Abstract
Tyrosine kinase inhibitors (TKIs) are effective anti-cancer therapies but resistance to these agents eventually develops. Several models of resistance to TKIs have been studied including resistance to EGFR inhibitors. Recent studies in EGFR-dependent A431 cells found upregulation of the IGF1R pathway as a mechanism to overcome blockade of EGFR. This was associated with amplification of IGF1R signaling and recovery of downstream PI3K-AKT activation. This work adds to a growing body of cell lines in culture that have provided insights into mechanisms of resistance that can be interrogated in primary tumors in patients. In this review, these model systems and their applicability to human cancers, as well as strategies to identify and overcome resistance to TKIs, are discussed.
Keywords: breast cancer, HER2, EGFR, tyrosine kinase inhibitors, TKI resistance, PI3K
Protein tyrosine kinases are critical activators of signal transduction pathways that control cell proliferation, differentiation, migration, metabolism, and survival. Tyrosine phosphorylation signaling cascades are tightly regulated in quiescent cells, but are deregulated and inappropriately activated through mutation or amplification of tyrosine kinases in cancer.1 Inhibitors of tyrosine kinases especially of the ErbB family, a group of transmembrane receptors that includes EGFR (ErbB1), HER2 (Neu, ErbB2), HER3 (ErbB3), and HER4 (ErbB4), have been an important advance in the treatment of cancer. Two types of therapeutic inhibitors against ErbB receptors have been developed. The first type includes small-molecule ATP-mimetics that compete for ATP binding to the receptor’s tyrosine kinase and thus interrupt the phosphorylation of cellular substrates. Additionally, there are monoclonal antibodies that bind the receptors’ ectodomains to block ligand binding and subsequent activation of the receptor tyrosine kinase.2 Interruption of these pathways in cancer cells with these antagonists results in potent anti-tumor effects. However, most cancers that exhibit an initial clinical response to these drugs eventually become resistant and progress.
Recently, Guix et al identified a pathway of acquired resistance to inhibitors of the EGFR tyrosine kinase.3 Gefitinib (Iressa, AstraZeneca Pharmaceuticals) and erlotinib (Tarceva, OSI Pharmaceuticals) are two TKIs that have been shown to produce significant clinical responses in a subset of non-small cell lung cancers (NSCLC) and prolong the survival of patients with NSCLC harboring activating mutations or amplification of EGFR.4–7 However, despite these benefits, most patients treated with these inhibitors eventually become refractory to treatment.
Guix et al used two models systems that show dependence on EGFR signaling, the A431 and HN11 squamous cancer cell lines. When cancer cells are addicted to EGFR, both the phosphoinositide 3-kinase (PI3K) and the ERK1/2 pathways are under strict regulation by EGFR. Thus, when the cells are exposed to an EGFR inhibitor like gefitinib, these pathways are turned off and the cells undergo apoptosis. Resistance was modeled by chronic exposure to gradually increasing concentrations of the TKI over a period of months, until cells were able to grow in the presence of clinically achievable concentrations of the inhibitor. Upon treatment with gefitinib, the resistant cells showed inhibition of EGFR and disruption of the association of EGFR with HER3; this coupling of EGFR to HER3 is required for maximal engagement of the PI3K-AKT survival pathway. Despite inhibition of the target receptor and downregulation of ERK1/2, the resistant cells maintained activation of PI3K-AKT. The authors then identified the IGF1 receptor pathway as an activator of PI3K-AKT in the resistant but not in the parental, drug-sensitive cells. Only combined inhibition of the IGF1R and EGFR kinases (the latter with gefitinib) resulted in inhibition of PI3K-AKT signaling and cell growth. Gene expression profiling identified downregulation of IGFBP-3 and IGFBP-4, binding proteins that attenuate IGF1-induced activation of IGF1R, as the mechanism whereby the IGF1R pathway becomes engaged in gefitinib-resistant cells. Addition of IGFBP-3 back to resistant cells restored sensitivity to gefitinib, but as with other pharmacologic inhibitors of IGF1R, IGFBP-3 alone had no effect. The upregulation of the IGF1R pathway to escape the effects of EGFR inhibition was not unique to this cell line model as it was also observed in A431 cells treated with the EGFR monoclonal antibody cetuximab (Erbitux, ImClone) and in HN11 head and neck cancer cells selected in gefitinib. In these, resistance to gefitinib was also accompanied by restoration of AKT activity and signaling to PI3K-AKT through the IGF1R pathway. In the case of the cetuximab-resistant A431 cells, IGFBP-3 was downregulated whereas in gefitinib-resistant HN11 cells, IGFBP-4 was downregulated compared to parental, gefitinib-sensitive cells.
