was more extensive than in previous investigations of IL-6.
Conclusion
Our findings that both racial and age-specific segregation are associated with an objective biological marker of chronic inflammation suggest a need to further investigate high-risk areas and determine whether these findings are generalizable to other nationally and internationally representative geographical areas and to other types of age-associated health outcomes. More detailed analyses also arc needed to identify specific types of environmental and gene–environmental risks that might contribute to inflammation and subsequent poor health in minority groups and in vulnerable seniors.
Future perspective
While previous studies have explored the association of environmental and census tract characteristics with population level health conditions and health service use, this is the first longitudinal study to examine the effect of census tract characteristics (the environment in which people live) on a biomarker of health status (IL-6), which may be an indicator of future coronary heart disease [43]. We anticipate that there will increasingly be attempts to link longitudinal environmental and census track data to longitudinal person level data in order to better ascertain the long-term effects of the residential environment on health, and thus identify potential areas of intervention that could result in broad improvements in health at the population level.
Executive summary
The USA Environmental Protection Agency and the National Institute for Environmental Health Sciences have highlighted a critical need for research on environmental risks to older adults and on physiological biomarkers of those effects.
Currently, few studies identify elders at high and low environmental risk or identify biological mechanisms by which environments differentially impact vulnerable elders.
Work is needed to spatially locate environmental exposures that might contribute to, interact with or enhance existing individual predisposition to adverse environmental effects.
The present study shows that it is possible to link geographically based measures of community level socioeconomic and sociodemographic exposures from the USA Census to a longitudinal cohort study of older adult health that includes biological markers of age-associated physiological dysregulation.
Although geospatial coding of persons with protected health information requires careful privacy-protection procedures, it is feasible nevertheless. Given the increasing interest in and feasibility of obtaining biomarkers, and the availability of longitudinal studies of representative samples of the population, increasing use of geospatial linkage should be anticipated.
Typically, interventions to improve health status have focused on individuals, and occasionally population-affecting intervention has been used (e.g., fluoridation of water, supplementation of milk and flour). The present study demonstrates that an alternative approach – identification of socially adverse geographic areas – may provide an additional indicator of the likelihood of poorer health, and thus indicate an alternative source of intervention.
Acknowledgments
The authors would like to acknowledge Sharon Edwards from the Children’s Environmental Health Initiative (CEHI) at Duke University for her assistance with mapping census-based exposures in North Carolina Counties and linking census data to the Duke EPESE health data, and Qiushi Feng for his graphical assistance. The content of this publication is solely the responsibility of the authors and does not necessarily reflect the official view of the NICHD or the National Institutes of Health.
Financial & competing interests disclosure
This work was partially supported by the Duke University Claude D Pepper Older Americans Independence Center, NIH grant number 5P30AG028716, by NICHD/NCMRR K01HD049593, and by NIH grant 1-P30-ES011961. The data were originally collected as part of the Duke Established Populations for Epidemiologic Studies of the Elderly NIA, contract number N01 AG12102, NIA R01 AG12765 and NIA R01 AG17559. The work was also supported in part by a contract with Lisa Berkman, PhD (5R01 AG18369–03) at the Harvard School of Public Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Website
101. US EPA (Environmental Protection Agency); The Aging Initiative. www.epa.gov/aging/Index.htm *Website for the USA EPA’s new Aging Initiative, which summarizes programmatic area of emphasis, lists new requests for proposals and available funding and summarizes recent research.
