Abstract
This review describes available methods for the diastereoselective and asymmetric synthesis of quaternary prolines. The focus is on the preparation of α-functionalized prolines with the pyrrolidine moiety not embedded in a polycyclic frame. The diverse synthetic approaches are classified according to the bond which is formed to complete the quaternary skeleton.
Keywords: α,α-Disubstituted amino acid; Constrained amino acid; α-Substituted proline; Diastereoselectivity; Enantioselectivity
1. Introduction
Biologically active peptides and proteins regulate most vital physiological processes and are therefore targets for potential medical applications across the full spectrum of human disease.[1] However, the pharmacological applicability of native peptides is greatly limited by profound factors such as lack of selectivity for a specific receptor, enzymatic instability or low bioavailability.[2] The diverse functionalities of peptides are based on the physical and chemical properties of specific amino acid side chains and on their three-dimensional structure, the folding. The modification of native peptides through the incorporation of rigid amino acid surrogates into their structures constitutes a powerful means to overcome their shortcomings since a limited conformational freedom often prevents from proteolytic degradation and sometimes leads to improved selectivity and potency.[3]
In this context, proline analogues[4] are of special interest given the key role of proline in nucleating the secondary structure and hence, the biological behavior of peptides.[5] Such significance is due to proline exceptional conformational rigidity granted by its cyclic structure and the hinge-like behavior displayed at the peptide bonds involving the pyrrolidine nitrogen. Particularly, quaternary proline analogues are of ongoing interest as modulators of proline conformational constraint for peptide engineering purposes.
Albeit little is explored on their conformational preferences,[6,7] α-substituted prolines are also attractive templates in structure-function relationship studies aimed at elucidating biologically active conformations likewise proline chimeras.[8] The large number of papers and patents dealing with the incorporation of the α-methylated[7,9,10] analogues of proline into bioactive peptides and other biologically relevant systems reveal the enormous potential of this type of proline analogues. Their increasing interest is also linked to potential uses in regulation of protein-protein interactions mediated via proline-rich binding domains[11] and to the peptidomimetic design based on mimicking protein-recognition motifs such as β-turns.[12] Moreover, their utility expands further into the design of new organic catalysts and auxiliaries for asymmetric synthesis.[13]
Despite their growing interest, the exploitation of α-substituted proline derivatives relies on a ready accessibility to their enantiomerically pure forms. For this reason, we wish to illustrate the progress of the enantio- and diastereoselective synthetic methods utilized for their construction. Although, the stereoselective synthesis of α,α-disubstituted amino acids has been broadly collected by us [4b,14] and others,[15] just few reports have particularly covered the preparation of α-substituted proline analogues.[4a,4b]
In this review, the synthetic approaches have been classified according to the bond which is formed to complete the quaternary proline skeleton. Well established strategies, such as the alkylation of enolate derivatives of existing α-amino acids or 1,3-dipolar cycloadditions, as well as more recent asymmetric catalytic approaches have been collected. Due to the abundant literature in the field, reactions leading to polycyclic proline-type compounds have not been comprised.
