Abstract
Aims
Cyclin D1 and cyclin-dependent kinases are commonly activated in colorectal cancer. Microsatellite instability (MSI) and CpG island methylator phenotype (CIMP) are important molecular classifiers in colorectal cancer. However, the relationship between cyclin D1, MSI and CIMP has been uncertain.
Methods and Results
Among 865 colorectal cancers with MSI and CIMP data, 246 tumors (28.4%) showed cyclin D1 overexpression by immunohistochemistry. We quantified DNA methylation in p14 and 8 CIMP-specific promoters (CACNA1G, CDKN2A (p16), CRABP1, IGF2, MLH1, NEUROG1, RUNX3 and SOCS1) by real-time PCR (MethyLight). Both MSI-high and CIMP-high were associated with cyclin D1 overexpression (p<0.0001). After tumors were stratified by MSI and CIMP status, the relation between MSI-high and cyclin D1 persisted (p≤0.02), whereas the relation between CIMP-high and cyclin D1 did not. Cyclin D1 overexpression was correlated with BRAF mutation (p=0.0001), p27 loss (p=0.0007) and p16 loss (p=0.02), and inversely with p53 expression (p=0.0002) and p21 loss (p<0.0001). After stratification by MSI status, the inverse relation between cyclin D1 and p21 loss still persisted (p<0.008).
Conclusions
cyclin D1 activation is associated with MSI and inversely with p21 loss in colorectal cancers. Cyclin D1 may play an important role in the development of MSI-high tumors, independent of CIMP status.
Keywords: colon cancer, CCND1, CpG island methylator phenotype, CIMP, microsatellite instability, MSI, p21, CDKN1A, cyclin, DNA methylation
Introduction
Cyclin D1 plays a key role in cell cycle control, particularly in the transition from G1 to S phase, which is regulated by cyclin-dependent kinases.1 Cyclin D1 overexpression (at both mRNA and protein levels) occurs in one-third or more of colorectal cancers.2–4 The ability of cyclin D1 to drive the cell cycle forward can be blocked by cyclin-dependent kinase (CDK) inhibitors, such as p21 (CDKN1A) and p27 (CDKN1B).1 In addition, cyclin D1 is a target gene of the WNT/β-catenin (CTNNB1) pathway. It has been suggested that cyclin D1 transactivation secondary to APC or β-catenin mutations participates in colon cancer initiation.5,6 Molecular correlates with cyclin D1 activation are important in understanding carcinogenic mechanisms in various molecular subtypes of colorectal cancer. However no study has comprehensively examined the relationship between cyclin D1, CDK inhibitors and microsatellite instability (MSI) in colorectal cancer.
Epigenetic aberrations are important mechanisms in human carcinogenesis.7 A number of tumor suppressor genes are silenced by promoter methylation during colorectal cancer development,8 and a subset of colorectal cancers exhibit widespread promoter methylation, which is referred to as the CpG island methylator phenotype (CIMP).9 CIMP-high colorectal tumors have distinct features, such as associations with proximal tumor location, female gender, BRAF mutation10 and wild-type TP53, independent of MSI status.11,12 In addition, CIMP-high is associated with intact p21 expression and loss of p27, independent of MSI.13,14 A molecular classification based on MSI and CIMP status is increasingly important,15 because MSI and CIMP reflect global genomic and epigenomic aberrations, respectively, in tumor cells.16
In this study, using quantitative DNA methylation analysis (MethyLight technology) and a large number of population-based colorectal cancers, we examined cyclin D1 expression, in relation to various clinicopathologic and molecular features, including expressions of CDK inhibitors (p21, p27 and p16), MSI and CIMP status. We have found that cyclin D1 overexpression is common in MSI-high tumors with intact p21 expression.
Materials and methods
Study group
We utilized the databases of two large prospective cohort studies; the Nurses’ Health Study (N = 121,700 women followed since 1976),17,18 and the Health Professional Follow-up Study (N = 51,500 men followed since 1986).18 Informed consent was obtained from all participants who returned initial questionnaires and subsequently developed colorectal cancer. A subset of the cohort participants developed colorectal cancers during prospective follow-up. Thus, these colorectal cancers represented population-based, relatively unbiased samples (compared to retrospective or single-hospital-based samples). Previous studies on the Nurses’ Health Study and Health Professionals Follow-up Study have described baseline characteristics of cohort participants and incident colorectal cancer cases, and confirmed that our colorectal cancers were well representative as a population-based sample.17,18 Follow-up of these cohorts are ongoing. Although survival data have not yet been available, it will be available in the future. We collected paraffin-embedded tissue blocks from hospitals where cohort participants with colorectal cancers had undergone resections of primary tumors. Based on availability of adequate tissue specimens, a total of 865 colorectal cancers (377 from men’s cohort and 488 from women’s cohort) were included (the mean age at diagnosis, 66.2 years old with standard deviation 8.4). Among our cohort studies, there was no significant difference in demographic features between cases with tissue available and those without available tissue.18 Many of the cases have been previously characterized for statuses of CIMP, MSI, p21, p27 and p53.13,14 However, we have not examined cyclin D1 expression in our tumors. Tissue collection and analyses were approved by the Dana-Farber/Harvard Cancer Center and Brigham and Women’s Hospital Institutional Review Boards.
