Abstract
Rationale
Since the discovery of endogenous cannabinoid signaling, the number of studies exploring its role in health and disease has increased exponentially. Fatty acid amide hydrolase (FAAH), the enzyme responsible for degradation of the endocannabinoid anandamide, has emerged as a promising target for anxiety-related disorders. FAAH inhibitors (e.g. URB597) increase brain levels of anandamide and induce anxiolytic-like effects in rodents. Recent findings, however, questioned the efficacy of URB597 as an anxiolytic.
Objectives
We tested here the hypothesis that conflicting findings are due to variations in the stressfulness of experimental conditions employed in various studies.
Results
We found that URB597 (0.1–0.3mg/kg) did not produce anxiolytic effects when the aversiveness of testing procedures was minimized by handling rats daily before experimentation, by habituating them to the experimental room, or by employing low illumination during testing. In contrast, URB597 had robust anxiolytic effects when the aversiveness of the testing environment was increased by eliminating habituation to the experimental room or by employing bright lighting conditions. Unlike URB597, the benzodiazepine chlordiazepoxide (5 mg/kg) had anxiolytic effects under all testing conditions. The anxiolytic effects of URB597 were abolished by the cannabinoid CB1-receptor antagonist AM251, showing that they were mediated by CB1 receptors. Close inspection of experimental conditions employed in earlier reports suggests that conflicting findings with URB597 can be explained by different testing conditions, such as those manipulated in the present study.
Conclusions
Our findings show that FAAH inhibition does not affect anxiety under mildly-stressful circumstances but protects against the anxiogenic effects of aversive stimuli.
Keywords: FAAH, URB597, anxiety, stress, aversive, elevated plus-maze, rat
Introduction
Since the discovery of the endocannabinoid system, a growing body of research has emerged focusing on the role of this system in major psychiatric disorders, including anxiety (Leweke and Koethe 2008; Pacher et al. 2006). Although it has been clearly demonstrated that cannabinoid signaling is involved in the control of anxiety, it has been difficult to define the exact role of this signaling because both anxiolytic- and anxiogenic-like effects have been reported with both cannabinoid agonists and antagonists (Viveros et al. 2005; Witkin et al. 2005). These discrepancies might be explained by the brain distribution of cannabinoid CB1 receptors. Such receptors have been shown to affect both glutamatergic and GABAergic mechanisms (Hajos et al. 2001; Hajos and Freund 2002; Piomelli 2003), which play opposite roles in anxiety (Millan 2003). Therefore, the anxiety-related effects of cannabinoids depend largely on the equilibrium between their effects on glutamatergic and GABAergic neurotransmission (Haller et al. 2007).
In this context, indirect stimulation of cannabinoid signaling via inhibitors of enzymes responsible for endocannabinoid degradation represents a promising approach for therapy. Fatty acid amide hydrolase (FAAH) is the primary enzyme responsible for degrading anandamide but does not degrade the other main endocannabinoid 2-arachidonyl glycerol, 2-AG. The indirect stimulation of anandamide signaling by FAAH inhibitors is more selective than direct cannabinoid agonism for two reasons. First, FAAH inhibitors do not directly affect 2-AG signaling, although there is recent evidence that increased anandamide signaling can lead to compensatory decreases in 2-AG signaling under some conditions (Di Marzo and Maccarrone 2008; Justinoval et al. 2008; Maccarrone et al. 2008). Second, FAAH inhibitors promote anandamide signaling only when and where it is already occurring, and do not directly induce it. It has been suggested that URB597 enhances the tonic actions of anandamide on a subset of CB1 receptors that are normally engaged in controlling emotions (Piomelli et al. 2006).
In the first study testing the hypothesis that URB597 should have therapeutic effects on anxiety, the compound met these expectations as it reduced anxiety-like behavior of Wistar rats in the elevated zero-maze test, and also reduced pups’ ultrasonic vocalizations (Kathuria et al. 2003). Later research confirmed these early findings, showing that URB597 can reduce anxiety-like behavior in a variety of species and strains, and in a variety of anxiety tests (Cippitelli et al. 2008, Scherma et al. 2008; Moreira et al. 2008; Hill et al. 2007; Patel and Hillard 2006; Rubino et al. 2008).
Although these findings provide strong evidence that URB597 can have anxiolytic effects, there have also been discrepant findings in certain studies, as follows: (i) URB597 was sometimes found to have no effect on anxiety, or produced anxiolytic effects only under very specific conditions (Naderi et al. 2008; Naidu et al. 2007). (ii) The compound was sometimes found to decrease anxiety in certain tests but was ineffective in others, even within the same study. For example, Moreira et al. (2008) found that URB597 decreased anxiety in the elevated plus-maze but not in the light/dark box. This failure to detect anxiolytic effects cannot be attributed to the light/dark test paradigm per se, as the compound did have anxiolytic effects in the light/dark test in other studies (Scherma et al. 2008). (iii) When the compound was found to decrease anxiety, there were sometimes discrepant findings regarding the effective doses (Cippitelli et al. 2008; Hill et al. 2007; Kathuria et al. 2003; Patel and Hillard 2006; Scherma et al. 2008; Moreira et al. 2008).
