Abstract
Blockade of T cell costimulatory pathways represents a potent and highly specific method of preventing naïve anti-donor T cell responses following transplantation in mouse, monkey, and man. However, numerous studies have shown that the presence of donor-reactive memory T cells in the recipient poses a sometimes insurmountable barrier to long-term graft survival and tolerance induction. Here, we discuss the ways in which donor-reactive memory T cells may arise from environmental exposure to pathogens. Pathogen-specific memory T cells, by virtue of the inherent degeneracy of TCR recognition of peptide:MHC ligands, may exhibit cross-reactivity with allogeneic peptide:MHC complexes and thereby mediate graft rejection. From the recent explosion in knowledge of the heterogeneity of memory T cell resulting from variations in frequency and duration of antigen exposure, cytokine milieu, site of priming, and a host of other factors, it is becoming increasingly well-appreciated that different memory T cell populations may exhibit differential susceptibilities to tolerance induction. Thus, the immune history of a transplant recipient and frequencies of donor-cross-reactive memory T cells within the various compartments may dictate the likelihood of success or failure of tolerance induction.
Keywords: transplant tolerance, T cell memory, heterologous immunity, alloreactivity
One of the hallmark features of the mammalian adaptive immune response is the formation of immunological memory. The existence of pre-formed humoral and cellular immunity to an invading pathogen thus provides the host, at both the individual and population level, with a large evolutionary advantage. The cardinal features of immunologic memory, including increased antigen-specific precursor frequency, lower activation threshold, and rapid effector function, provide a clear benefit for protection against re-infection with the same pathogen. However, for precisely the same reasons that they provide host protection against pathogen threats, they pose a potential barrier to transplant tolerance induction, and threaten long-term graft survival.
Of mice and men: immune history impacts susceptibility to tolerance induction
While targeting the CD28 and CD40 costimulatory pathways to induce transplantation tolerance in laboratory mice met with wide success (1, 2), the initial application of these approaches proved less successful in non-human primates. It is now widely recognized that the immune history of a transplant recipient may be a major determinant of the success or failure of tolerance induction strategies (3-10). To underscore this point, memory T cells (CD44hi) comprise only ~5-10% of the T cell compartment of specific pathogen-free experimental mice. In contrast, while absent at birth, memory T cells compromise 40-50% of the T cell pool in socially housed non-human primates by three years of age and in adult humans (11-13). In human transplant recipients, higher levels of donor-specific memory T cells are associated with higher rejection rates (14). Given the fact that it has been estimated that 1-10% of the peripheral T cell compartments of naïve mice are allo-reactive (15), it stands to reason that this large pool of memory T cells in adult humans would stochastically contain a high frequency of donor-reactive memory T cells. The question of whether the alloreactive precursor frequency is in fact similar between memory and naïve T cell compartments has not been fully addressed, and warrants further study. However, it is likely that the relative presence of memory T cells in a given individual is a major factor in determining the relative success of tolerance induction protocols during transplantation.
Memory T cells are refractory to tolerance induction through a variety of therapeutic modalities
Secondly, memory T cell populations seem to exhibit an increased resistance to depletional therapy, either by anti-lymphocyte serum (ALS) in mice (25), or via alemtuzumab (Campath-1H) in humans (26). In a recent study by Pearl et al., it was demonstrated that memory CD4+ T cells were selectively spared from depletion with Campath-1H in human recipients of renal allografts, and these T cells persisted upon repopulation of the peripheral T cell compartment (26). Third, memory T cells are relatively resistant to the effects of regulation, as demonstrated in a recent study by Jones and colleagues (27). While CD4+ CD25+ FoxP3+ regulatory T cells were capable of attenuating naïve T cell responses in this model, memory T cell responses were less inhibited by the activity of regulatory T cells (27). In a final and perhaps most dramatic example, memory T cells appear to be refractory to the tolerizing effects of agonistic anti-CD28 monoclonal antibody therapy. While agonistic anti-CD28 based regimens were shown to be efficacious in preventing allograft rejection in murine models of fully MHC disparate bone marrow transplantation (28, 29), Phase I clinical trials ended in tragedy when agonistic anti-CD28 mAbs induced a systemic inflammatory response accompanied by lung injury, renal failure, and disseminated intravascular coagulation in six out of six recipients of the drugs (30). The reason for this dichotomous result relative to the murine studies was speculated to have been caused by the presence of a high frequency of memory T cells in the human patients, as opposed to the 2-10% observed in mice (30).