Histopathologic evaluations
Hematoxylin and eosin (H&E) stained tissue sections were examined by a pathologist (S.O.) blinded from clinical and other laboratory data. The following features were evaluated. (1) The degree of tumor differentiation was categorized as well/moderate (≥50% gland formation) vs. poor (<50% gland formation). (2) The presence and extent of extracellular mucin were categorized as 0% (no mucin), 1–49% or ≥ 50% of the tumor volume. (3) The presence and extent of signet ring cells were categorized as 0% (no signet ring cells), 1–49% or ≥ 50% of the tumor volume.
Genomic DNA extraction and sequencing of KRAS and BRAF
Genomic DNA was extracted from dissected tumor tissue sections.19 Normal DNA was obtained from colonic tissue at resection margins. Whole genome amplification (WGA) of genomic DNA was performed by PCR using random 15-mer primers.19 PCR and Pyrosequencing targeted for KRAS codons 12 and 13,19 and BRAF codon 600 were performed as previously described.20
Analyses for microsatellite instability (MSI) and TGFBR2 and BAX mutations
MSI analysis was performed as previously described.21 In addition to D2S123, D5S346, D17S250, BAT25 and BAT26,22 we used BAT40, D18S55, D18S56, D18S67 and D18S487 (i.e., 10-marker panel).21 A “high degree of MSI” (MSI-H) was defined as the presence of instability in ≥ 30% of the markers. A low degree of MSI (MSI-L) was defined as instability in < 30% of the markers, and “microsatellite stable (MSS)” tumors were defined as tumors without an unstable marker. Methods to detect mutations in TGFBR223 and BAX mononucleotide repeats have been previously described.24
Real-time PCR (MethyLight) for quantitative DNA methylation analysis
Sodium bisulfite treatment on genomic DNA and subsequent real-time PCR (MethyLight25) were validated and performed as previously described.26 We quantified DNA methylation in 8 CIMP-specific promoters (CACNA1G, CDKN2A (p16), CRABP1, IGF2, MLH1, NEUROG1, RUNX3 and SOCS1),27 all of which were selected from screening of 195 CpG islands in the human genome.10,12 Primers and probes were previously described.10 In addition, we quantified p14 (CDKN2A/ARF) methylation with primers and probe; P14-F, 5'-TTGGAGGCGGCGAGAATAT-3' (Genbank No. L41934, nucleotide Nos. 238–256); P14-R, 5'-CCCCGTAAACCGCGAAATA-3' (nucleotide Nos. 332–350); P14-probe, 6FAM-5'-CGGTTCGTCGCGAGTGAGGGTT-3'-BHQ-1 (nucleotide Nos. 299–320). The PCR condition was initial denaturation at 95C for 10 min followed by 45 cycles of 95C for 15 sec and 60C for 1 min, and performed as previously described.24
CIMP-high was defined as the presence of ≥6 of 8 methylated promoters (excluding p14), CIMP-low as the presence of 1/8–5/8 methylated promoters, and CIMP-0 as the absence (0/8) of methylated promoters, according to the previously established criteria.27
Tissue microarrays (TMAs) and immunohistochemistry for p53, p21, p27, p16, β-catenin and cyclin D1
TMAs were constructed as previously described.28 We examined two to four tumor tissue cores for each marker. We examined whole tissue sections in all cases (for p21, p27 and p16), and in cases (for other markers) in which no tissue block was available for TMAs or results were equivocal in TMAs. Methods for immunohistochemistry were previously described; p21,29 p27,29 p16,21 p53,14 and β-catenin.30 For p53, moderate/strong nuclear staining in ≥50% of tumor cells was interpreted as positive. For p21 and p27, nuclear staining in <5% and <10% of tumor cells, respectively, was interpreted as protein loss.
For cyclin D1, antigen retrieval was performed, and deparaffinized tissue sections in Target Retrieval Solution (Dako, Carpinteria, CA) were treated with microwave in a pressure cooker for 15 min. Tissue sections were incubated with 3% H2O2 (10 min) to block endogenous peroxidase, and with 10% normal goat serum (Vector Laboratories, Burlingame, CA) in phosphate-buffered saline (10 min). Primary antibody against cyclin D1 (rabbit monoclonal [SP4] to cyclin D1, 1:100 dilution; Abcam, Cambridge, MA) was applied, and the slides were maintained overnight at room temperature. Next, we applied an anti-rabbit IgG antibody (Vector Laboratories) for 30 min, followed by an avidin–biotin complex conjugate (Vector Laboratories) for 30 min. The immunochemical reaction was revealed by diaminobenzidine (5 min) and methyl-green counterstain. Nuclear cyclin D1 expression was recorded as no expression, weak expression, or moderate/strong expression. Cyclin D1 positivity (i.e., overexpression) was defined as ≥50% of tumor cells with weak nuclear staining or ≥20% of tumor cells with moderate/strong nuclear staining. The antigen retrieval procedure for examination of cyclin D1 was considered to be uniform across samples by virtue of the large number of cases, and we could secure enough number of cases in various strata of expression level (for example, expression in <10% of tumor cells, 10–30% of tumor cells, 30–50% of tumor cells, ≥50% of tumor cells, etc.). Then, we could see where the optimal cutoff was. Thus, our cut off for Cyclin D1 was not totally arbitrary. In any case any random misclassification would drive the results towards the null hypothesis of no difference in the frequencies of cyclin D1 overexpression. Despite such a possible misclassification, we still obtained statistically very significant results for many of the correlations.
Appropriate positive and negative controls were included in each run of immunohistochemistry. All immunohistochemically-stained slides were interpreted by a pathologist (S.O., except for cyclin D1 and β-catenin by K.N.) blinded from any other clinical and laboratory data.