Discrepancies such as these are not unusual in anxiety research (see Haller et al. 2004a, a nd Rodgers 1997 for reviews). However, the reasons for these conflicting results are less understood in the case of URB597 than with other compounds. Some of the discrepancies might be explained by an interaction between anandamide and the transient receptor potential vanilloid 1 (TRPV1) receptor (Rubino et al. 2008). Yet this does not explain discrepant results reported by the same group in the same study performed in similar subjects or within the same kind of test. A more likely explanation is that the effects of URB597 depend largely on the experimental conditions. This conclusion is directly supported by the study performed by Naidu et al. (2007), where the anxiolytic effect of URB597 became evident only when the illumination differed in the open and closed arms of the plus-maze. A context-dependent effect of URB597 was also reported by Trezza and Vanderschuren (2008) who found that the increase in social play induced by the FAAH inhibitor URB597 was influenced by the level of social activity exhibited by the partner, whereas that induced by WIN-55,212 and morphine was not.
The manipulation of cannabinoid neurotransmission by means other than FAAH inhibition can also lead to context dependent effects on anxiety. Such context-dependent effects were noticed in CB1-KO mice, as well as with the cannabinoid agonist HU-210, and the antagonist rimonabant (Haller et al. 2004b; Hill and Gorzalka 2004; Rodgers et al. 2003). In tests unrelated to anxiety, cannabinoids have been found to have context-dependent effects on a host of measures, including cocaine sensitization, hypothalamic-pituitary axis (HPA) modulation, locomotion and basal ganglia activation, central amygdala activation, and place preference in the water maze (Gerdeman et al. 2007; Patel et al. 2004; Patel et al. 2005; Robinson et al. 2003; Shi et al. 2005).
Taken together, the studies briefly reviewed above suggest that the effects of cannabinoid treatments (including those induced by FAAH inhibition) are strongly dependent on environmental factors. Thus, we hypothesized that discrepancies in the findings previously obtained with URB599 in tests of anxiety can be explained by differences in testing conditions. To test this hypothesis, we studied the anxiety-related effects of URB597 in environments with differing degree of aversiveness. To determine whether the effects of these manipulations are specific to URB597 or whether they also apply to the anxiolytic effects of drugs from other pharmacological classes, we also tested the efficacy of the benzodiazepine chlordiazepoxide under non-aversive and aversive conditions. Finally, we checked whether the aversive environments used in this study were able to affect HPA-axis responses to plus-maze exposure.
Materials and Methods
Animals
Subjects were 3 month-old male Sprague-Dawley rats provided by Charles River Laboratories (Budapest, Hungary), and weighing 319.5±2.1 g. The weight of rats employed in different experiments was similar; the largest weight difference was between the rats used in Experiment 1 (305g on average) and those used in Experiment 4 (327g on average), a difference of only about 7%. In all experiments, laboratory food (Charles River Laboratories) and water were available ad libitum, while temperature and relative humidity were kept at 22±2 °C and 60±10%, respectively. A light/dark cycle of 12 hours was ensured with lights on at 0700h. All rats were housed in 1354G Eurostandard Type 4 cages (59.5×38×22 cm) in groups of 4. Acclimatization to housing conditions lasted at least one week. All rats were handled for 2–3 min on each of the 5 days that preceded behavioral testing. All subjects were experimentally naive and used in only one experiment each (with no drug history prior to the experiment).
Experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and were reviewed and approved by the Animal Welfare Committee of the Institute of Experimental Medicine.
Behavioral testing
The elevated plus-maze was made of dark grey painted wood (arm length 50 cm, arm width 17 cm, wall height 30 cm and platform height 80cm). The apparatus was illuminated either by four 40W neon lamps (high light condition; 200 lx at maze level) or by a red lamp of 40 W (low light condition; approximately 1 lx at maze level). Subjects were placed in the central area of the apparatus with head facing a closed arm. Exposure lasted 5min. Closed-arm entries (expressed as counts/5 min) were considered indicators of locomotor activity, whereas open-arm exploration was used as a measure of anxiety (Pellow et al 1985). Open arm exploration was characterized by two variables: the duration of open arm visits (expressed as % of total time), and % open arm entries (open arm entries/total arm entries*100). Behavior was video recorded by a camera placed 1.8m above the elevated plus-maze, and was scored later by an experimenter blind to the treatments. All experiments were scored by the same experimenter. Intra-rater reliability was over 90%.
Drugs and doses
URB597 was dissolved in 0.2 ml dimethylsulfoxide (DMSO), which was diluted to the final volume with saline that contained 0.4% methylcellulose, a biologically neutral solvent. The doses employed (vehicle, 0.1, and 0.3 mg/kg) were selected based on earlier findings (Kathuria et al. 2003; Naderi et al. 2008; Scherma et al. 2008; Hill et al. 2007; Naidu et al. 2007, Patel and Hillard 2006). AM-251 was also dissolved in 0.2 ml DMSO diluted to the final volume with 0.4% methylcellulose in saline. Earlier we observed that two consecutive injections slightly alter behavior compared to when one injection is given; therefore, AM251 and URB597 were administered via the same injection when given as a combined treatment. The injected volume was similar to that of single treatments. The AM251 dose (1 mg/kg) was selected based on earlier experience (Haller et al. 2007) in which this dose of AM251 per se was without effect in rats, but interfered with the effects of the cannabinoid agonist WIN-55,212. Chlordiazepoxide was dissolved in 0.4% methylcellulose in saline. The doses applied were 1, 2, 3, 4, 5 and 6 mg/kg. All drugs were obtained from Sigma (Budapest, Hungary), and were injected intraperitoneally in a volume of 1 ml/kg.