These results suggested the possibility that combined blockade of EGFR and amplified IGF1R signaling would abrogate or prevent drug escape in parental cells. Therefore, the authors treated A431 cells with a combination of gefitinib and the IGF1R TKI AEW541 (Novartis Pharmaceuticals). In contrast to treatment with gefitinib alone, where resistance emerged after a couple of months in culture, combined treatment did not allow the emergence of resistant clones. Further, treatment with gefitinib in mice bearing A431 xenografts resulted in tumor shrinkage. However, in the majority of cases tumors re-grew while on therapy or shortly after stopping treatment. Treatment with an anti-IGF1R monoclonal antibody alone caused a minor and very short-lived partial response. When the two drugs were combined, all mice achieved a complete response that was maintained for several months after discontinuation of treatment. Thus, initial treatment with an inhibitor of the acquired drug resistance pathway had minimal activity by itself but, in combination with primary anti-EGFR therapy, completely prevented the emergence of drug resistance. These studies have yet to be validated in primary tumors from patients treated with EGFR inhibitors. While the IGF1R pathway is an important emerging therapeutic target with several inhibitors in clinical trials, the role of this pathway in resistance to EGFR inhibitors and, in particular, the downregulation of IGFBP-3 and IGFBP-4 as the mechanism of activation of this pathway will need to be tested in patients who have relapsed or progressed after treatment with EGFR antagonists.
This work adds to a growing list of resistance mechanisms to targeted agents that were first identified in cancer cells in culture and later confirmed to occur in patients. Recently, another resistance pathway to EGFR TKIs was found by Engelman and colleagues in cancers with activating EGFR mutations.8 Similar to the studies by Guix et al, gefitinib resistance was accompanied by reactivation of the PI3K-AKT pathway, in this case as a result of amplification of the MET receptor tyrosine kinase and engagement of HER3. Treatment with the EGFR inhibitor or a MET inhibitor alone did not affect cell viability, but the combination of both drugs resulted in cell death. Most importantly, in tumor samples from patients who had developed resistance to gefitinib, almost a quarter showed MET gene amplification. Paired tumor samples were available from a few of the patients prior to treatment and at the time they became resistant. Notably, in some of these paired specimens, amplification of the MET gene was not detected in the pre-treatment biopsy. Thus, MET amplification developed or was selected upon pressure by treatment with the EGFR TKI. This study underscores the need to biopsy and profile post-treatment tumor recurrences to interrogate acquired mechanisms of resistance.