Statistical analysis
For categorical data, chi-square test (or Fisher’s exact test when any expected cell count was less than 5) was performed and odds ratio (OR) with 95% confidence interval (CI) was computed, using SAS program (Version 9.1, SAS Institute, Cary, NC). All p values were two-sided, and statistical significance was set at p ≤ 0.05.
Results
Cyclin D1 overexpression in colorectal cancers
Among the 865 colorectal cancers that we examined cyclin D1 expression by immunohistochemistry, 246 (28.4%) tumors overexpressed cyclin D1 (Figure 1). Table 1 summarizes the frequencies of cyclin D1 overexpression in relation to various clinical and pathologic features. Cyclin D1 overexpression was more common in proximal tumors (34%=117/343) than distal tumors (excluding rectum) (24%=62/252, p=0.009) and rectal tumors (21%=36/169, p=0.003). However, after tumors were stratified by MSI status, cyclin D1 was no longer correlated with tumor location (data not shown).
Figure 1.
Cyclin D1 and p21 expressions in colorectal cancer.
A. Heterogeneous nuclear cyclin D1 expression in carcinoma (arrowheads) and very weak cyclin D1 expression in normal colonic mucosa (empty arrows). B. Strong and diffuse nuclear cyclin D1 overexpression in carcinoma (arrow). C. Loss of nuclear p21 in carcinoma (empty arrows). Inflammatory cells serve as internal positive control (solid arrowhead). D. Intact nuclear p21 expression in carcinoma cells (arrows).
Table 1.
Frequency of cyclin D1 overexpression in colorectal cancer according to various clinical and pathologic features
| Clinical/pathologic feature | Total N | Cyclin D1(+) | OR (95% CI) | P value | |
|---|---|---|---|---|---|
| All cases | 865 | 246 (28%) | |||
| Gender | Men | 377 | 102 (27%) | 1 | |
| Women | 488 | 144 (30%) | 1.13 (0.84–1.52) | ||
| Age | ≤59 | 185 | 48 (26%) | 1 | |
| 60–69 | 326 | 99 (30%) | 1.24 (0.83–1.87) | ||
| ≥70 | 267 | 70 (26%) | 1.01 (0.66–1.55) | ||
| Location* | Proximal | 343 | 117 (34%) | 1 | Referent |
| Distal (not Rectum) | 256 | 62 (24%) | 0.62 (0.43–0.89) | 0.009 | |
| Rectum | 169 | 36 (21%) | 0.52 (0.34–0.80) | 0.003 | |
| Stage | I | 177 | 47 (27%) | 1 | |
| II | 234 | 77 (33%) | 1.36 (0.88–2.09) | ||
| III | 208 | 52 (25%) | 0.92 (0.58–1.46) | ||
| IV | 92 | 21 (23%) | 0.82 (0.45–1.48) | ||
| Tumor differentiation | Well/moderate | 747 | 209 (28%) | 1 | |
| Poor | 79 | 23 (29%) | 1.06 (0.63–1.76) | ||
| Mucinous component | 0% | 463 | 128 (28%) | 1 | |
| 1–49% | 175 | 60 (34%) | 1.37 (0.94–1.98) | ||
| ≥50% | 110 | 34 (31%) | 1.17 (0.74–1.84) | ||
| Signet ring cell | 0% | 644 | 194 (30%) | 1 | Referent |
| Component | 1–49% | 45 | 14 (31%) | 1.05 (0.55–2.01) | |
| ≥50% | 15 | 0 (0%) | 0ˆ | 0.01 | |
Only significant p values are described.
Proximal colon includes cecum to transverse colon, and distal colon (not rectum) includes splenic flexure to sigmoid colon.
Because of the presence of 0 cases in a category, 95% CI was not calculated. CI, confidence interval; OR, odds ratio.
Cyclin D1 overexpression was less frequent ≥50% signet ring cell carcinoma (0%=0/15, p=0.01) than carcinoma with no signet ring cells (30%=194/644). However, after the tumors were stratified by MSI status, the correlation of cyclin D1 with signet ring cells did not persist (data not shown). Cyclin D1 was not correlated with any of the other clinical or pathologic features examined.
Cyclin D1 overexpression is associated with MSI-H, CIMP-high and BRAF mutation
Table 2 summarizes the frequencies of cyclin D1 overexpression in relation to molecular alterations in colorectal cancer. Cyclin D1 overexpression was significantly more common in microsatellite instability-high (MSI-H) tumors (54%=63/117, p<0.0001) than in microsatellite stable (MSS) tumors (24%=156/640). We determined CpG island methylator phenotype (CIMP) status using MethyLight assays on a panel of 8 CIMP-specific promoters (CACNA1G, CDKN2A, CRABP1, IGF2, MLH1, NEUROG1, RUNX3 and SOCS1) 10,27. Cyclin D1 overexpression was more common in CIMP-high tumors (48%=57/118, p<0.0001) than in CIMP-0 tumors (21%=81/384). Cyclin D1 overexpression was more common in BRAF-mutated tumors (44%=46/105, p=0.0001) than BRAF wild-type tumors (26%=184/715).
Table 2.
