Abstract
Clean sulfenylations are observed upon reaction of activated methylenes with phenyl succinimidyl sulfide. When working with diethyl benzylmalonate, the sulfenylated product can be selectively oxidized and thermally fragmented affording phenylsulfenic acid, initially, and diethyl benzylidenemalonate. The developed method was applied using a polymer-supported thioanisole derivative (JandaJel). Formation of the enedicarboxylate documents proof of principle of polymer-supported sulfides as sulfenylating agents onto activated methylenes.
Keywords: polymer-supported synthesis, sulfenylation
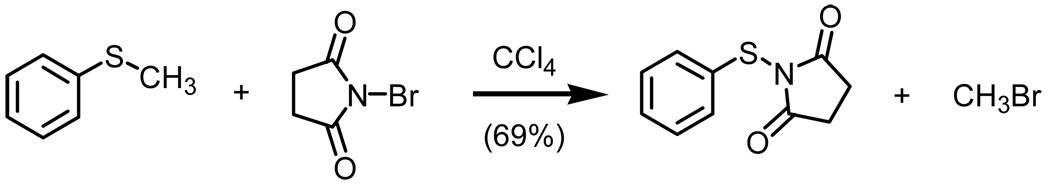
Since Groebel’s seminal work almost fifty years ago (Scheme 1),1 there have been numerous reports on the preparation and use of N-(organothio)succinimide derivatives.2,3 These activated organosulfides are most commonly used as sulfenylating reagents. While not all N-(organothio)succinimide derivatives are prepared via a demethylation process as first reported by Groebel, subsequent reports do present strategies based upon convenience, such as the avoidance of sulfenyl chloride, or use of readily accessible starting materials.
Scheme 1.
Sulfenylations using either N-(organothio)succinimide or N-(organothio)phthalimide derivatives with a host of nucleophiles (neutral, anionic, and heteroatomic) have been shown to proceed with high efficiency.3 The most common class of nucleophiles employed are activated methylenes. When considering cost, stability of the activated organosulfide, and the avoidance of toxic materials, the use of an S-imido derivative is the reagent of choice when performing sulfenylation chemistry.
The novelty of this process is how the sulfur atom can serve the role of both nucleophile and electrophile. Upon formation of phenyl succinimidyl sulfide using a demethylation process, the sulfur atom of thioanisole serves the role of nucleophile upon bromination with NBS. This role is then reversed upon expulsion of the bromide with succinimide. Similar strategies have been used toward the preparation of not only S-imidosulfides but S-thio-, S-seleno-, S-stannyl-, and S-halosulfides. As activated organosulfides, the relatively weak S-linkage is then exploited upon reaction with a wide spectrum of nucleophiles.
The use of organosulfides has been a very attractive strategy in assembly of both complex and simple organic molecules. The major synthetic use is in the preparation of α-thiocarboxylates.4 Since our research centers on the use of α-thiocarboxylates in S-ylide aziridination and epoxidation reactions,5 the recent commercial availability of polymer-supported thioanisole derivatives caught our attention and reported herein are our findings on the proof of concept of polymer-supported sulfides as sulfenylating agents onto activated methylenes.
The advantages of developing such technologies are clear. The materials needed for this process are cheap, readily available, and easy to work with even when considering on scale. The strategy utilizing polymer-supported technologies (polymer-supported organic synthesis (PSOS)) is evident given the numerous benefits associated with a process involving solid-phase chemistry. While we have made great progress with S-methylene transfers onto a host of carbonyl derivatives, limitations do exist. One significant advancement in the development of S-ylide chemistries would be the incorporation of polymer-supported technologies in the assembly of α-thiocarboxylates.
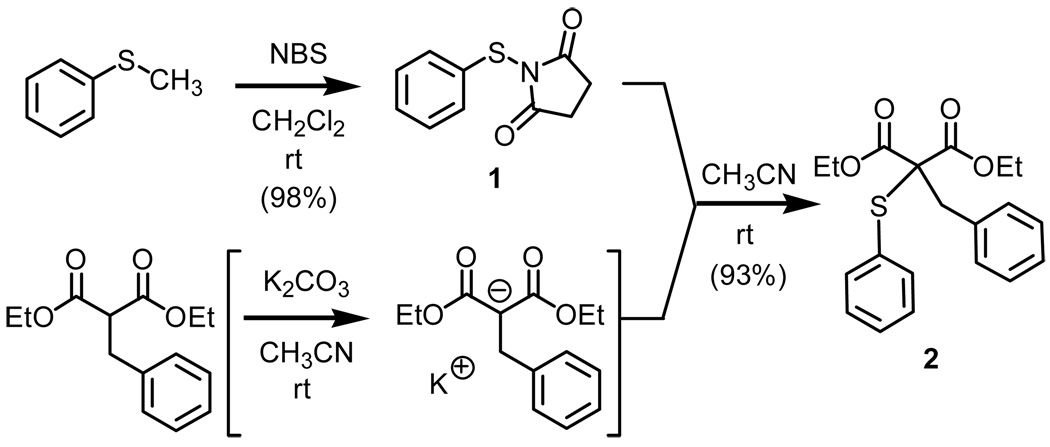
Prior to exploring the use of solid-phase chemistry (polymer-supported sulfide), we embarked upon a solution phase study (Scheme 2).