2. Syntheses from Proline Derivatives
A number of strategies devised for the synthesis of α-substituted proline derivatives involve the α-functionalization of l- proline itself. Most often, the assembly of the fully substituted stereocenter is accomplished by electrophilic alkylation of l- proline enolate equivalents. Some of these approaches have been the subject of recent reviews.[4a,4b]
2.1 Self-Reproduction of Chirality
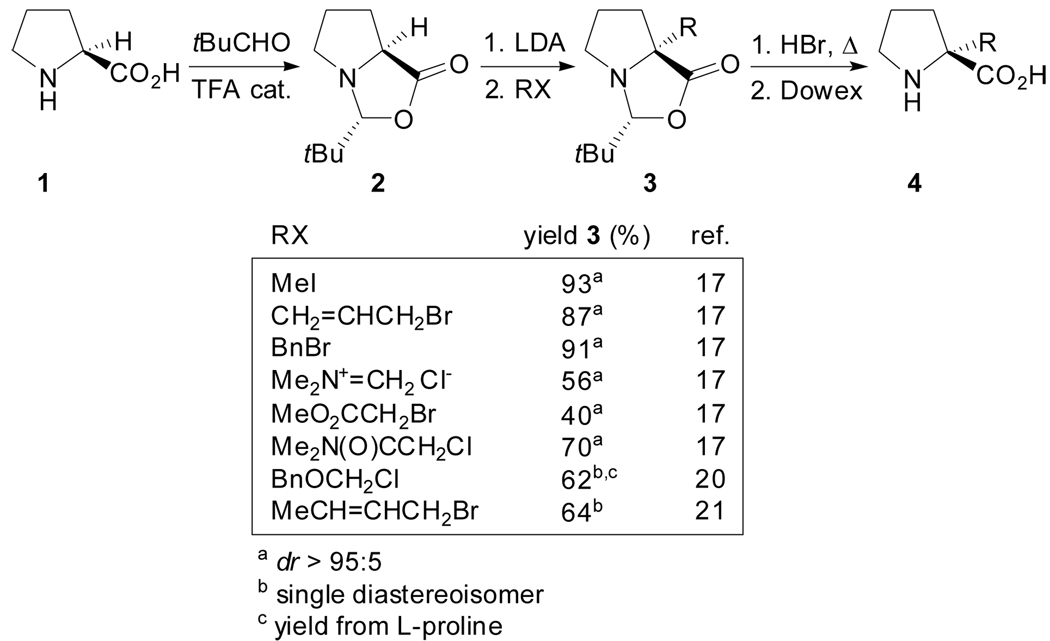
In 1983, Seebach et al. reported a methodology that formally allows the direct α-alkylation of l- proline without loss of the optical purity and with retention of configuration,[16–18] thus being a show-case of their concept self-reproduction of chirality.[19] l- Proline was condensed with pivalaldehyde to give a single stereoisomer of 2-tert-butyl-1-aza-3-oxabicyclo[3.3.0]octan-4-one 2 which, upon deprotonation with LDA, gave a chiral non-racemic enolate that combined with alkyl halides afforded the corresponding α-alkylated compounds in moderate to high yields and with essentially complete diastereoselection (Scheme 1).[17,20,21] In addition, the intermediate 2 was also phenylated with (benzene)(tricarbonyl)chromium and thiolated with diphenyl disulfide.