Frequency of cyclin D1 overexpression in colorectal cancer according to various molecular features
| Molecular feature | Total N | Cyclin D1(+) | OR (95% CI) | P value | |
|---|---|---|---|---|---|
| MSI status | MSS | 640 | 156 (24%) | 1 | Referent |
| MSI-low | 69 | 14 (20%) | 0.79 (0.43–1.46) | ||
| MSI-high | 117 | 63 (54%) | 3.62 (2.41–5.43) | <0.0001 | |
| CIMP status | CIMP-0 | 384 | 81 (21%) | 1 | Referent |
| CIMP-low | 301 | 86 (29%) | 1.50 (1.05–2.12) | 0.02 | |
| CIMP-high | 118 | 57 (48%) | 3.50 (2.26–5.41) | <0.0001 | |
| KRAS mutation | (−) | 532 | 178 (33%) | 1 | |
| + | 302 | 86 (28%) | 0.79 (0.58–1.08) | ||
| BRAF mutation | (−) | 715 | 184 (26%) | 1 | Referent |
| + | 105 | 46 (44%) | 2.25 (1.48–3.43) | 0.0001 | |
| p53* | (−) | 474 | 157 (33%) | 1 | Referent |
| + | 377 | 82 (22%) | 0.56 (0.41–0.77) | 0.0002 | |
| p21 (CDKN1A)* | 2+ (intact) | 89 | 54 (61%) | 1 | Referent |
| 1+ | 220 | 95 (43%) | 0.54 (0.32–0.89) | 0.01 | |
| Loss | 479 | 84 (18%) | 0.14 (0.085–0.22) | <0.0001 | |
| p27 (CDKN1B)* | + (intact) | 221 | 46 (21%) | 1 | Referent |
| Loss | 471 | 157 (33%) | 1.90 (1.30–2.77) | 0.0007 | |
| p16 expression* | + (intact) | 432 | 107 (25%) | 1 | Referent |
| Loss | 98 | 36 (37%) | 1.76 (1.11–2.81) | 0.02 | |
| CDKN2A (p16) | (−) | 598 | 146 (24%) | 1 | Referent |
| methylation | + | 246 | 91 (37%) | 1.82 (1.32–2.50) | 0.0002 |
| p14 (CDKN2A/ARF) | (−) | 654 | 162 (25%) | 1 | Referent |
| methylation | + | 163 | 67 (41%) | 2.12 (1.48–3.04) | <0.0001 |
| Nuclear β-catenin* | (−) | 335 | 100 (29.9%) | 1 | |
| + | 417 | 120 (28.8%) | 0.95 (0.69–1.30) | ||
| TGFBR2 | (−) | 44 | 20 (45%) | 1 | |
| mononucleotide mutation (only MSI-H tumors) | + | 90 | 46 (51%) | 1.25 (0.61–2.59) | |
| BAX | (−) | 101 | 45 (45%) | 1 | |
| Mononucleotide mutation (only MSI-H tumors) | + | 33 | 21 (64%) | 2.18 (0.97–4.90) |
Only significant p values are described.
p53, p21, p27, p16 and β-catenin statuses were determined by immunohistochemistry.
CI, confidence interval; CIMP, CpG island methylator phenotype; MSI, microsatellite instability; OR, odds ratio.
The relationship between cyclin D1 overexpression and MSI is independent of CIMP status
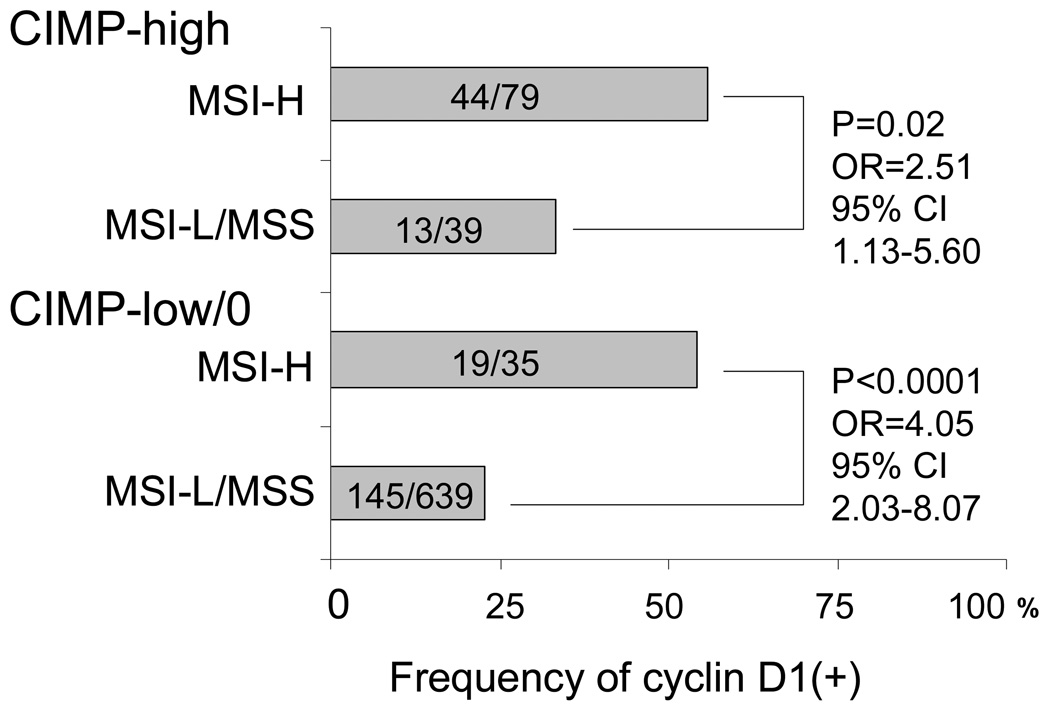
In order to examine effect of MSI (or CIMP) on cyclin D1 expression independent of CIMP (or MSI) status, we classified tumors into 4 subtypes according to combined MSI and CIMP status (Figure 2). The relationship between cyclin D1 overexpression and MSI-H persisted (p≤0.02) after stratification by CIMP status. In contrast, the relationship between CIMP and cyclin D1 expression did not persist after stratification by MSI status.