Scheme 2.
Using as our baseline the demethylation chemistry first reported by Groebel, we were successful in improving the isolated yield of organosulfide 1 from 69% to 98%. After examining solvent systems, stoichiometry, and temperature, we found that upon reaction of a slight excess of NBS with thioanisole in dichloromethane at room temperature, we were successful in obtaining the desired product in excellent yield when allowed to react over a period of 24h. While higher reaction temperatures (>50°C) did result in shorter reaction times based upon the consumption of thioanisole, the higher reaction temperatures did result in decomposition of organosulfide 1. Furthermore, using either excess quantities of NBS or alternative dipolar aprotic solvents did not result in significant improvements when considering reaction time.
Diethylbenzylmalonate was one of several activated methylenes we surveyed. When working with diethylmalonate, bis(sulfenylation), not suprising, using phenyl succinimidyl sulfide was observed. Surprising however was bis(sulfenylation) of diethylmalonate using just 0.5 equivalents of organosulfide 1. For this example, we confirmed the challenges associated with monosulfenylations with systems bearing two acidic protons. Observed with this example was bis(sulfenylation) in 44% conversion using phenyl succinimidyl sulfide and diethylmalonate. This however does not detract from the chemistry at hand. Even with the predisposition of double sulfenylation, reaction of sodium ethanethiolate with the bissulfenylated product has been shown to cleanly reduce the material to the corresponding monosulfenylated derivative.6
With our model system, the desired sulfenylated product (2) was obtained in excellent yield starting from the diethylbenzylmalonate and organosulfide 1.7 For this transformation, the order of addition and allowing for formation of the potassium salt prior to addition of the activated organosulfide was key.
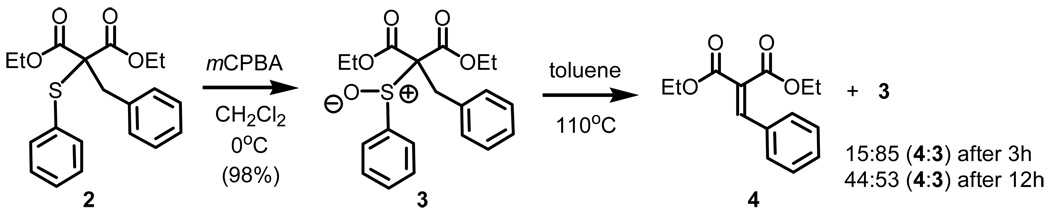
With a series of organosulfides in hand, we next explored the aspect of cleaving the C-S bond. The development of a fragmentation process was needed in that once a solution phase method was established; we were to go blind using identical reaction procedures with not thioanisole but a polymer-supported thioanisole derivative. Scheme 3 presents the key steps of our approach. While a β-hydrogen is needed for a β-elimination, selective cleavage of the C-S bond using an oxidant which preferentially reacts with the sulfur atom makes this an attractive method. Even though this strategy results in the formation of an alkene upon β-elimination, it does avoid the use of less than desirable reagents such as n-Bu3SnH/AIBN or Na/NH3 which can as well be used to perform cleavage of the C-S bond directly.
Scheme 3.
Sulfide 2 was oxidized using mCPBA and resulted in formation of sulfoxide 3 in 98% isolated yield.8 While high boiling solvents are most commonly used to perform fragmentation processes involving β-eliminations,9 we found this process with our system (sulfoxide 3) to be sluggish. Using toluene as solvent, the percent conversion of 3 to 4 never exceeded 50%. This was observed while monitoring the reaction at three separate intervals (3h, 12h, and 48h). Odd was that the mass balance of the reaction mixture exceeded 90% on the 1.0 mmol scale and when monitoring the reaction, no evidence of any secondary processes was observed. One known process being polymerization of enedicarboxylate 4.10 While disappointed with the overall levels of conversion, the reaction conditions are unoptimized. As stated above, while we did not observe any secondary processes, we cannot dismiss the fact that as the reaction proceeds, the buildup of phenylsulfenic acid (PhSOH) may play an important role in the overall outcome of this process. While secondary when considering the proof of principle of sulfenylation chemistry using polymer-supported materials, efforts which address the failure of this reaction to complete are underway.