Hormone assays
Blood was collected on ice-cold Eppendorf tubes containing 20 μl 20% sodium-EDTA and centrifuged at 3000 rpm/min for 20 min at −4 °C. The plasma was stored at −20 °C till the hormone assays. Plasma ACTH was measured by RIA in 50 μl unextracted plasma as described earlier (Zelena et al., 1999). The ACTH antibody was raised in rabbit in the Institute of Experimental Medicine, Hungarian Academy of Sciences (Budapest, Hungary) and was directed against the middle part of the h-ACTH1–39 molecule. The sample no. 8514 was used. The antibody is highly specific, showing 0.2% cross-reaction with α-MSH, and no significant cross-reaction with γ-MSH, corticotropin-like intermediate lobe peptide, ACTH11–24, ACTH25–39, ACTH1–14, and ACTH1–19. The intra-assay coefficient of variation was 7.23%. All samples were assessed in the same RIA.
Plasma corticosterone was measured in 10 μl unextracted plasma by a RIA using a specific antibody developed in our institute as described earlier (Zelena et al., 2003). The corticosterone antibody was raised in rabbits against B-carboxymethyloxime BSA. 125I-labeled B-carboxymethyloxime-tyrosine methyl ester was used as tracer. The interference from plasma transcortin was eliminated by inactivating transcortin at a low pH. Assay sensitivity was 1 pmol. The intra-assay coefficient of variation was 7.5%. All samples were measured in one RIA.
Experimental design
General procedure
The general experimental conditions applied here were similar to those in which anxiolytic effects of URB597 were demonstrated earlier (Scherma et al. 2008). Rats were acclimatized to housing conditions for at least one week. On the last 5 days of acclimatization, each rat was handled daily for 2–3 min. The experiments were performed in the early hours of the light phase, i.e. between 0900–1200h. A subset of rats was habituated to the test room for 2h (i.e. between 0700–0900h) on the test day. Habituation consisted of transferring the wheeled cage racks from the maintenance room to the testing room. After 2h of habituation, rats received treatments intraperitoneally, and were exposed to the elevated plus-maze 40min later. Treatments were administered in a random order. Non-habituated rats remained in their maintenance rooms where they received treatments, and were transferred to the test room immediately prior to testing. The timing of treatments and testing was similar in the two groups.
Experiment 1 was designed to test whether the experimental conditions under which URB597 decreased anxiety in the light/dark box would lead to a similar effect in the elevated plus maze. In this experiment, all rats were habituated to the testing environment and were tested under high light. Sample size was 13–14 per group.
In Experiment 2, we mimicked the light intensity changes to which rats were inherently exposed in the light-dark box. Since URB597 had minimal effects on behavior in Experiment 1, and illumination has been reported to strongly influence the anxiety-related effects of URB597-treated rats in various contexts (see e.g. Naidu et al. 2007), we hypothesized that the anxiolytic effects of URB597 would be revealed in the plus maze if the lighting-related aspects of the light/dark test were incorporated into the experimental conditions. Rats were again habituated to the test room, and their plus-maze testing started under high light. One min later, however, the white lamps were switched off, while red lamps were switched on. As a consequence, rats underwent dramatic light intensity changes during testing (from 200 lx light intensity down to 1 lx). Sample size was 13–14 per group.
Experiment 3 tested the impact of habituation to the testing environment on the anxiolytic efficacy of URB597. In Experiment 2, the compound had a robust anxiolytic effect. We hypothesized that this increase in efficacy was not related to light per se, but was induced by the aversiveness of the testing environment. In Experiment 3, aversiveness of the testing environment was increased by omitting the habituation to the test room rather than by changing lighting conditions. The rats tested here were transferred to the testing room immediately before testing, and were studied under high light conditions. Sample size was 12–14 per group.
Experiment 4 was performed to assess the CB1 receptor dependency of the anxiolytic effects noticed in Experiment 3. In this experiment, rats received the following treatments: vehicle, the CB1-receptor antagonist AM-251 (1 mg/kg), URB597 (0.3 mg/kg), and combination treatment with 1 mg/kg AM-251 and 0.3 mg/kg URB597. Sample size was 9–11 per group.
In Experiment 5, we studied the effects of URB597 under both low and high light conditions in rats that were not habituated to the testing room. We hypothesized that anxiolytic effects would not be detected under the less aversive low light condition, but would occur under the more aversive high light condition. Rats received either vehicle or URB 0.3 mg/kg, with half of the rats tested under low light (1 lx) and the other half tested under high light (200 lx) condition. Both pharmacological treatment and illumination schemes were applied in a random order using a factorial design. Sample size was 10 per group.
Experiment 6 aimed at testing the effects of the benzodiazepine chlordiazepoxide under the conditions employed for URB597 in Experiment 5. Rats were not habituated to the testing room. In Experiment 6a, we established the dose-response curve of chlordiazepoxide by treating rats with 1, 2, 3, 4 and 6 mg/kg chlordiazepoxide under the high light (200 lx) condition. Thirty min after treatments, rats were tested in the plus-maze without habituation to the testing environment. Sample size was 14 per group. In Experiment 6b, rats received 5 mg/kg chlordiazepoxide or vehicle and were then tested either under the low (1 lx) or under the high light (200 lx) condition. Treatments and light conditions were randomized. Sample size was 10 per group.