Figure 2.
Frequency of cyclin D1 overexpression in four MSI/CIMP subtypes of colorectal cancer.
The relationship between MSI and cyclin D1 overexpression is independent of CIMP status.
CI, confidence interval; CIMP, CpG island methylator phenotype; MSI, microsatellite instability; OR, odds ratio.
Cyclin D1 overexpression, MSI, and BRAF and KRAS mutation
To examine the interrelationship between MSI, BRAF, KRAS and cyclin D1, we stratified tumors according to combined MSI, BRAF and KRAS status (Figure 3). No significant relationship between BRAF mutation and cyclin D1 was present in MSI-H. However, among the MSI-L/MSS group, cyclin D1 was more commonly overexpressed in BRAF-mutated tumors than in KRAS/BRAF-wild-type tumors (p=0.003).
Figure 3.
Frequency of cyclin D1 overexpression in colorectal cancer according to MSI, KRAS (K), and BRAF (B) statuses.
The relationship between BRAF mutation and cyclin D1 does not persist in MSI-H tumors.
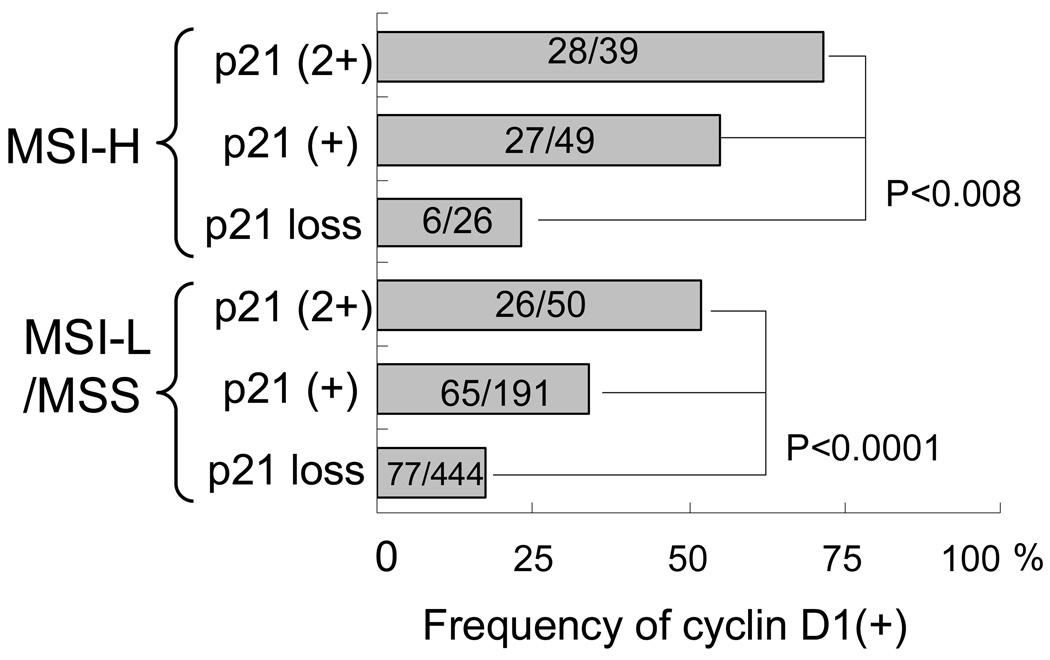
Cyclin D1 overexpression is inversely associated with p21 loss, independent of MSI status
We examined the relation between cyclin D1 and various cyclin-dependent kinase (CDK) inhibitors in colorectal cancer, because cyclin D1 is regulated by various CDK inhibitors (Figure 4). Cyclin D1 overexpression was significantly associated with p27 loss (p=0.0007), p16 loss (p=0.02), CDKN2A (p16) methylation (p=0.0002) and p14 methylation (p<0.0001), and inversely with p21 loss (p<0.0001) and p53 expression (p=0.0002) (Table 2). Considering the relationship between cyclin D1 and MSI (shown above) and the associations of MSI with p53, p21, p27 and p16,13,14,27 we stratified tumors by MSI status. The inverse relationship between p21 loss and cyclin D1 overexpression persisted (p<0.008) after stratification by MSI status (Figure 5). In contrast, the association of cyclin D1 with p27 loss, p16 loss, CDKN2A (p16) methylation, p14 methylation or p53 expression did not persist after stratification by MSI status (data not shown). We also stratified tumors by p53 and p21 status, because p21 is known to be induced by wild-type p53.31 It was evident that p53 status had no effect on cyclin D1 after tumors were stratified by p53 and p21 (Figure 6).
Figure 4.
Cyclin D1, cyclin-dependent kinase (CDK) inhibitors (p21, p27, p16 and p14), p53 and the cell cycle pathway.
Note the close relationship between cyclin D1 and p21.
Figure 5.
Frequency of cyclin D1 overexpression in colorectal cancer according to MSI and p21 statuses.