How do transplant recipients develop alloreactive memory T cells?
Donor-specific memory T cells arise from prior exposures to alloantigens via transfusion, transplantation, or pregnancy. In addition to these traditional conceptions of alloantigen exposure, in more recent years it has become increasingly apparent that several other mechanisms exist by which donor-reactive memory T cells might be generated. First, memory T cells specific for a pathogen-derived epitope may express a second T cell receptor (TCR) that could be alloreactive against donor tissue. Dual receptor T cells are the result of incomplete allelic exclusion of the TCR α locus, and have been purported to comprise up to 30% of the peripheral T cells in adult humans (31). Second, alloreactive memory T cells may be generated by homeostatic proliferation of alloreactive precursors following lymphopenia in the host (32, 33). This may be clinically relevant in that following lymphopenia induced by a viral pathogen such as HIV, or by therapeutic depletion of T cells for the treatment of autoimmunity or transplantation, as residual T cells are induced to undergo rapid division and the acquisition of a memory-like phenotype. Memory cells generated in this manner have also been shown to constitute a barrier to tolerance induction (34).
In addition, studies have shown that prior infections generate memory T cells that are allo-cross-reactive in a process termed heterologous immunity (4, 22, 35). First thought to be exquisitely specific for a given peptide:MHC complex, T cell receptors (TCRs) are now considered to undergo degenerate recognition of their ligands, in that each TCR possess intrinsic cross-reactivity to a wide spectrum of related peptide:MHC ligands. Early studies using variations of the antigenic peptide with amino acid substitutions at TCR contact residues demonstrated that each TCR can respond to a range of peptides that vary widely in their sequences, and that these peptides elicit a spectrum of responses in the T cell (36, 37). The implications of this intrinsic cross-reactivity supports the concept of heterologous immunity, suggesting that microbial pathogens might activate antigen-specific T cells that then cross-react with allogeneic tissue and result in graft rejection. Mathematical modeling of the T cell repertoire suggests that TCR cross reactivity is required for complete coverage of pathogens; for example, it has been estimated that a single TCR may be capable of recognizing up to 106 related ligands (38). Recently, Allen and colleagues proposed that T cells may not only possess intrinsic cross-reactivity and degeneracy but may be capable of recognizing multiple, structurally unrelated peptide:MHC complexes (15).
Cross-reactive T cell stimulation history can impact tolerance induction
Studies in murine models of infection prior to or following transplantation have revealed that both the timing and type of infection can play a large role in determining the impact on tolerance induction. For example, concurrent infection with LCMV Armstrong or with Listeria monocytogenes accelerates rejection and abrogrates tolerance induction (39, 40). However, infection with LCMV Armstrong after tolerance has already been established does not break tolerance (39). These data suggest that the inflammatory milieu of a viral or bacterial infection may override the effects of costimulation blockade during tolerance induction, but may not affect donor-reactive T cell responses once they are already anergized or deleted. Furthermore, while prior infection with LCMV Armstrong results in inhibition of tolerance induction in only 7% of mice, prior infection with LCMV clone 13, which persists for the life of the host, completely abrogates tolerance induction in 100% of the recipients (8). Thus, the type of infection, whether it persists in the host, and the frequency and timing of T cell stimulation with antigen are all likely to play a role in determining the impact of those T cell populations on tolerance induction.
The tropism of the virus is also likely to play a role in determining its impact on T cell tolerance induction. If the virus infects the transplanted organ, as in hepatitis C virus or BK virus, viral-specific T cells may play a greater role in inhibiting tolerance induction. For example, infection with mouse polyoma virus (a relative of human BK virus, which infects the kidney) resulted in acute rejection of allogeneic but not syngeneic transplanted kidneys (41). This was accompanied by an increase in donor-reactive T cell responses in the CD8+ T cell compartment (41). Thus, these results suggest that either viral-specific T cells were cross-reactive with alloepitopes, or that the inflammatory milieu generated by the viral infection in the kidney increased the activation and differentiation of the allo-reactive T cell clones.