In the examples presented thus far, resistance to the TKI developed through activation of alternative pathways, such as MET and IGF1R, but the drug target itself remained sensitive to drug-induced inhibition. Another proven mechanism of escape is genetic alteration of the drug target itself to overcome drug inhibition. This was first observed in imatinib-resistant chronic myelogenous leukemia (CML) and gastrointestinal stromal tumors (GIST), where the molecular targets of imatinib are BCR-ABL and c-KIT, respectively. Upon the development of resistance, the target kinases acquired a ‘gatekeeper’ mutation in the ATP binding pocket that decreased the affinity of their interaction with the TKI.9, 10 Other imatinib-resistant leukemia models have shown gene amplification of BCR-ABL that developed after chronic exposure to the drug in culture (reviewed in ref. 11), a finding that was later confirmed in patient samples.10 A secondary resistance-associated mutation analogous to the gatekeeper mutation in BCR-ABL and c-KIT was found in the kinase domain of EGFR. First identified in tumor samples from patients who developed resistance to gefitinib or erlotinib,12, 13 the T790M resistance-associated mutation in the EGFR tyrosine kinase was also identified in NSCLC lines chronically selected in gefitinib.14, 15 Interestingly, the T790M mutant receptor remained sensitive to irreversible covalent TKIs such as HKI-272 (Wyeth Pharmaceuticals)16 and CL387,785.14 Thus, modeling resistance in cell lines has identified mechanisms of drug escape that occurred in patients’ specimens and has been useful for testing inhibitors designed to thwart such compensatory mechanisms of escape.
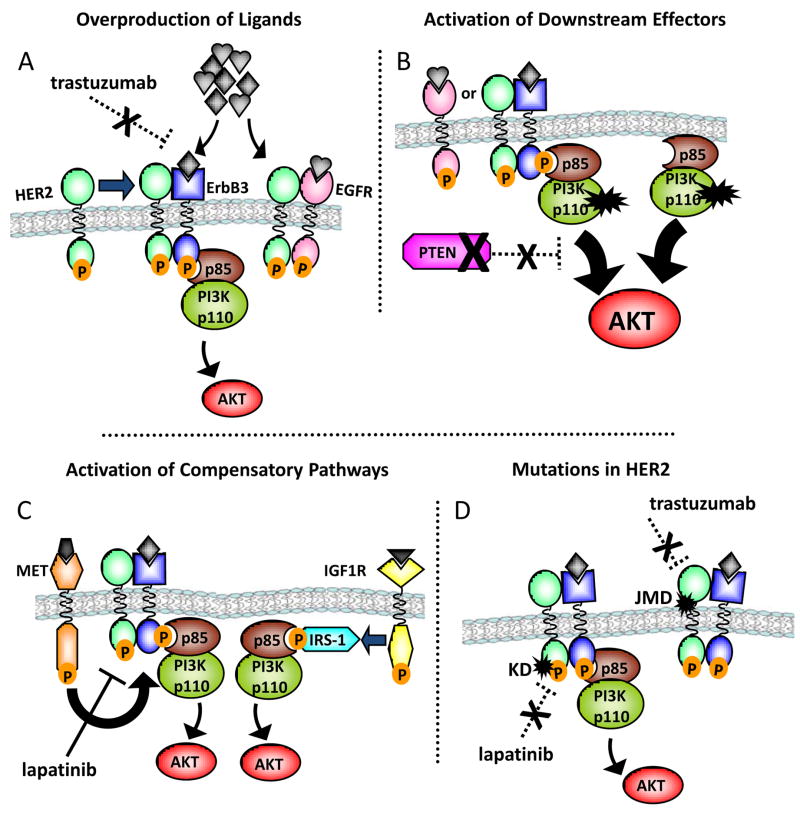
The lessons learned from studies with cancer cell lines selected for resistance in the presence of EGFR antagonists have important implications for other oncogene-dependent neoplasias, such as breast cancers with HER2 gene amplification. A monoclonal antibody, trastuzumab (Herceptin, Genentech), and a small molecule TKI, lapatinib (Tykerb, GlaxoSmithKline), are FDA-approved therapies for HER2 gene amplified breast cancers.17–21 As with other oncogene-targeted therapies, drug resistance eventually develops in the majority of patients with HER2+ breast cancer. Based on the resistance paradigms discussed above, we would expect to find several potential mechanisms of resistance to HER2 inhibition (Figure 1). Much work has already been done to investigate some of these possibilities (reviewed in ref. 22). An important theme that has emerged from these resistant model systems is the critical role of the PI3K-AKT pathway in mediating resistance. In the case of EGFR, reactivation of PI3K-AKT through IGF1R or MET was the common downstream mediator of resistance. Importantly, the antitumor activity of EGFR and HER2 inhibitors requires downregulation of the PI3K-AKT axis,14, 23–25 further suggesting that reactivation of this pathway will likely be important in mediating escape from drug action. Consistent with this notion, HER2 gene amplified BT-474 xenografts that grew in mice under continuous trastuzumab treatment demonstrated recovery of PI3K-AKT activity as a result of compensatory signaling mediated by upregulated EGFR and ErbB receptor ligands26 (Figure 1A). The PI3K-AKT pathway can also be abnormally activated by loss of the PTEN phosphatase or by activating mutations in the p110α catalytic