The inverse relationship between p21 loss and cyclin D1 overexpression is independent of MSI status. The relation between MSI and cyclin D1 somewhat persists after stratification by p21 status. Within p21 (1+) tumors, cyclin D1(+) is higher in MSI-H tumors (55%) than MSI-L/MSS tumors (34%, p=0.007), and within p21 (2+) tumors, cyclin D1(+) is higher in MSI-H (72%) than in MSI-L/MSS (52%, p=0.06).
Figure 6.
Frequency of cyclin D1 overexpression in colorectal cancer according to p53 and p21 statuses.
The inverse relationship between p21 loss and cyclin D1 overexpression is independent of p53 status, while p53 is not related to cyclin D1 after stratification by p21 status.
Cyclin D1 and other molecular changes
Cyclin D1 was not significantly associated with nuclear β-catenin. Among MSI-high tumors, cyclin D1 was not significantly associated with mutation in TGFBR2 or BAX (Table 2), in agreement with a previous study on cyclin D1 and TGFBR2.32
Discussion
We conducted this study to examine the relationship between cyclin D1 overexpression and various molecular alterations in colorectal cancers. Molecular correlates with abnormalities of cyclins, cyclin-dependent kinases (CDK) and CDK inhibitors are important in understanding carcinogenic mechanisms in various subtypes of colorectal cancer.13,14,32,33 In addition, a molecular classification based on MSI and CIMP status is increasingly important,15 because MSI and CIMP reflect global genomic and epigenomic aberrations, respectively, in tumor cells.16 We have demonstrated that cyclin D1 overexpression is associated with MSI-H and inversely with p21 loss, independent of CIMP status. Our data suggest that cyclin D1 may play an important role in the development of MSI-H tumors, especially when p21 is not down-regulated.
Our resource of a large number of colorectal cancers derived from the two prospective cohort studies has enabled us to precisely estimate the frequency of colorectal cancers with a specific molecular feature (such as cyclin D1 overexpression, MSI-H, etc.) at a population level. The large number of cases has also provided a sufficient power for rare subtypes of colorectal cancer such as MSI-H CIMP-low/0. For quantitative DNA methylation analysis on p14 and 8 CIMP-specific promoters including CDKN2A (p16), we have utilized MethyLight technology, which is robust and can reproducibly differentiate low-level methylation from high-level methylation.26,34
Cyclin D1 (CCND1, the official gene symbol) activation has been implicated in carcinogenesis,1 among many other molecular changes in colorectal cancer.35–39 Previous studies have reported that cyclin D1 overexpression occurs in 30–40% of colorectal cancers and adenomas,3,40 but not in hyperplastic polyps or normal mucosa.3 CCND1 mRNA is overexpressed in colon cancer cell lines, but not in normal cells.41 A common polymorphism (so-called 870G>A) of CCND1 appears to modify risks of colorectal cancer and adenoma42,43 and the age of onset of hereditary nonpolyposis colorectal cancer (HNPCC),44 although other studies have shown no association of the CCND1 polymorphism with age of onset of HNPCC.45,46 Relative amounts of CCND1 transcript variants in blood/normal mucosa appear to modify the age of onset of HNPCC.45 In addition to the pivotal role of cyclin D1 in cell cycle progression,1 these epidemiologic data collectively support the importance of CCND1 in colorectal carcinogenesis.
The inverse correlation between p21 down-regulation and cyclin D1 overexpression has been reported in colorectal cancers;40,47 however, MSI or CIMP status has not been examined in these studies. Previous studies have shown that p21 loss is inversely correlated with MSI-H in colorectal cancers,13,33 and we have shown that MSI-H is correlated with cyclin D1 overexpression (in this study). Thus, to support a pathogenetic interaction between p21 and cyclin D1, one must examine p21 and cyclin D1 in relation to MSI status. In the current study, we have indeed shown the inverse correlation between p21 loss and cyclin D1 overexpression independent of MSI status. Our data support a possible pathogenetic interaction between p21 and cyclin D1.
We have also demonstrated that the associations between cyclin D1 overexpression and various pathologic features (signet ring cell component, and tumor-infiltrating lymphocytes) appear to be mediated through MSI. Our findings seem to be reasonable. MSI status reflects genomic aberrations in tumor cells and determines molecular features and phenotypes of colorectal cancer. Both pathologic features and cyclin D1 expression are likely influenced by genomic status of tumor cells. A previous study on endometrial carcinoma has also reported the relation between cyclin D1 overexpression and MSI.48 Concerning with colorectal cancers, a recent study has reported that cyclin D1 expressions were generally low in 11 MSI-high tumors and higher expression levels were noted in 37 MSS/MSI-low tumors.49 This discrepancy might be due to differences in the sample sizes and the methods and criteria for cyclin D1 interpretation.
In conclusion, cyclin D1 overexpression is associated with MSI and inversely with p21 down-regulation, independent of CIMP status in colorectal cancer. Our data imply that cyclin D1 may play an important role in the development of MSI-H tumors. An exact mechanism of the possible pathogenetic link between cyclin D1 activation and MSI remains to be investigated.