[17] The hydrolytic cleavage of the products 3, which can be difficult under acidic conditions, could be performed under mild conditions using a suspension of silica gel in methanol/water[22] or, alternatively, carboxamide derivatives could be obtained after treatment with lithium amides.[17]
Scheme 1.
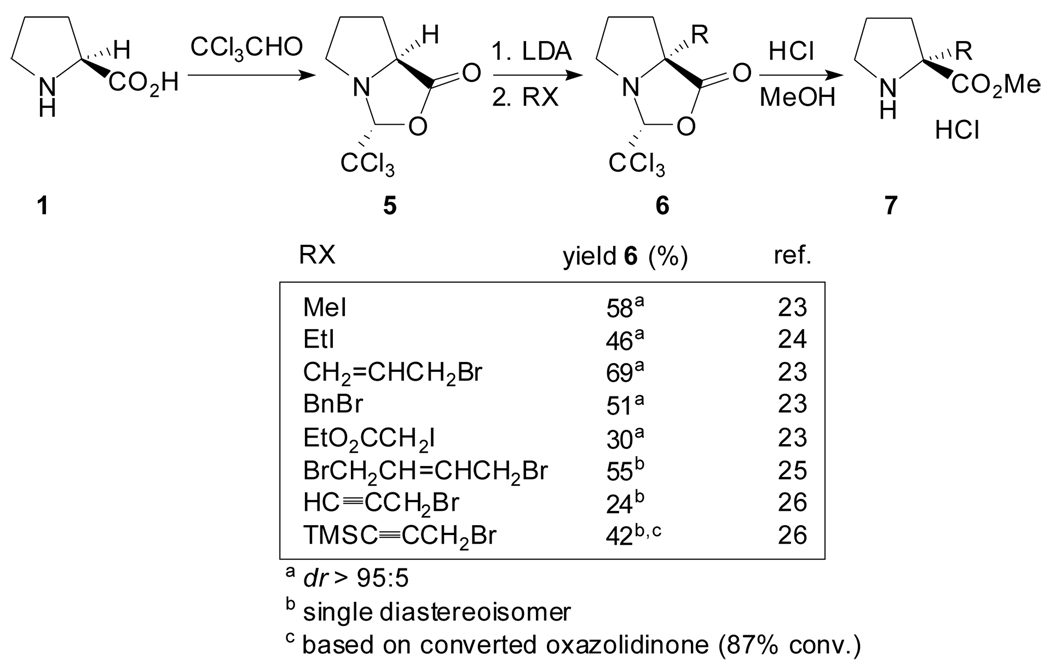
A related strategy was pursued by Germanas et al. and involved the use of 4-alkyl-2-trichloromethyloxazolidin-5-one 5 as the precursor to enantiomerically pure α-substituted prolines (Scheme 2).[23–26] In terms of the stereochemical course of the reaction their results were similar to those reported by Seebach, however, the use of a trichloromethyloxazolidinone is preferable due to its greater stability and lower cost of production. The cleavage of the chloral auxiliary often required refluxing hydrochloric acid/methanol to produce the desired methyl ester hydrochloride salt. Interestingly, Williams et al. described that the exposure of the lactone 6 (R= allyl) to Na in methanol followed by the addition of acetyl chloride to the solution and heating to reflux readily removes the trichloroacetaldehyde auxiliary.