subunit of PI3K.27, 28 Both of these alterations have been found to correlate with worse patient outcome following treatment with trastuzumab29, 30 (Figure 1B). Other models have also implicated IGF1R signaling and MET gene amplification in trastuzumab resistance, again providing alternate pathways to activation of PI3K-AKT31–34 (Figure 1C). Finally, the role of HER2 kinase domain mutations analogous to the resistance-associated secondary mutations observed in EGFR, BCR-ABL, and c-Kit remains to be investigated (Figure 1D). Several studies have identified HER2 mutations in lung cancer35–39 or head and neck cancer.40 Only one report has identified a low frequency of HER2 mutation in breast cancer.41 Interestingly, one of these mutations, a YVMA insertion at G776 in exon 20, was found to confer de novo resistance to trastuzumab and lapatinib.42 These alterations need to be confirmed in primary breast tumors recurring after an initial response to trastuzumab.
Figure 1. Mechanisms of resistance to HER2 inhibitors.
Several mechanisms of resistance to HER2 inhibitors have been proposed or discovered, many of which are characterized by reactivation of PI3K-AKT signaling. A. Upregulation of other ErbB family receptors and/or ligands bypasses the inhibition of the primary target (in this example, trastuzumab) and allows for recovery of PI3K-AKT signaling and emergence of drug-resistant cells. B. Mutation or alteration in the downstream effectors (e.g., mutation in p110α PI3K or loss of PTEN) confers a gain of function that is independent of HER2 receptor blockade and allows for PI3K-AKT signaling despite inhibition of HER2/HER3. C. Compensatory signaling pathways that still activate HER3 or signal to PI3K directly, such as MET and IGF1R, are engaged to bypass the primary inhibition of the HER2 receptor-directed therapy. D. Mutations in HER2 alter the drug target. Acquired mutations in the kinase domain (KD) of HER2 decrease binding of the small-molecule TKI. Acquired mutations in the juxtamembrane region (JMD) of the receptor that contains the binding epitope of trastuzumab may abolish antibody binding and allow recovery of receptor activation and downstream signaling.
In addition to mediating resistance to trastuzumab, we would expect to find a critical role for PI3K-AKT signaling in escape from the HER2 TKI lapatinib. However, in HER2-overexpressing BT474 human breast cancer cells selected for acquired resistance to lapatinib, the resistant cells continued to show inhibition of both HER2 and AKT phosphorylation upon treatment with lapatinib.43 In these cells, signaling through the estrogen receptor (ER) was the proposed mechanism of escape, and co-treatment of the cells with lapatinib and an anti-estrogen prevented the outgrowth of resistant cells. This interesting result requires confirmation in the clinic especially in light of the fact that the majority of HER2-positive tumors are ER-negative. Other studies have shown recovery of HER3 phosphorylation and downstream PI3K-AKT signaling after short-term inhibition of HER2 with gefitinib.44 We should note, however, that this escape from gefitinib action may well be prevented by lapatinib, a more potent inhibitor of HER2 compared to gefitinib. The role for activating PI3K mutations, PTEN loss, and alternative signaling pathways to activate PI3K-AKT as a mechanism for escape from lapatinib are also important areas of continued investigation.