Acknowledgments
This work was supported by The U.S. National Institute of Health (NIH) grants P01 CA87969, P01 CA55075, P50 CA127003 and K07 CA122826 (to S.O.), and in part by grants from the Bennett Family Fund and the Entertainment Industry Foundation (EIF) National Colorectal Cancer Research Alliance (NCCRA). K.N. was supported by a fellowship grant from the Japanese Society for Promotion of Science. These funding sponsors had no role or involvement in the study design, the collection, analysis and interpretation of data, or writing and submission of the manuscript. We deeply thank the Nurses’ Health Study and Health Professionals Follow-up Study cohort participants who have generously agreed to provide us with biological specimens and information through responses to questionnaires. We thank Walter Willett, Sue Hankinson and many other staff members who implemented and have maintained the cohort studies.
Abbreviations and HUGO Gene Nomenclature Committee (HGNC)-approved official gene symbols
- CCND1
cyclin D1
- CDK
cyclin-dependent kinase
- CDKN1A
cyclin-dependent kinase inhibitor 1A (p21/CIP1)
- CDKN1B
cyclin-dependent kinase inhibitor 1B (p27/KIP1)
- CDKN2A
cyclin-dependent kinase inhibitor 2A (p16/INK4A)
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- CTNNB1
catenin (cadherin-associated protein), beta 1 (β-catenin)
- MSI
microsatellite instability
- MSS
microsatellite stable
- OR
odds ratio
- TGF
transforming growth factor
- TGFBR2
transforming growth factor-β receptor type 2
- TMA
tissue microarray
Footnotes
Conflicts of interest: None declared.
Referenaces
- 1.Alao J. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmqvist R, Stenling R, Oberg A, Landberg G. Expression of cyclin D1 and retinoblastoma protein in colorectal cancer. Eur J Cancer. 1998;34:1575–1581. doi: 10.1016/s0959-8049(98)00162-2. [DOI] [PubMed] [Google Scholar]
- 3.Arber N, Hibshoosh H, Moss SF, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–674. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- 4.Palmqvist R, Rutegard JN, Bozoky B, Landberg G, Stenling R. Human colorectal cancers with an intact p16/cyclin D1/pRb pathway have up-regulated p16 expression and decreased proliferation in small invasive tumor clusters. Am J Pathol. 2000;157:1947–1953. doi: 10.1016/S0002-9440(10)64833-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 6.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jass JR, Baker K, Zlobec I, et al. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a 'fusion' pathway to colorectal cancer. Histopathology. 2006;49:121–131. doi: 10.1111/j.1365-2559.2006.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 11.Samowitz W, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Ogino S, Cantor M, Kawasaki T, et al. CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut. 2006;55:1000–1006. doi: 10.1136/gut.2005.082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogino S, kawasaki T, Kirkner GJ, et al. Down-regulation of p21 (CDKN1A/CIP1) is inversely associated with microsatellite instability and CpG island methylator phenotype (CIMP) in colorectal cancer. J Pathol. 2006;210:147–154. doi: 10.1002/path.2030. [DOI] [PubMed] [Google Scholar]
- 14.Ogino S, kawasaki T, Kirkner GJ, Yamaji T, Loda M, Fuchs CS. Loss of nuclear p27 (CDKN1B/KIP1) in colorectal cancer is correlated with microsatellite instability and CIMP. Mod Pathol. 2007;20:15–22. doi: 10.1038/modpathol.3800709. [DOI] [PubMed] [Google Scholar]
- 15.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 16.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10:13–27. doi: 10.2353/jmoldx.2008.070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 18.Chan AT, Ogino S, Fuchs CS. Aspirin and the Risk of Colorectal Cancer in Relation to the Expression of COX-2. New Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 19.Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagn. 2005;7:413–421. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogino S, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagn. 2006;8:582–588. doi: 10.2353/jmoldx.2006.060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogino S, Brahmandam M, Cantor M, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19:59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- 22.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 23.Ogino S, kawasaki T, Ogawa A, Kirkner GJ, Loda M, Fuchs CS. TGFBR2 mutation is correlated with CpG island methylator phenotype in microsatellite instability-high colorectal cancer. Hum Pathol. 2007;38:614–620. doi: 10.1016/j.humpath.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki T, Nosho K, Ohnishi M, et al. IGFBP3 promoter methylation in colorectal cancer: relationship with microsatellite instability, CpG island methylator phenotype, and p53. Neoplasia. 2007;9:1091–1098. doi: 10.1593/neo.07760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eads CA, Danenberg KD, Kawakami K, et al. MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000;28:E32. doi: 10.1093/nar/28.8.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogino S, kawasaki T, Brahmandam M, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagn. 2006;8:209–217. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogino S, kawasaki T, Kirkner GJ, Kraft P, Loda M, Fuchs CS. Evaluation of markers for CpG island methylator phenotype (CIMP) in colorectal cancer by a large population-based sample. J Mol Diagn. 