Bibliography
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.US EPA (Environmental Protection Agency) Aging and Toxic Response: Issues Relative to Risk Assessment. National Center for Environmental Assessment; Washington, DC, USA: 2006. pp. 564–3261. Available from the National Technical Information Service, Springfield. VA (202) [Google Scholar]
- 2.Geller AM, Zenick H. Aging and the environment: a research framework. Env Health Perspect. 2005;113(9):1257–1262. doi: 10.1289/ehp.7569. **Summarizes the USA Environmental Protection Agency’s (EPA’s) proposed framework for studying exposure, health effects and risk communication concerns for the USA EPA’s evolving research program on older adults as a susceptible subpopulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood E. Toward new understanding of aging. Env. Health Perspect. 2003;111(14):A756–A758. doi: 10.1289/ehp.111-a756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albertini R, Bird M, Doerer N, et al. The use of biomonitoring data in exposure and human health risk assessment. Env Health Perspect. 2006;114(11):1755–1762. doi: 10.1289/ehp.9056. *Summarizes the International Biomonitoring Workshop, which was sponsored by the Health and Environmental Sciences Institute, the USA EPA, the CDC, the Agency for Toxic Substances and Disease Registry and International Council of Chemical Associations in September 2004. The paper discusses the use of biomonitoring data for risk assessment purposes and emphasizes the integration of biomarker measurements of exposure, internal dose and potential health outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morbidity and Mortality Weekly Report. Surveillance Summaries: USA 1997–2001. 2003;52(SS10):1. [PubMed] [Google Scholar]
- 6.Miranda ML, Dolinoy D, Overstreet MA. Mapping for prevention: GIS models for directing childhood lead poisoning prevention programs. Env Health Perspect. 2002;110(9):947–953. doi: 10.1289/ehp.02110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornoni-Huntley J, Blazer D, Lafferty M, et al. Established Populations for Epidemiologic Studies of the Elderly: Resource Data Book (Volume 2) Washington, DC, USA: 1990. PHS, NIH Publication No.: 90–495. [Google Scholar]
- 8.Blazer DG, Burchett B, Service C, George LK. The association of age and depression among the elderly: an epidemiologic exploration. J Gerontal Med Sci. 1991;46(6):M210–M215. doi: 10.1093/geronj/46.6.m210. [DOI] [PubMed] [Google Scholar]
- 9.Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality. Am J Med. 2003;114(3):180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 10.Cohen HJ, Pieper CF, Harris T, Rao KMK, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol Med Sci. 1997;52A(4):M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 11.Dentino AN, Pieper CF, Rao MK, et al. Association of interleukin-6 and other biologic variables with depression in older people living in the community. J Am Geriatr Soc. 1999;47(1):6–11. doi: 10.1111/j.1532-5415.1999.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 12.Wilson CJ, Cohen HJ, Pieper CF. Cross-linked fibrin degradation products (D-dimer), plasma cytokines, and cognitive decline in community-dwelling elderly persons. J Am Geriatr Soc. 2003;51(10):1374–1381. doi: 10.1046/j.1532-5415.2003.51454.x. [DOI] [PubMed] [Google Scholar]
- 13.Visser M, Pahor M, Taaffe D, et al. Relationship of IL-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the Health ABC study. J Gerontol Med Sci. 2002;57A(5):M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 14.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor-1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115(6):429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6(3):295–299. doi: 10.1097/01.mco.0000068965.34812.62. [DOI] [PubMed] [Google Scholar]
- 16.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Int Med. 1998;128(2):127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition: the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50(12):2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 18.Bruunsgaard H, Pedersen B. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23(1):15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 19.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging An evolutionary perspective on immunosenescence. Ann NY Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 20.Franceschi C, Valensin S, Fagnoni F, Barbi C, Bonafe M. Biomarkers of immunosenescence within an evolutionary perspective: the challenge of heterogeneity and the role of antigenic load. Exp Gerontol. 1999;34(8):911–921. doi: 10.1016/s0531-5565(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 21.Franceschi C, Bonafe M. Centenarians as a model for healthy aging. Biochem Soc Trans. 2003;31:457–461. doi: 10.1042/bst0310457. [DOI] [PubMed] [Google Scholar]