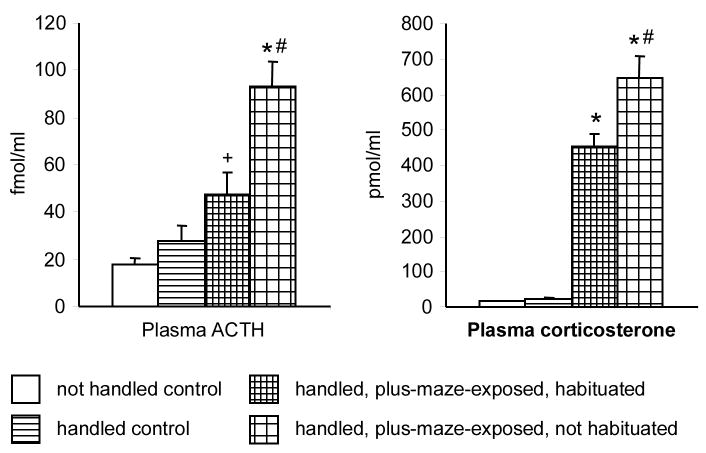
The interaction between the aversiveness of the testing environment and stress responses was studied in Experiment 7. The following groups were employed: non-handled controls (rats left undisturbed in their home-cage throughout); handled control (rats daily handled as described above, but not exposed to the plus-maze); habituated, plus-maze-exposed (handled rats habituated to the testing environment as described in Experiment 1, and exposed to the plus-maze); non-habituated, plus-maze exposed (handled rats transferred to the testing room immediately before testing). The experiment was performed under high light. Sample size was 9–10 per group. Trunk blood was sampled 15 min after the start of plus-maze exposure to determine the plasma levels of corticosterone and ACTH. Blood sampling from non-exposed controls was intercalated. The order of testing and blood sampling was randomized.
Statistics
Data were presented as mean ± the standard error of the mean. Behavioral differences were evaluated by Kruskal-Wallis ANOVA for Experiments 1–4 and 6a. Experiment 5 and 6b required a two-factorial approach. Data were square root transformed to fulfill ANOVA requirements. Two factor ANOVA was performed on transformed data. Factor 1 was pharmacological treatment, whereas Factor 2 was the light condition. Hormone levels (Experiment 7) were evaluated by ANOVA without data transformation. P values lower than 0.05 were considered to indicate statistically significant differences. Pairwise comparisons were also run when the ANOVA analysis revealed significant main effects. Post-hoc comparisons with the Bonferroni-Holm correction procedure(maintaining an experiment-wise significance level of .05) were performed via the Mann-Whitney U test (Experiments 1–4 and 6a), and the Fischer LSD test (Experiment 5, 6b and 7).
Results
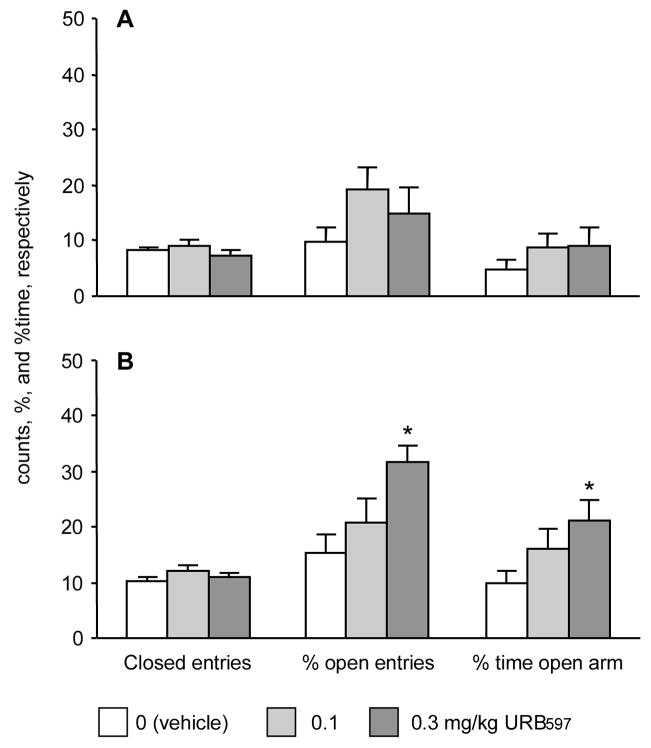
In rats habituated to the testing environment for 2h and tested under high light, URB597 did not affect plus-maze behavior significantly (Experiment 1; Fig. 1A). Closed arm entries showed minimal changes (H(2,40)= 3.61; p> 0.2). Open arm exploration was not significantly affected (%time open arms: H(2,40)= 1.61; p> 0.4; % open arm entries: H(2,40)= 2.66; p> 0.2).
Fig. 1.
The effects of URB597 in the elevated plus-maze. A, Effects seen under continuous high light (Experiment 1); B, Behavior shown when the level of light underwent a sudden change during testing (Experiment 2). *, significantly different from vehicle control.
In contrast, URB597 treatment significantly decreased anxiety-like behavior when the illumination dropped from 200 to 1 lx during testing (Experiment 2; Fig. 1B). Under these conditions, the compound dose-dependently increased both measures, the duration of open arm visits (expressed as % of total testing time; H(2,42)= 6.46; p< 0.04), and % open arm entries (H(2,42)= 8.83; p< 0.02). Closed arm entries were not affected (H(2,42)= 2.07; p> 0.4).