What then should be our strategy for identifying and overcoming resistance to TKIs in cancer? Clearly, the use of oncogene-addicted, highly drug-sensitive cell line models to identify escape mechanisms has been fruitful. When confirmed in tumor material from drug-refractory patients, this information should lead to therapeutic strategies that can be tested in clinical trials. Thus, it is of paramount importance that when medically possible, we biopsy cancer recurrences in patients that become resistant to targeted therapies so that these mechanisms can be confirmed or refuted. By identifying how a patient’s cancer becomes refractory to targeted therapies, we will be well positioned to devise rational treatment strategies to re-induce remissions. With the expanding knowledge of these mechanisms of escape, the availability of drugs that therapeutically target those escape pathways, and examples where such molecular data informed a treatment decision leading to a good clinical outcome, biopsy and molecular profiling of recurrent drug-resistant tumors may become widely practiced.
As predominant resistance pathways are identified, the goal may shift to determine if therapies that block the resistance mechanisms can be used earlier in the natural history of tumors to prevent or delay cancer recurrences. Two important challenges to this strategy exist. First, currently there are no good biomarkers to prospectively define the likely mechanism of acquired escape. In addition, overcoming resistance may well require adding an inhibitor to an EGFR TKI but not replacing it as it was in the case where IGF1R or MET mediated such resistance to the primary anti-EGFR therapy.3, 8 The preclinical data in the case of EGFR TKI-resistant cells suggest that, as single agents, these inhibitors may have no activity but, in combination with the primary therapy, will significantly prolong response and/or induce complete remissions. Thus, it is entirely possible that their efficacy would be missed if the emphasis is to test them as single agents. In turn, this low single-agent activity may limit enthusiasm for patient accrual into trials testing their tolerability and efficacy in combination with the primary anti-EGFR therapy. With all these caveats, the last few years have witnessed important advances in the discovery of mechanisms of de novo and acquired resistance to oncogene-targeted therapies. We firmly believe that this knowledge should eventually unleash the full potential of combinations of TKIs in the treatment of cancer.
Acknowledgments
Supported in part by NCI R01 CA62212 (CLA), R01 CA80195 (CLA), ACS Clinical Research Professorship Grant CRP-07-234 (CLA), a grant from the Entertainment Industry Foundation (CLA), NCI T32 CA119910 (BNR), Breast Cancer Specialized Program of Research Excellence (SPORE) P50 CA98131, Vanderbilt-Ingram Comprehensive Cancer Center Support Grant P30 CA68485, NIH K08 grant CA120060-01 (JAE), an American Association for Cancer Research Grant (JAE), the DF/HCC Lung Cancer Specialized Program SPORE grant P50 CA090578 (JAE), and the DF/HCC Gastrointestinal Cancer SPORE grant P50 CA127003 (JAE).