[27] In this manner, (R)-allylproline methyl ester hydrochloride salt was prepared on a 20 g scale and was used as a building block for the total synthesis of the fungal metabolites (−)-Stephacidin A, (+)-Stephacidin B and (+)-Notoamide B. Besides this, the trichloromethyloxazolidinones could also be readily transformed into amides by nucleophilic ring opening with amines.[28]
Scheme 2.
Both versions of this methodology have been profusely applied to the synthesis of enantiomerically pure α-alkylproline derivatives to be used as intermediates in the synthesis of more complex structures. In particular, enantiomerically pure α-allylproline has proven plentiful applications towards the synthesis of type II[29] and type VI[30] β-turn[31] mimetics, spirolactams[25,32] as conformationally restricted pseudopeptides, biologically active natural products[21,27,33] and peptidomimetics.[24,34]
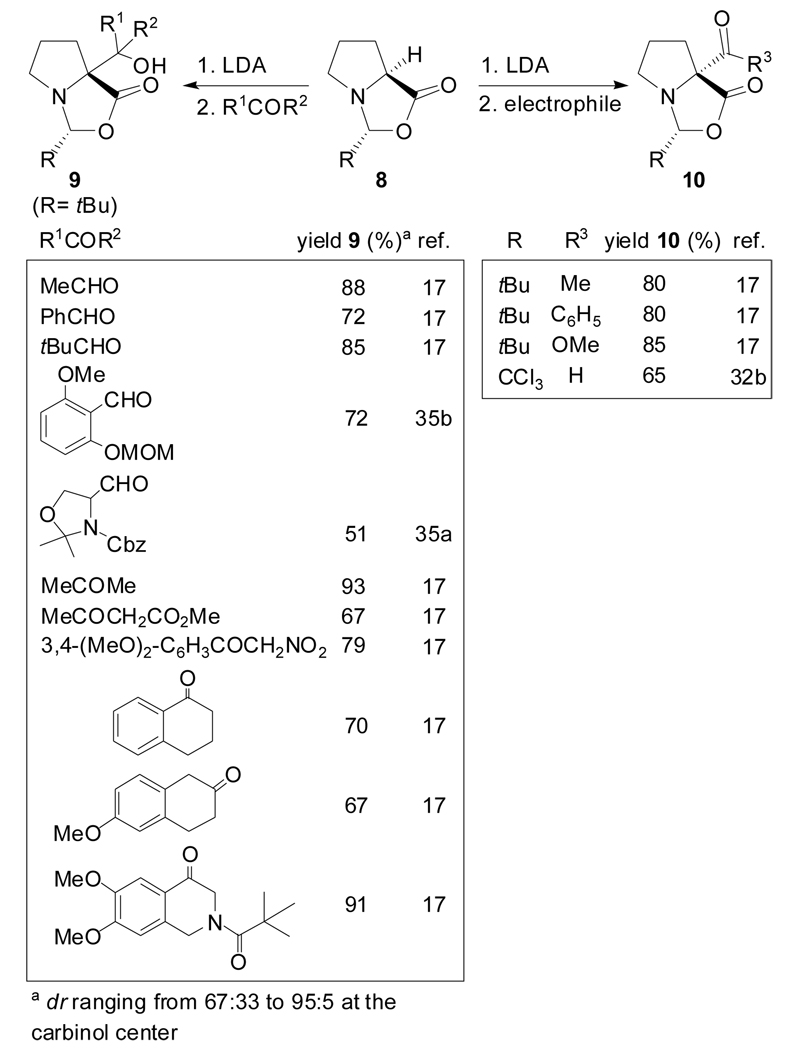
Aside from alkylation reactions, additions to carbonyl groups of aldehydes[17,35] and ketones,[17] Michael additions[17] and acylation reactions[17,32b] have also been reported (Scheme 3). The additions to aldehydes and ketones afforded isomers epimeric at the carbinol center, the Michael additions gave rise to the formation of constitutional isomers and the acylation reactions generated compounds quite unstable.[17]