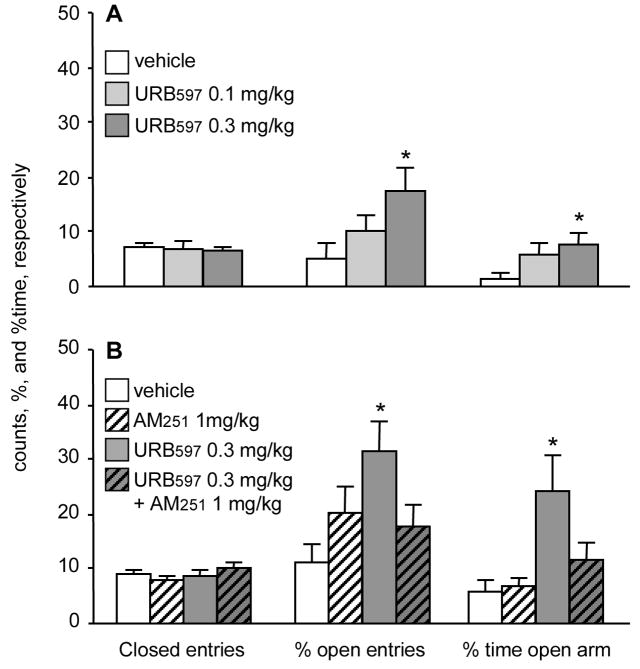
The strong interaction between a stressful event (change in illumination) and the efficacy of URB597 prompted the next study (Experiment 3), where the aversiveness of the testing environment was increased by eliminating habituation to the testing environment. Under these conditions, URB597 again significantly reduced anxiety-like behaviors (Fig. 2A). The duration of open arm visits was significantly increased (H(2,39)= 6.17; p< 0.05), and a similar trend was noticed for % open arm entries (H(2,39)= 5.06; p= 0.08). Closed arm entries were not affected (H(2,39)= 0.29; p> 0.8). The effect of URB597 in non-habituated rats was CB1 receptor dependent (Experiment 4), as the CB1 blocker AM-251 abolished it (Fig. 2B). Closed arm entries were not affected by either treatment (H(3,40)= 2.7; p> 0.4), while open arm exploration was significantly changed (time spent in open arms: H(3,40)= 8.06; p< 0.04; % open arm entries: H(3,40)= 8.31; p< 0.04). In post-hoc comparisons, URB-597 (0.3 mg/kg) significantly increased both measures. AM-251 (1 mg/kg) alone had no significant effect on any of the behaviors, but abolished the effects of URB597 when the two compounds were administered together (Fig. 2B).
Fig. 2.
The elevated plus-maze behavior of rats that were not habituated to the testing environment. A, the effects of URB597 (Experiment 3); B, the effects of the cannabinoid antagonist AM251 on anxiolysis produced by URB597 (Experiment 4). *, significantly different from vehicle control (p< 0.05).
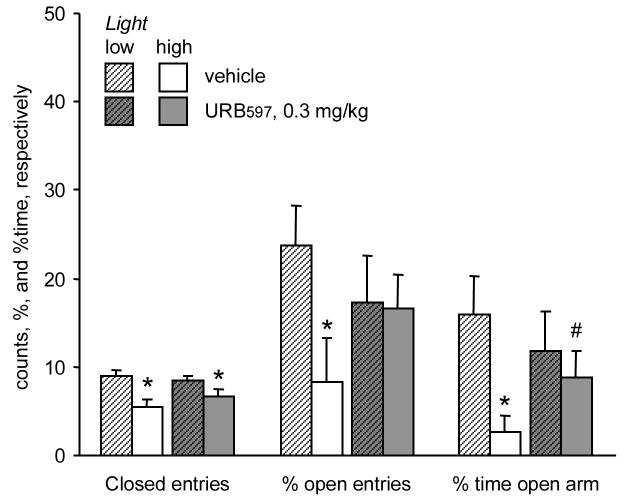
In Experiment 5, we tested the effects of 0.3 mg/kg URB597 under high light and low light conditions in rats that were not habituated to the testing environment. Closed arm entries, indicators of locomotor activity, were higher under low light as compared with high light (Flighting(1,35)= 13.27; p< 0.001). URB597 did not affect locomotion (Ftreatment(1,35)= 0.13; p> 0.7; Finteraction(1,35)= 1.27; p> 0.2) (Fig. 3). The effects of the lighting condition and URB597 interacted significantly in the case of open arm exploration (% time open arms: Finteraction(1,35)= 3.99; p= 0.05; and % open arm entries (Finteraction(1,35)= 4.45; p< 0.04). Post-hoc comparisons revealed that in controls, both measures (the duration of open arm visits and % open entries) were higher under low light as compared with high light (p values were between 0.002 and 0.0001 in post-hoc comparisons). In contrast, under low light, there was no difference between the level of open arm exploration shown by vehicle and URB597-treated rats. Thus, URB597 only produced anxiolytic-like effects in high light in Experiment 5.
Fig. 3.
The effects of URB597 in the elevated plus-maze under low and high illumination (Experiment 5). *, significant effect of light; #, significant effect of URB597 treatment (p< 0.05).