- 22.Baren E, Gershwin ME. Inflamm-aging: autoimmunity, and the immune-risk phenotype. Autoimmune Rev. 2004;3(5):401–406. doi: 10.1016/j.autrev.2004.03.004. *Discusses concepts immunosenescence as relates to the concept of a specific cluster of cytokine profiles, colned an immune-risk phenotype. These concepts bridge between basic immune function and clinical immunology and could help generate effective interventions to improve immune function in the elderly. [DOI] [PubMed] [Google Scholar]
- 23.Ness RB, Catherine L, Haggerty CL, Hager G, Ferrell R. Differential distribution of allellc variants in cytokine genes among African–Americans and White Americans. Am J Epidemiol. 2004;160(11):1033–1038. doi: 10.1093/aje/kwh325. [DOI] [PubMed] [Google Scholar]
- 24.Zabaleta J, Schneider BG, Ryckman K, et al. Ethnic difference in cytokine gene polymorphisms: potential implications for cancer development. Cancer Immunol Immunother. 2008;57:107–114. doi: 10.1007/s00262-007-0358-4. *Discusses ethnic differences in allele frequencies for inflammatory genetic loci that could potentially contribute to an immune-risk phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan MI, Aschner Y, Manning CH, Xu J, Aschner JL. Racial differences in selected cytokine allelic and genotypic frequencies among healthy, pregnant woman in North Carolina. Cytokine. 2003;21:10–16. doi: 10.1016/s1043-4666(02)00489-1. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine. Toward environmental Justice: Research, Education and Health Policy Needs. National Academy Press; Washington, DC. USA: 1999. [PubMed] [Google Scholar]
- 27.Satcher D. Ethnic disparities in health: the public’s role in working for equality. PLos Med. 2006;10:1683–1685. doi: 10.1371/journal.pmed.0030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payne-Sturgess D, Gee GC, Crowder K, et al. Workshop summary: connecting social and environmental factors to measure and track environmental health disparities. Environ Res. 2006;102:146–153. doi: 10.1016/j.envres.2005.11.001. *Summarizes a recent workshop focused on the environment as it impacts environmental health disparities. [DOI] [PubMed] [Google Scholar]
- 29.Rao KM, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102(6):802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- 30.United States Bureau of the Census. US Department of Commerce, Economics, and Statistics; Washington, DC, USA: 1991. [Google Scholar]
- 31.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area-based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (USA) J Epidemiol Community Health. 2003;57(3):186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krabbe KS, Pedersen M, Bruunsgard H. Inflammatory mediators in the study. Exp Gerontol. 2004;39(5):687–699. doi: 10.1016/j.exger.2004.01.009. ** Discusses the factors that mediate chronic inflammation in older adult. [DOI] [PubMed] [Google Scholar]
- 33.US Census Bureau, Statistical Abstract of the United States (Table 702) Purchasing power of the dollar: 1950–2006. Washington, DC, USA: 2008. p. 467. [Google Scholar]
- 34.WHO consultation on Obesity. WHO Technical Report Series 894. World Health Organization; Geneva, Switzerland: 2000. [Google Scholar]
- 35.Blazer DG, Fillenbaum GG, Gold DT, Burchett BM, Hays JC. APOE ε4 as a predictor of subjective quality of life in a biracial older person community sample. J Aging Health. 2003;15(4):645–660. doi: 10.1177/0898264303256216. [DOI] [PubMed] [Google Scholar]
- 36.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Services. 1976;6(3):493–507. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 37.Fillenbaum GG. Screening the elderly: a brief instrumental ADL measures. J Am Geriatr Soc. 1985;33(10):698–706. doi: 10.1111/j.1532-5415.1985.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 38.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21(4):556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 39.Radioff L. The CES-D scale: a self report depression scale for research in the general population. Appl Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 40.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deflcit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 41.Dixon WJ, editor. BMDP Statistical Software. University of California Press; Berkeley, CA, USA: 1983. [Google Scholar]
- 42.Jenks GF, Caspall FC. Error on choroplethic maps: definition, measurement, reduction. Ann Assoc Am Geogr. 1971;61:217–244. [Google Scholar]
- 43.Office of Minority Health and Health Disparities and State Center for Health Statistics, North Carolina Department of Health and Human Services. Racial and Ethnic Health Disparities in North Carolina Report Card. 2006. [Google Scholar]
- 44.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5(4):600–610. doi: 10.1371/journal.pmed.0050078. ** Presents two new prospective studies on the effects of IL-6 on heart disease outcomes and also presents a meta-analysis of associations between IL-6 and heart disease that corrects for within-person as well as between-person variability. [DOI] [PMC free article] [PubMed] [Google Scholar]