Heterogeneity among memory T cell subsets: implications for tolerance induction
Over the past decade, major advances have helped unravel the intricacies of T cell memory. It is now recognized that subsets of memory T cells exhibit an array of phenotypes, functional properties and recirculation patterns and may serve specialized roles in protection. Memory CD4+ and CD8+ T cells are often segregated into two subsets, central (TCM) and effector (TEM) memory. TCM express lymph node homing receptors (CD62L or CCR7), whereas TEM lack these markers but express other chemokine receptors (CCR5 and CCR6), which direct them to peripheral tissues (42, 43). In addition, TCM have high proliferative potential, express CD27, produce IL-2 upon Ag recognition, but require a longer period to re-acquire cytolytic function upon rechallenge. In contrast, TEM have a lower proliferative potential reside primarily in nonlymphoid tissues, are immediately cytolytic upon Ag re-exposure, and are poor producers of IL-2 (42, 44 , 45-47). Current thinking holds that depending on the route of exposure, dose, replication rate, and tropism of the infectious challenge, either memory T cell subset may be retained to a greater or lesser degree and subsequently play a more or less dominant role in protective immunity (48). Furthermore, there is evidence that memory CD8+ T cell differentiation into TCM and TEM is dictated by the cumulative history of Ag exposure with repeated exposure favoring TEM>TCM (48.).
Given that memory subsets play distinct roles in protective immunity during recall responses and differ in expression of key costimulatory, cytokine and chemokine receptors, they are likely to vary in their susceptibilities to blockade of costimulatory pathways. For example, our studies as well as those of others suggest that CD4+ memory cells may be more susceptible to the effects of CD28/CD40 costimulation blockade during recall than CD8+ memory T cells (22, 49). Furthermore, we have shown in a fully allogeneic model that TCM that were generated by prior exposure to BALB/c antigen through a skin graft posed a greater barrier to tolerance induction than TEM in a fully allogeneic murine model of skin graft rejection (22). However, in a model of donor-reactive memory T cells generated via a latent viral infection, TEM generated by a latent EBV homologue posed a robust barrier to the tolerance induction (50). A recent study by Pearl et al. implicated CD4+ TEM as being relatively resistant to the effects of therapeutic depletion via either anti-thymocyte globulin or Campath-H1. This study also highlights the fact that subsets reside in anatomically different locations may also impact their relative access to therapeutic agents.
Increased precursor frequency alone may contribute to the costimulation blockade resistance of memory T cells
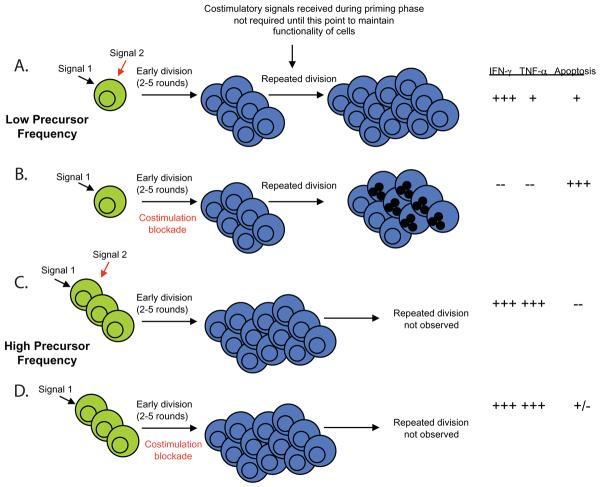
What properties of donor-reactive memory T cell populations confer costimulation independence? We recently uncovered a role for naïve CD4+ and CD8+ T cell precursor frequency in determining the degree of proliferation and differentiation of responding donor-reactive T cell populations during transplantation, and in mediating costimulation blockade-resistant rejection (51, 52). Naive graft-specific CD8+ T cells stimulated at high frequency proliferated and accumulated even in the presence of CTLA-4 Ig and anti-CD154, resulting in more “high-quality,” multi-cytokine producing effector cells and the precipitation of graft rejection (51). In contrast, naïve graft-specific CD8+ T cells stimulated at lower frequency failed to accumulate and did not differentiate into high quality effectors that were capable of rejecting a skin graft (Figure 1). Thus, even naïve T cells present at high frequency appear to obviate the need for costimulation during priming. Furthermore, previous studies have shown that a critical frequency of memory T cells is also required to generate costimulation blockade-resistant rejection. For example, adoptive transfer of 105 but not 104 alloreactive memory cells into naïve recipients resulted in graft loss despite treatment with costimulation blockade (22). Therefore, in addition to their clearly reduced activation threshold, a critical component involved in the costimulation blockade resistance of memory T cells may be their heightened precursor frequency. Taken together, these studies demonstrate that high-frequency T cell responses, present either as naïve alloreactive populations or as memory T cell populations cross-reactive for an alloantigen, may obviate the need for costimulation and play a large role in mediating costimulation blockade-resistant allograft rejection.
Figure 1.