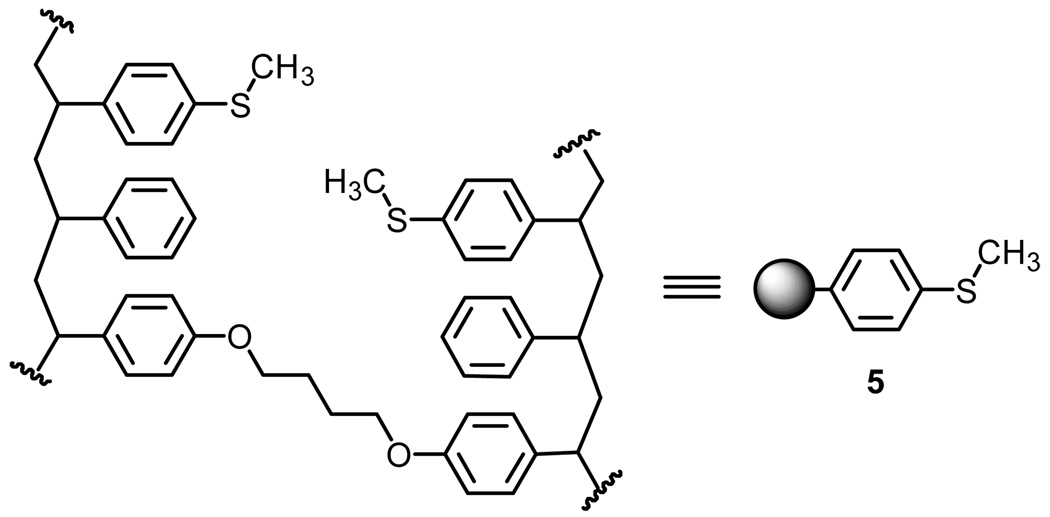
Using solution phase chemistry, we developed a method of sulfenylation using activated organosulfides and cleavage upon thermolysis of sulfoxide 3. For the polymer-supported chemistry, we explored the use of commercially available thioanisole functionalized JandaJel 5 (Figure 1). Aside from the commercial availability of this material, we were excited about the potential of using this material in particular given its homogeneous properties when compared to traditional solid-phase synthesis supports.11
Figure 1.
Commercially available thioanisole functionalized JandaJel 5.
Duplicating the reaction sequence presented in Scheme 2 and Scheme 3 resulted in isolation of enedicarboxylate 4 upon heating the toluene suspension containing the oxidized JandaJel derivative. Starting with 1.0 g of the thioanisole derived JandaJel 5 we obtained 30mg, unoptimized, of enedicarboxylate 4. The only difference with this work when considering the mechanics of running the reaction itself was that once the beads were given sufficient time to drop to the bottom of the flask, the solvent system containing unreacted starting material and by-product were removed by pipet.
While it is unfortunate that the by-product of the thermolysis reaction, phenylsulfenic acid, does rapidily dimerize to form a thiosulfinate,12 we were pleased to see that each malonate intermediate prepared in solution could be unambiguously identified using just the benzylic proton(s) by 1H NMR thus adding the value of this synthetic sequence as a teaching exercise. Had the dimerization process be slower under these reaction conditions, a direct method toward the regeneration of organosulfide 1 both in solution and as a derivative bound to polymer-support would be possible. Efforts which address reuse of this polymer-supported material and application toward the grafting, assembly, and releasing of materials in multi-step processes are currently underway and will be reported in due course.
Acknowledgements
DCF would like to thank the helpful suggestions made during the review process of this manuscript. DCF would like to recognize NIGMS (NIH NIGMS 1R15GM085936), NSF (CHE 0514004), and the Camille and Henry Dreyfus Foundation (TH-06-008) for partial funding of this research. DCF would like to thank Professor Patrick Toy (University Hong Kong) for his generous donation of a thioanisole grafted JandaJel. BPF would like to thank NSF for summer sponsorship over the 2007 and 2008 summer months. JAK would like to acknowledge financial support through the Alabama Space Grant Scholars Program and the University of South Alabama (UCUR and University Honors Program).
References
- 1.Groebel W. Chem. Ber. 1959;92:2887. [Google Scholar]
- 2.For preparation, see: Abe Y, Nakabayashi T, Tsurugi J. Bull. Chem. Soc. Jpn. 1973;46:1898. Büchel KH, Conte A. Chem. Ber. 1967;100:1248. doi: 10.1002/cber.19671000321. Pant BC, Noltes JG. Inorg. Nucl. Chem. Lett. 1971;7:63. Harpp DN, Aida T, DeCesare J, Tisnes P, Chan TH. Syn. Commun. 1984:1037.