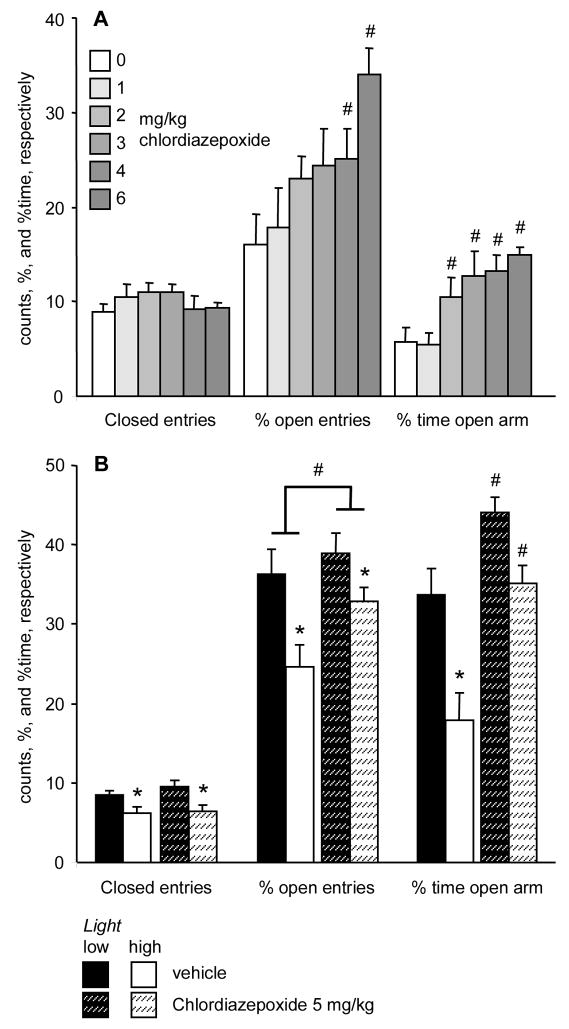
Chlordiazepoxide dose-dependently decreased anxiety (% open entries: F(5,86)= 15.51; p< 0.001; %time open arms: F(5,86)= 24.57; p< 0.001) without affecting locomotion (F(5,86)= 5.57; p> 0.3) (Fig. 4A). In Experiment 6b, high light decreased locomotion, a variable that was not affected by chlordiazepoxide (Flight condition(1,36)= 13.84, p< 0.001; Ftreatment(1,36)= 0.45, p> 0.4; Finteraction(1,36)= 0.30, p> 0.5) (Fig. 4B). % open arm entries were significantly affected by light (Flight condition(1,36)= 10.46; p< 0.01) and treatment (Ftreatment(1,36)= 4.84; p< 0.4), but the interaction between factors was not significant (Finteraction(1,36)= 1.52; p> 0.2). Light decreased while chlordiazepoxide increased this variable. The time spent on the open arms was significantly affected by both light (Flight condition(1,36)= 18.86; p< 0.001) and treatment (Ftreatment(1,36)= 23.85; p< 0.001). The interaction showed a trend (Finteraction(1,36)= 3.03; p= 0.09). Chlordiazepoxide increased the duration of open arm exploration under both low and high light conditions (p< 0.03 and p< 0.001, respectively).
Fig. 4.
The effects of chlordiazepoxide in the elevated plus-maze under low and high illumination. A, dose-response curve (Experiment 6a); B, interaction with light conditions (Experiment 6b). *, significant effect of light; #, significant effect of chlordiazepoxide (p< 0.05).
Experiment 7 showed that the aversiveness of the testing environment significantly affected stress responses (plasma ACTH: F(3,33)= 18.83; p< 0.0001; plasma corticosterone: F(3,33)= 79.08; p< 0.0001) (Fig. 5). Handling history had no effect on the plasma levels of stress hormones. Plus-maze exposure increased plasma ACTH and corticosterone levels in both habituated and not habituated rats. Yet, the increase was significantly larger in rats not habituated to the testing environment as compared to those that were habituated.
Fig. 5.
The effect of experimental conditions on plasma ACTH and corticosterone levels (Experiment 7). *, significantly different from both controls (p< 0.0001); +, significantly different from not handled control (p< 0.001) but not different from handled control; #, significantly different from habituated, plus-maze-exposed (p< 0.01).
Discussion
A continuing challenge for preclinical research on anxiolytic drugs is to capture the affective dimension that characterizes anxiety. It has been proposed that exposure to aversive environmental events provides unique insight into the affective features of behavior (Miczek et al., 1995). Here we investigated the impact of the environment on the anxiolytic efficacy of the FAAH inhibitor URB597 and found that this efficacy was strongly dependent on the conditions of testing. Anxiolytic effects were not detected under non-challenging conditions, for example when rats were tested under low light (Experiment 5) or were habituated to the testing environment (Experiment 1). In contrast, robust anxiolytic effects of URB597 were observed when rats were tested under high light without habituation (Experiment 3, 4, 5), or when habituated rats were submitted to sudden changes in illumination during testing (Experiment 2). It is noteworthy that non-habituation to the testing environment significantly increased the activation of the HPA-axis, confirming the aversiveness of the conditions employed (Experiment 7). Furthermore, the effects of URB597 were mediated by CB1 receptors, as the CB1 antagonist AM-251 abolished the anxiolytic effects of URB597. Locomotion was not affected by URB597 under any condition. Thus, FAAH inhibition did not affect behavior under non-stressful circumstances but protected against the anxiogenic effects of stressful stimuli and circumstances (e.g. sudden changes in light and unfamiliar environments). In contrast, the aversiveness of the testing environment did not affect the efficacy of chlordiazepoxide. Taken together, these findings suggest that FAAH inhibition by URB-597 normalizes stress-induced anxiety while CDP is similarly effective in high and low anxiety-provoking conditions.
A potential explanation for some of these results is that the reduced response to stress may be due to changes in light perception, as in 2 of the 5 experiments the stress factor involved the manipulation of lighting (Experiments 2 and 5). Lighting also affected the efficacy of cannabinoid treatments in earlier experiments (Haller et al. 2004b; Naidu et al. 2007). Furthermore, cannabinoid signaling may indeed affect vision via CB1 receptors (Lalonde et al. 2006; Opere et al. 2006; Romano and Lograno 2007). However, in our experiments the anxiety-related effects of cannabinoids were also strongly affected by a factor not related to lighting, namely habituation, which involves at least three sensory modalities, vision, hearing, and olfaction. Most importantly, URB597 did not alter the locomotor suppressant effects of high light, even when it abolished its anxiogenic effects (Experiment 5). Taken together, these findings indicate that the context-dependent anxiolytic effect of URB597 was not due to impaired light perception.