List of abbreviations
- EGFR
epidermal growth factor receptor
- HER2
human epidermal growth factor receptor 2
- TKI
tyrosine kinase inhibitor
- PI3K
phosphoinositide 3-kinase
- ERK
extracellular signal regulated kinase
- IGF1
insulin-like growth factor 1
- IGF1R
insulin-like growth factor receptor 1
- IGFBP
insulin-like growth factor binding protein
- PTEN
phosphatase and tensin homolog
- ER
estrogen receptor
References
- 1.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 2.Sergina NV, Moasser MM. The HER family and cancer: emerging molecular mechanisms and therapeutic targets. Trends in molecular medicine. 2007;13:527–34. doi: 10.1016/j.molmed.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–19. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 9.Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–90. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 10.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 11.Shah NP, Sawyers CL. Mechanisms of resistance to STI571 in Philadelphia chromosome-associated leukemias. Oncogene. 2003;22:7389–95. doi: 10.1038/sj.onc.1206942. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 13.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelman JA, Mukohara T, Zejnullahu K, Lifshits E, Borrás AM, Gale CM, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116:2695–706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogino A, Kitao H, Hirano S, Uchida A, Ishiai M, Kozuki T, et al. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non small cell lung cancer cell line. Cancer Res. 2007;67:7807–14. doi: 10.1158/0008-5472.CAN-07-0681. [DOI] [PubMed] [Google Scholar]
- 16.Yuza Y, Glatt KA, Jiang J, Greulich H, Minami Y, Woo MS, et al. Allele-dependent variation in the relative cellular potency of distinct EGFR inhibitors. Cancer Biol Ther. 2007;6:661–7. doi: 10.4161/cbt.6.5.4003. [DOI] [PubMed] [Google Scholar]
- 17.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 18.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 19.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 20.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 21.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 22.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Annals of Oncology. 2007;18:977. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 23.Engelman JA, Jänne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci USA. 2005;102:3788–93. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le XF, Lammayot A, Gold D, Lu Y, Mao W, Chang T, et al. Genes affecting the cell cycle, growth, maintenance, and drug sensitivity are preferentially regulated by anti-HER2 antibody through phosphatidylinositol 3-kinase-AKT signaling. J Biol Chem. 2005;280:2092–104. doi: 10.1074/jbc.M403080200. [DOI] [PubMed] [Google Scholar]
- 25.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–41. [PubMed] [Google Scholar]
- 26.Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–19. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 27.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Current Opinion in Oncology. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 28.Sansal I, Sellers W. The Biology and Clinical Relevance of the PTEN Tumor Suppressor Pathway. Journal of Clinical Oncology. 2004;22:2954. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 29.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–27. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–7. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 32.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nature clinical practice Oncology. 2006;3:269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 33.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–28. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 34.Shattuck DL, Miller JK, Carraway KL, 3rd, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68:1471–7. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- 35.Buttitta F, Barassi F, Fresu G, Felicioni L, Chella A, Paolizzi D, et al. Mutational analysis of the HER2 gene in lung tumors from Caucasian patients: mutations are mainly present in adenocarcinomas with bronchioloalveolar features. Int J Cancer. 2006;119:2586–91. doi: 10.1002/ijc.22143. [DOI] [PubMed] [Google Scholar]
- 36.Mounawar M, Mukeria A, Le Calvez F, Hung RJ, Renard H, Cortot A, et al. Patterns of EGFR, HER2, TP53, and KRAS mutations of p14arf expression in non-small cell lung cancers in relation to smoking history. Cancer Res. 2007;67:5667–72. doi: 10.1158/0008-5472.CAN-06-4229. [DOI] [PubMed] [Google Scholar]
- 37.Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65:1642–6. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 38.Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431:525–6. doi: 10.1038/431525b. [DOI] [PubMed] [Google Scholar]
- 39.Willmore-Payne C, Holden JA, Layfield LJ. Detection of epidermal growth factor receptor and human epidermal growth factor receptor 2 activating mutations in lung adenocarcinoma by high-resolution melting amplicon analysis: correlation with gene copy number, protein expression, and hormone receptor expression. Hum Pathol. 2006;37:755–63. doi: 10.1016/j.humpath.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Willmore-Payne C, Holden JA, Layfield LJ. Detection of EGFR- and HER2-activating mutations in squamous cell carcinoma involving the head and neck. Mod Pathol. 2006;19:634–40. doi: 10.1038/modpathol.3800552. [DOI] [PubMed] [Google Scholar]
- 41.Lee JW, Soung YH, Seo SH, Kim SY, Park CH, Wang YP, et al. Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res. 2006;12:57–61. doi: 10.1158/1078-0432.CCR-05-0976. [DOI] [PubMed] [Google Scholar]
- 42.Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10:25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA. 2006;103:7795–800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–41. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]