- 3.For synthetic use, see: Mukaiyama T, Kobayashi S, Kumamoto T. Tetrahedron Lett. 1970;59:5115. Furukawa M, Suda T, Hayashi S. Synthesis. 1974:282. Mukaiyama T, Kobayashi S, Kamio K, Takei H. Chem. Lett. 1972:237. Walker KAM. Tetrahedron Lett. 1977;51:4475. Torii S, Sayo N, Hideo T. Chem. Lett. 1980:695. Huang C-H, Liao K-S, De SK, Tsai Y-M. Tetrahedron Lett. 2000;41:3911.
- 4.(a) Shcherbakova I, Pozharskii AF. In: In Comprehensive Organic Functional Group Transformations II. Katritzky AR, Taylor RJK, editors. Volume 2. Oxford: UK: Elsevier Ltd.; 2005. p. 89. [Google Scholar]; (b) Taylor PC, Parrett MR. Annu. Rep. Prog. Chem., Sect. B: Org. Chem. 2007;103:47. [Google Scholar]; (c) Skabara PJ. Annu. Rep. Prog. Chem., Sect. A. 2004;100:113. [Google Scholar]
- 5.(a) Forbes DC, Standen MC, Lewis DL. Org. Lett. 2003;5:2283. doi: 10.1021/ol034612a. [DOI] [PubMed] [Google Scholar]; (b) Forbes DC, Amin SR, Bean CJ, Standen MC. J. Org. Chem. 2006;71:8287. doi: 10.1021/jo061370u. [DOI] [PubMed] [Google Scholar]; (c) Forbes DC, Bettigeri SV, Patrawala SA, Pischek SC, Standen MC. Tetrahedron. 2009;65:70. doi: 10.1016/j.tet.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Forbes DC, Bettigeri SV, Amin SR, Bean CJ, Law AM, Stockman RA. Syn. Commun. doi: 10.1080/00397910802654898. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grossert JS, Dubey PK. J. Chem. Soc., Chem. Commun. 1982:1183. [Google Scholar]
- 7. Representative Reaction Procedure: To a dichloromethane (5 mL) solution consisting of thioanisole (0.12 g, 1.0 mmol) was added NBS (0.27 g, 1.5 mmol) portionwise at room temperature. The reaction mixture was allowed to stir for a period of 24h at room temperature at which time all volatiles were removed in vacuo. The resulting slurry was then recrystallized using CH2Cl2:hexanes (1:6) to afford 0.20 g (0.98 mmol) of phenyl succinimidyl sulfide (1) in 98% yield. Organosulfide 1 was then added neat to a solution of acetonitrile (5 mL) containing diethyl benzylmalonate (0.25 g, 1.0 mmol) and potassium carbonate (0.20 g, 1.5 mmol) having been allowed to react for a period of 30 min at room temperature. After addition of the sulfide (1.0 mmol), the reaction mixture was allowed to stir at room temperature for a period of 60h at which time 30 mL of dichloromethane was added and subsequently washed with water. The organic phase was then dried using MgSO4, concentrated in vacuo and purified by column chromatography to afford 0.34 g (0.93 mmol) diethyl benzyl(phenylthio)malonate in 93% yield.
- 8. Representative Reaction Procedure: A dichloromethane (5 mL) solution consisting of organosulfide 2 (0.36 g, 1.0 mmol) was cooled to 0°C at which time mCPBA (0.21 g, 1.2 mmol) was added portionwise. The reaction mixture was allowed to gradually warm to room temperature and stir for a period of 30min. The reaction mixture was then neutralized using NaHCO3 and washed with water. The organic phase was dried using MgSO4, concentrated in vacuo, and purified by column chromatography to afford 0.37 g (0.98 mmol) of organosulfoxide 3 in 98% yield. Organosulfoxide 1 was then redissolved in toluene (5 mL) and externally warmed to reflux using a sand bath. The refluxing solution was monitored by NMR. After 12h, NMR analysis revealed 44% conversion to the desired product. The analytical data of diethylbenzylidenemalonate obtained matched that of commercial material.
- 9.Kametani T, Yukawa H, Honda T. J. Chem. Soc., Chem. Commun. 1988:685. [Google Scholar]
- 10.Hoye TR, Caruso AJ, Magee AS. J. Org. Chem. 1982;47:4152. [Google Scholar]
- 11.(a) Solinas A, Taddei M. Synthesis. 2007;16:2409. [Google Scholar]; (b) Kan JW, Toy PH. J. Sulfur Chem. 2005;26:509. and references cited therein. [Google Scholar]
- 12.Davis FA, Billmers RL. J. Org. Chem. 1985;50:2593. [Google Scholar]