Impact of antigen-specific T cell precursor frequency on susceptibility to costimulation blockade. A, Cells stimulated at low precursor frequency are required to undergo multiple rounds of division in order to generate a threshold number of effector cells needed to mediate graft rejection. B, Cells that undergo multiple rounds of division in the absence of costimulation fail to differentiate into competent effectors and undergo increased cell death at later rounds of division. C, In contrast, cells that are stimulated at high naïve T cell precursor frequency must undergo many fewer rounds of division in order to a sufficient number of effector cells to mediate graft rejection. D, Populations that had undergone fewer rounds of division in the absence of costimulation had better effector function and reduced death as compared to those that underwent more rounds of division in the absence of costimulation.
Natural history of a given patient may therefore influence memory T cell repertoire
It is becoming increasingly well-recognized that stimulation history may play an important role in the quantity and quality of antigen-specific T cell memory. For example, T cells that had been exposed to antigen three times (so-called tertiary memory cells) exhibited significantly increased proliferation and effector function and decreased cell death as compared to cells that had been previously exposed only once (so-called primary memory cells) (48). Thus, the functional properties of memory T cells generated by a single brief encounter with antigen (a single vaccine), repeated brief encounters (the common cold/corona viruses and influenza), or sustained exposure (latent pathogens such as EBV and CMV) may differ in the relative composition of their memory subsets as well as the function of cells within each subset. As discussed above, the panel of pathogens to which a given patient has been exposed may dictate the relative composition and frequency of CD4+ and CD8+ TEM and TCM subsets that are cross-reactive for alloantigens. Thus, the immune history of a transplant recipient and frequencies of donor-cross-reactive memory T cells within the various compartments may dictate the likelihood of success or failure of tolerance induction or even immunosuppression. By understanding the function, costimulatory and signaling requirements for recall responses mediated by the various memory T cell subsets, we may be able to tailor tolerance induction approaches to control the predominant forms of memory for specific donor-recipient combinations.
Conclusions
The presence of pre-existing anti-donor T cells in transplant recipients is increasingly being recognized as a potentially formidable barrier to tolerance induction via a variety of therapeutic modalities, including costimulation blockade, T cell depletion, and regulatory T cells. As discussed above, however, memory T cell populations contain a high degree of heterogeneity, and may vary considerably in terms of their ability to traffic into the graft, to resistant depletion, and to mediate costimulation blockade-resistant rejection. Advances in understanding the costimulatory, cytokine, adhesion, and survival requirements of different types of donor-reactive memory T cells will allow us to better tailor immunosuppression/tolerance induction protocols for a given individual based on their memory T cell profile, and will direct the future development of novel therapeutics to better target donor-reactive memory T cell populations.
References
- 1.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 2.Linsley PS, Wallace PM, Johnson J, et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 3.Neujahr DC, Chen C, Huang X, et al. Accelerated memory cell homeostasis during T cell depletion and approaches to overcome it. J Immunol. 2006;176:4632. doi: 10.4049/jimmunol.176.8.4632. [DOI] [PubMed] [Google Scholar]
- 4.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 5.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 6.Welsh RM, Markees TG, Woda BA, et al. Virus-induced abrogation of transplantation tolerance induced by donor- specific transfusion and anti-CD154 antibody [In Process Citation] J Virol. 2000;74:2210. doi: 10.1128/jvi.74.5.2210-2218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams AB, Shirasugi N, Jones TR, et al. Conventional immunosuppression is compatible with costimulation blockade-based, mixed chimerism tolerance induction. Am J Transplant. 2003;3:895. doi: 10.1034/j.1600-6143.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams MA, Onami TM, Adams AB, et al. Cutting edge: persistent viral infection prevents tolerance induction and escapes immune control following CD28/CD40 blockade-based regimen. J Immunol. 2002;169:5387. doi: 10.4049/jimmunol.169.10.5387. [DOI] [PubMed] [Google Scholar]
- 9.Valujskikh A, Lakkis FG. In remembrance of things past: memory T cells and transplant rejection. Immunol Rev. 2003;196:65. doi: 10.1046/j.1600-065x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 10.Lakkis FG, Sayegh MH. Memory T cells: a hurdle to immunologic tolerance. J Am Soc Nephrol. 2003;14:2402. doi: 10.1097/01.asn.0000085020.78117.70. [DOI] [PubMed] [Google Scholar]