2007;9:305–314. doi: 10.2353/jmoldx.2007.060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogino S, Brahmandam M, kawasaki T, Kirkner GJ, Loda M, Fuchs CS. Combined analysis of COX-2 and p53 expressions reveals synergistic inverse correlations with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Neoplasia. 2006;8:458–464. doi: 10.1593/neo.06247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino S, Meyerhardt JA, Cantor M, et al. Molecular alterations in tumors and response to combination chemotherapy with gefitinib for advanced colorectal cancer. Clin Cancer Res. 2005;11:6650–6656. doi: 10.1158/1078-0432.CCR-05-0738. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki T, Nosho K, Ohnishi M, et al. Correlation of beta-catenin localization with cyclooxygenase-2 expression and CpG island methylator phenotype (CIMP) in colorectal cancer. Neoplasia. 2007;9:569–577. doi: 10.1593/neo.07334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papazoglu C, Mills AA. p53: at the crossroad between cancer and ageing. J Pathol. 2007;211:124–133. doi: 10.1002/path.2086. [DOI] [PubMed] [Google Scholar]
- 32.Grady WM, Willis JE, Trobridge P, et al. Proliferation and Cdk4 expression in microsatellite unstable colon cancers with TGFBR2 mutations. Int J Cancer. 2006;118:600–608. doi: 10.1002/ijc.21399. [DOI] [PubMed] [Google Scholar]
- 33.Edmonston TB, Cuesta KH, Burkholder S, et al. Colorectal carcinomas with high microsatellite instability: defining a distinct immunologic and molecular entity with respect to prognostic markers. Hum Pathol. 2000;31:1506–1514. doi: 10.1053/hupa.2000.20383. [DOI] [PubMed] [Google Scholar]
- 34.Bettstetter M, Dechant S, Ruemmele P, et al. Distinction of hereditary nonpolyposis colorectal cancer and sporadic microsatellite-unstable colorectal cancer through quantification of MLH1 methylation by real-time PCR. Clin Cancer Res. 2007;13:3221–3228. doi: 10.1158/1078-0432.CCR-06-3064. [DOI] [PubMed] [Google Scholar]
- 35.Makinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50:131–150. doi: 10.1111/j.1365-2559.2006.02548.x. [DOI] [PubMed] [Google Scholar]
- 36.Baker K, Chong G, Foulkes WD, Jass JR. Transforming growth factor-beta pathway disruption and infiltration of colorectal cancers by intraepithelial lymphocytes. Histopathology. 2006;49:371–380. doi: 10.1111/j.1365-2559.2006.02520.x. [DOI] [PubMed] [Google Scholar]
- 37.Prall F, Weirich V, Ostwald C. Phenotypes of invasion in sporadic colorectal carcinomas related to aberrations of the adenomatous polyposis coli (APC ) gene. Histopathology. 2007;50:318–330. doi: 10.1111/j.1365-2559.2007.02609.x. [DOI] [PubMed] [Google Scholar]
- 38.Korkolopoulou P, Saetta AA, Levidou G, et al. c-FLIP expression in colorectal carcinomas: association with Fas/FasL expression and prognostic implications. Histopathology. 2007;51:150–156. doi: 10.1111/j.1365-2559.2007.02723.x. [DOI] [PubMed] [Google Scholar]
- 39.Lugli A, Zlobec I, Minoo P, et al. Prognostic significance of the wnt signalling pathway molecules APC, beta-catenin and E-cadherin in colorectal cancer: a tissue microarray-based analysis. Histopathology. 2007;50:453–464. doi: 10.1111/j.1365-2559.2007.02620.x. [DOI] [PubMed] [Google Scholar]
- 40.McKay JA, Douglas JJ, Ross VG, et al. Cyclin D1 protein expression and gene polymorphism in colorectal cancer. Aberdeen Colorectal Initiative. Int J Cancer. 2000;88:77–81. doi: 10.1002/1097-0215(20001001)88:1<77::aid-ijc12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 41.Gerlach U, Kayser G, Walch A, et al. Centrosome-, chromosomal-passenger- and cell-cycle-associated mRNAs are differentially regulated in the development of sporadic colorectal cancer. J Pathol. 2006;208:462–472. doi: 10.1002/path.1914. [DOI] [PubMed] [Google Scholar]
- 42.Kong S, Wei Q, Amos CI, et al. Cyclin D1 polymorphism and increased risk of colorectal cancer at young age. J Natl Cancer Inst. 2001;93:1106–1108. doi: 10.1093/jnci/93.14.1106. [DOI] [PubMed] [Google Scholar]
- 43.Lewis RC, Bostick RM, Xie D, et al. Polymorphism of the cyclin D1 gene, CCND1, and risk for incident sporadic colorectal adenomas. Cancer Res. 2003;63:8549–8553. [PubMed] [Google Scholar]
- 44.Kong S, Amos CI, Luthra R, Lynch PM, Levin B, Frazier ML. Effects of cyclin D1 polymorphism on age of onset of hereditary nonpolyposis colorectal cancer. Cancer Res. 2000;60:249–252. [PubMed] [Google Scholar]
- 45.Bala S, Peltomaki P. CYCLIN D1 as a genetic modifier in hereditary nonpolyposis colorectal cancer. Cancer Res. 2001;61:6042–6045. [PubMed] [Google Scholar]
- 46.Kruger S, Engel C, Bier A, et al. Absence of association between cyclin D1 (CCND1) G870A polymorphism and age of onset in hereditary nonpolyposis colorectal cancer. Cancer Lett. 2006;236:191–197. doi: 10.1016/j.canlet.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Cheng M, Olivier P, Diehl JA, et al. The p21(Cip1) and p27(Kip1) CDK 'inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. Embo J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno-Bueno G, Rodriguez-Perales S, Sanchez-Estevez C, et al. Molecular alterations associated with cyclin D1 overexpression in endometrial cancer. Int J Cancer. 2004;110:194–200. doi: 10.1002/ijc.20130. [DOI] [PubMed] [Google Scholar]
- 49.Ortega P, Moran A, de Juan C, et al. Differential Wnt pathway gene expression and E-cadherin truncation in sporadic colorectal cancers with and without microsatellite instability. Clin Cancer Res. 2008;14:995–1001. doi: 10.1158/1078-0432.CCR-07-1588. [DOI] [PubMed] [Google Scholar]