Interactions between experimental conditions and cannabinoid treatments (including URB597) have been reported in a variety of behavioral paradigms (Gerdeman et al. 2007; Haller et al. 2004b, Hill and Gorzalka 2004; Naidu et al. 2007; Patel et al. 2004; Patel et al. 2005; Robinson et al. 2003; Shi et al. 2005; Trezza and Vanderschuren 2008). Earlier studies linking stress responses and cannabinoid signaling appear especially important for the present study. We previously showed that mice with genetically disrupted cannabinoid signaling show increased anxiety, but this effect was only seen under stressful conditions; moreover, anxiety was expressed in conjunction with an increased HPA axis function (Haller et al. 2004). Somewhat similar findings were obtained by Patel et al. (2004). In their studies with rats, CB1 receptor blockade enhanced glucocorticoid secretion induced by restraint stress, while the FAAH inhibitor URB597 strongly decreased glucocorticoid secretion induced by restraint stress. In a subsequent study, the same authors showed that endocannabinoid activation opposes the behavioral responses (i.e., active escape during restraint) and neuronal responses (i.e., restraint-induced c-Fos activation) to stress (Patel et al. 2005). These studies support the notion that cannabinoid neurotransmission moderates the effects of stress in experimental animals, and this ameliorating effect can be demonstrated at the behavioral, endocrine, and neural levels. Similar findings were obtained by Griebel et al. (2005) who compared the effects of rimonabant in various models of anxiety, and concluded that the endocannabinoid system may be primarily involved in the adaptive responses to unavoidable stressful stimuli.
Our study suggests that the anxiolytic effects of URB-597 are revealed by the combination of non-habituation and high light, because: (i) high light per se was insufficient to unmask the anxiolytic effects of the compound (Experiment 1), (ii) URB-597 was unable to decrease anxiety in non-habituated animals tested under low light (thus, non-habituation per se was not sufficient; see Experiment 5), but (iii) the compound was strongly anxiolytic when tested in non-habituated animals under high light (Experiment 3). Based on earlier studies, however, one can tentatively hypothesize that high light per se may be sufficient to reveal the anxiolytic effects of URB-597 in other strains or species. The open arms of the plus-maze were shown to become aversive only above 3 lx light intensity in Wistar rats; thus, the exploration of open and closed arms was similar in this strain at the light level applied in our experiments (1 lx) (Garcia et al., 2005). This was not seen in Sprague Dawley rats where open arm avoidance was not eliminated at 1 lx. Thus, the aversive nature of light may be strain-dependent, which suggests a similarly strain-dependent interaction between light as an aversive stimulus and URB597 efficacy.
Earlier studies indirectly support our findings on the dependence of URB-597 effects on the aversiveness of the testing environment. Naidu et al. (2007) and Naderi et al. (2008) allowed subjects to habituate to the testing environment and found no anxiolytic effects at doses which were effective in other studies. Anxiolytic effects were seen only when the testing environment was made aversive by bright light focused on the open arms of the plus-maze Naidu et al. (2007). In the study by Patel and Hillard (2006), subjects were habituated to the testing environment, and URB597 decreased anxiety. In these studies, however, the elevated plus-maze was lit by a 1.8 m standing light pole, which was placed next to a wall about 2–3 feet from the plus maze (personal communication by Patel S.). Thus, the light reached the plus-maze at an angle of 20–30 degrees. The walls of the closed arms shadowed the runway of the closed arms, which produced a visible light contrast between the open and closed arms. This probably made open arm visits more challenging than under center overhead lighting. In experiments where anxiolytic effects of URB597 were obtained, no habituation was allowed (Kathuria et al. 2003; Hill et al. 2007; Moreira et al. 2008), subjects were housed individually, which per se increases anxiety (Moreira et al. 2008; Lewejohann et al. 2006), or the compound was administered during alcohol withdrawal, which also increases anxiety (Cippitelli et al. 2008). In the study by Rubino et al. (2008), URB597 was microinjected into the prefrontal cortex, a procedure that was likely aversive as well. Somewhat surprisingly, URB597 decreased anxiety in the study by Scherma et al. (2008), where precautions were taken to minimize the aversiveness of the testing procedure by daily handling of rats before experiments and by familiarizing them with the experimental room. Yet, these authors employed the light-dark test of anxiety, which involves transitions from dimly to brightly lit compartments. When we mimicked these sudden changes in illumination, URB597 readily decreased anxiety in the elevated plus-maze as well (Experiment 2). Taken together, these considerations suggest that the anxiolytic efficacy of URB597 is strongly enhanced by aversive circumstances, like unfamiliarity with the testing environment, individual housing, sudden changes in light levels, drug withdrawal, and microinjection-related manipulations.
The findings reviewed above show that FAAH inhibition produces cannabinoid activation that dampens various effects of stress exposure, and a closer look at the experimental conditions of previous studies suggests that FAAH inhibition decreased anxiety in experiments where the testing environment or other experimental conditions were aversive. These two lines of reasoning imply that URB597 is especially effective as an anxiolytic under stressful conditions. Yet, this assumption has never been investigated systematically until now. Here we show for the first time that FAAH inhibition produces robust anxiolytic effects only when the testing procedure involves relatively aversive conditions, suggesting that the compound can effectively counteract the anxiogenic effects of stressful stimuli.
Acknowledgments
This study was supported in part by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Baltimore, MD, USA and OTKA (Hungarian Scientific Research Fund) grant No. K72621 to J. Haller. The authors thank Sachin Patel for help provided during preparation of the manuscript. Both funding institutions sponsor basic research. No income was obtained from commercial sponsors. The authors have full control of all primary data and they agree to allow the journal to review their data if requested.