- 11.McFarland RD, Douek DC, Koup RA, Picker LJ. Identification of a human recent thymic emigrant phenotype. Proc Natl Acad Sci U S A. 2000;97:4215. doi: 10.1073/pnas.070061597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690. doi: 10.1038/25374. see comments. [DOI] [PubMed] [Google Scholar]
- 13.Pitcher CJ, Hagen SI, Walker JM, et al. Development and Homeostasis of T Cell Memory in Rhesus Macaque. J Immunol. 2002;168:29. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Heeger PS, Greenspan NS, Kuhlenschmidt S, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267. [PubMed] [Google Scholar]
- 15.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 16.Lanzavecchia A, Sallusto F. From synapses to immunological memory: the role of sustained T cell stimulation. Curr Opin Immunol. 2000;12:92. doi: 10.1016/s0952-7915(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 17.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 18.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 20.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 21.Grayson JM, Murali-Krishna K, Altman JD, Ahmed R. Gene expression in antigen-specific CD8+ T cells during viral infection. J Immunol. 2001;166:795. doi: 10.4049/jimmunol.166.2.795. [DOI] [PubMed] [Google Scholar]
- 22.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Heeger PS, Valujskikh A. In Vivo Helper Functions of Alloreactive Memory CD4+ T Cells Remain Intact Despite Donor-Specific Transfusion and Anti-CD40 Ligand Therapy. Journal of Immunology. 2004;172:5456. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 25.Minamimura K, Sato K, Yagita H, Tanaka T, Arii S, Maki T. Strategies to induce marked prolongation of secondary skin allograft survival in alloantigen-primed mice. Am J Transplant. 2008;8:761. doi: 10.1111/j.1600-6143.2007.02143.x. [DOI] [PubMed] [Google Scholar]
- 26.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Brook MO, Carvalho-Gaspar M, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci U S A. 2007;104:19954. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert MH, Yu XZ, Martin PJ, Anasetti C. Prevention of lethal acute GVHD with an agonistic CD28 antibody and rapamycin. Blood. 2005;105:1355. doi: 10.1182/blood-2004-08-3305. [DOI] [PubMed] [Google Scholar]
- 29.Yu XZ, Albert MH, Martin PJ, Anasetti C. CD28 ligation induces transplantation tolerance by IFN-gamma-dependent depletion of T cells that recognize alloantigens. J Clin Invest. 2004;113:1624. doi: 10.1172/JCI20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 31.Padovan E, Casorati G, Dellabona P, Meyer S, Brockhaus M, Lanzavecchia A. Expression of two T cell receptor alpha chains: dual receptor T cells. Science. 1993;262:422. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- 32.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J Immunol. 2000;165:1733. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J Immunol. 2003;170:4077. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 36.Evavold BD, Sloan-Lancaster J, Allen PM. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunology Today. 1993;14:602. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- 37.Sloan-Lancaster J, Allen PM. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu Rev Immunol. 1996;14:1. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 39.Williams MA, Tan JT, Adams AB, et al. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J Immunol. 2001;167:4987. doi: 10.4049/jimmunol.167.9.4987. [DOI] [PubMed] [Google Scholar]
- 40.Wang T, Chen L, Ahmed E, et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol. 2008;180:5991. doi: 10.4049/jimmunol.180.9.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han Lee ED, Kemball CC, Wang J, et al. A mouse model for polyomavirus-associated nephropathy of kidney transplants. Am J Transplant. 2006;6:913. doi: 10.1111/j.1600-6143.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- 42.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 43.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 44.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005;201:579. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hislop AD, Gudgeon NH, Callan MF, et al. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J Immunol. 2001;167:2019. doi: 10.4049/jimmunol.167.4.2019. [DOI] [PubMed] [Google Scholar]
- 47.Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 49.Ndejembi MP, Teijaro JR, Patke DS, et al. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. J Immunol. 2006;177:7698. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 50.Stapler D, Lee ED, Selvaraj SA, et al. Expansion of Effector Memory TCR V{beta}4+CD8+ T Cells Is Associated with Latent Infection-Mediated Resistance to Transplantation Tolerance. J Immunol. 2008;180:3190. doi: 10.4049/jimmunol.180.5.3190. [DOI] [PubMed] [Google Scholar]
- 51.Ford ML, Koehn BH, Wagener ME, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ford ML, Wagener ME, Hanna SS, Pearson TC, Kirk AD, Larsen CP. A Critical Precursor Frequency of Donor-Reactive CD4+ T Cell Help is Required for CD8+ T Cell-Mediated CD28/CD154-Independent Rejection. J Immunol. 2008;180:7207. doi: 10.4049/jimmunol.180.11.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]