References
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology. 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Maccarrone M. FAAH and anandamide: is 2-AG really the odd one out? Trends Pharmacol Sci. 2008;29:229–233. doi: 10.1016/j.tips.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Garcia AM, Cardenas FP, Morato S. Effect of different illumination levels on rat behavior in the elevated plus-maze. Physiol Behav. 2005;85:265–270. doi: 10.1016/j.physbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Schechter JB, French ED. Context-Specific Reversal of Cocaine Sensitization by the CB(1) Cannabinoid Receptor Antagonist Rimonabant. Neuropsychopharmacology. 2007 Dec 5; doi: 10.1038/sj.npp.1301648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Haller J, Matyas F, Soproni K, Varga B, Barsy B, Nemeth B, Mikics E, Freund TF, Hajos N. Correlated species differences in the effects of cannabinoid ligands on anxiety and on GABAergic and glutamatergic synaptic transmission. Eur J Neurosci. 2007;25:2445–2456. doi: 10.1111/j.1460-9568.2007.05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Mátyás F, Soproni K, Varga B, Barsy B, Németh B, Mikics E, Freund TF, Hájos N. Correlated species differences in the effects of cannabinoid ligands on anxiety and on GABAergic and glutamatergic synaptic transmission. Eur J Neurosci. 2007;25:2445–2456. doi: 10.1111/j.1460-9568.2007.05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004b;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Halász J, Majercsik E. Psychosocial conditions and the efficacy of clinically available anxiolytics. Curr Drug Targets. 2004a;5:655–664. doi: 10.2174/1389450043345173. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Enhancement of anxiety-like responsiveness to the cannabinoid CB1 receptor agonist HU-210 following chronic stress. Eur J Pharmacol. 2004;499:291–295. doi: 10.1016/j.ejphar.2004.06.069. [DOI] [PubMed] [Google Scholar]
- Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32:350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty Acid Amide Hydrolase Inhibition Heightens Anandamide Signaling Without Producing Reinforcing Effects in Primates. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.08.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Lalonde MR, Jollimore CA, Stevens K, Barnes S, Kelly ME. Cannabinoid receptor-mediated inhibition of calcium signaling in rat retinal ganglion cells. Mol Vis. 2006;12:1160–1166. [PubMed] [Google Scholar]
- Lewejohann L, Reinhard C, Schrewe A, Brandewiede J, Haemisch A, Görtz N, Schachner M, Sachser N. Environmental bias? Effects of housing conditions, laboratory environment and experimenter on behavioral tests. Genes Brain Behav. 2006;5:64–72. doi: 10.1111/j.1601-183X.2005.00140.x. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addict Biol. 2008;13:264–275. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nature Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Weerts EM, Vivian JA, Barros HM. Aggression, anxiety and vocalizations in animals: GABAA and 5-HT anxiolytics. Psychopharmacology. 1995;121:38–56. doi: 10.1007/BF02245590. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Naderi N, Haghparast A, Saber-Tehrani A, Rezaii N, Alizadeh AM, Khani A, Motamedi F. Interaction between cannabinoid compounds and diazepam on anxiety-like behaviour of mice. Pharmacol Biochem Behav. 2008;89:64–75. doi: 10.1016/j.pbb.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology. 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Opere CA, Zheng WD, Zhao M, Lee JS, Kulkarni KH, Ohia SE. Inhibition of potassium- and ischemia-evoked [3H] D-aspartate release from isolated bovine retina by cannabinoids. Curr Eye Res. 2006;31:645–653. doi: 10.1080/02713680600762747. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology. 2005;30:497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic pituitary adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L, Hinder L, Pertwee RG, Riedel G. Effects of delta9-THC and WIN-55, 212-2 on place preference in the water maze in rats. Psychopharmacology. 2003;166:40–50. doi: 10.1007/s00213-002-1302-0. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Haller J, Halasz J, Mikics E. ‘One-trial sensitization’ to the anxiolytic-like effects of cannabinoid receptor antagonist SR141716A in the mouse elevated plus-maze. Eur J Neurosci. 2003;17:1279–1286. doi: 10.1046/j.1460-9568.2003.02548.x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ. Animal models of ‘anxiety’: where next? Behav Pharmacol. 1997;8:477–496. doi: 10.1097/00008877-199711000-00003. [DOI] [PubMed] [Google Scholar]
- Romano MR, Lograno MD. Evidence for the involvement of cannabinoid CB1 receptors in the bimatoprost-induced contractions on the human isolated ciliary muscle. Invest Ophthalmol Vis Sci. 2007;48:3677–3682. doi: 10.1167/iovs.06-0896. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Viganó D, Marras E, Petrosino S, Perletti G, Maccarrone M, Di Marzo V, Parolaro D. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, Mikics E, Haller J, Yasar S, Tanda G, Goldberg SR. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54:129–140. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LH, Luo F, Woodward DJ, Chang JY. Dose and behavioral context dependent inhibition of movement and basal ganglia neural activity by Delta-9-tetrahydrocannabinol during spontaneous and treadmill locomotion tasks in rats. Synapse. 2005;55:1–16. doi: 10.1002/syn.20088. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: Differential behavioral mechanisms. Eur Neuropsychopharmacol. 2008;18:519–530. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- Zelena D, Kiem DT, Barna I, Makara GB. α2-Adrenoreceptor subtypes regulate ACTH and β-endorphin secretions during stress in the rat. Psychoneuroendocrinology. 1999;24:333–343. doi: 10.1016/s0306-4530(98)00081-x. [DOI] [PubMed] [Google Scholar]
- Zelena D, Mergl Z, Foldes A, Kovacs KJ, Toth Z, Makara GB. Role of hypothalamic inputs in maintaining pituitary-adrenal responsiveness in repeated restraint. Am J Physiol Endocrinol Metab. 2003;285:E1110–E1117. doi: 10.1152/ajpendo.00219.2003. [DOI] [PubMed] [Google Scholar]