Scheme 3.
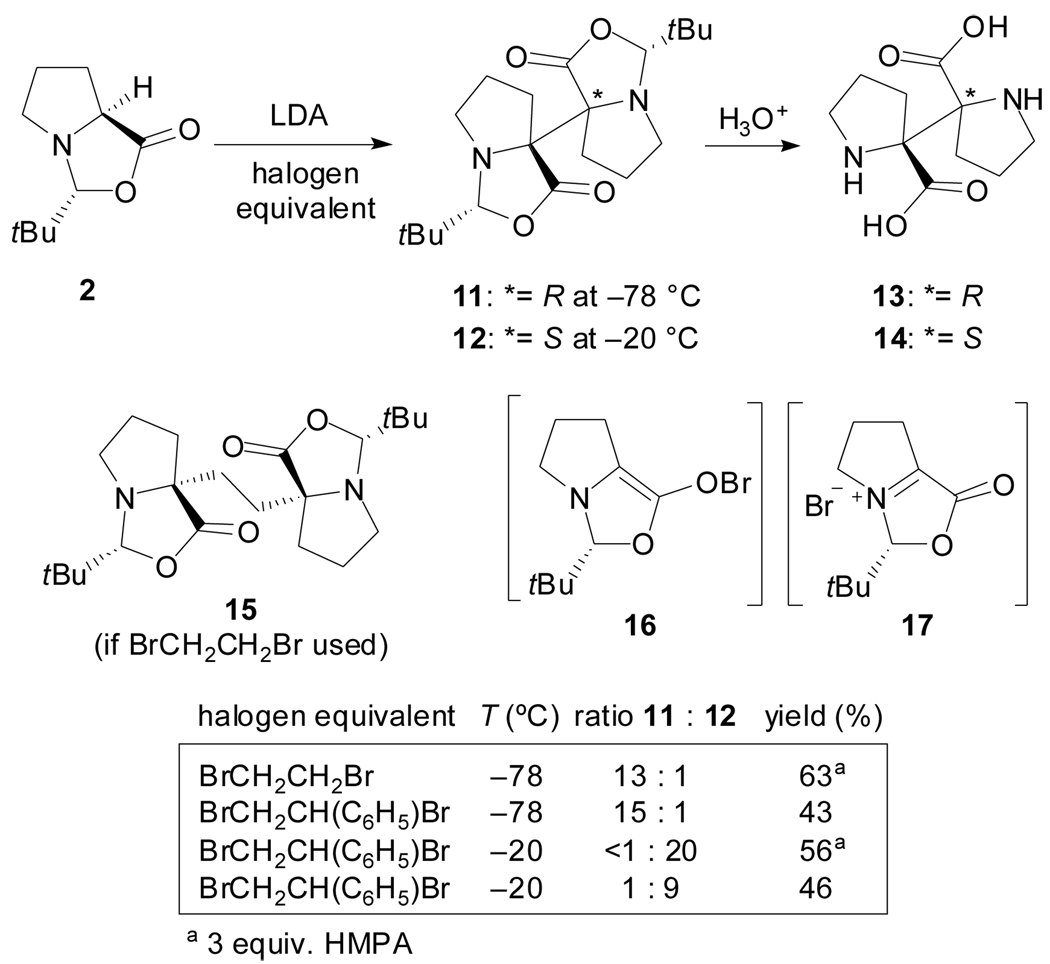
More recently, Johnson et al. reported the synthesis of the (R,R)-α,α’-biproline 13 and meso-α,α’-biproline 14via a temperature dependent diastereoselective dimerization of Seebach’s oxazolidinone (Scheme 4).[36] Instead of the C2-linked bisoxazolidinone 15, the alkylation of the lithium enolate of 2 with vicinal dihalides at −78 °C afforded primarily 11, while at −20 °C, the dimer 12 was obtained as the major diastereoisomer. The vic-dihalides essentially function as halogen equivalents in this oxidative process. To account for the temperature-dependent reversal in diastereoselectivity the authors suggest two different intermediates involved in the reaction. Thus, the organohypobromite 16 and the iminium halide 17 are proposed to serve as intermediates for the enolate of 2 to give 13 and 14 respectively after hydrolysis.
Scheme 4.
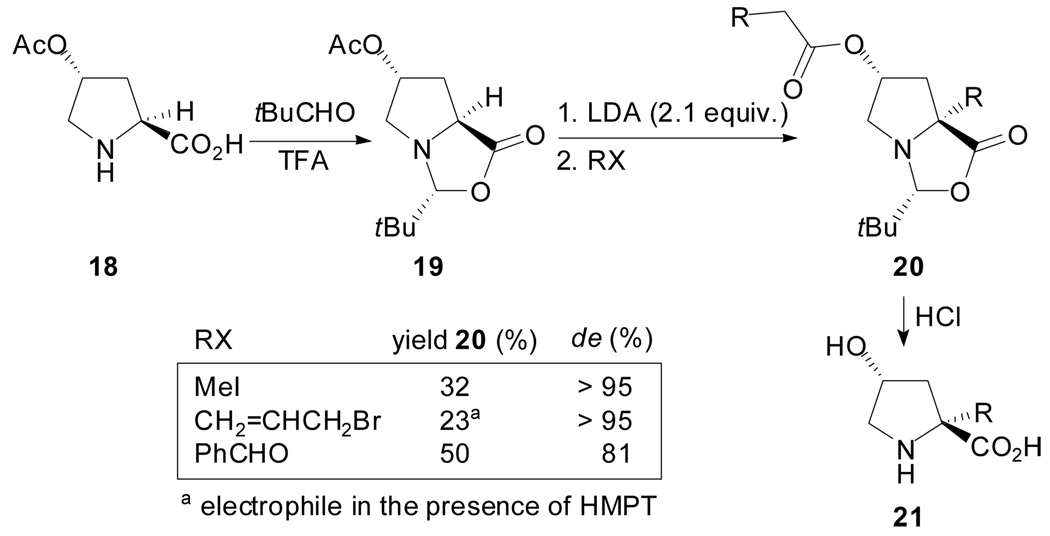
The self-reproduction of chirality principle has also been applied to the synthesis of 2-alkyl-4-hydroxyprolines starting from (2S,4R)-O-acetyl-4-hydroxyproline (Scheme 5).[37] The reaction of the dienolate generated by treatment of compound 19 with LDA, with different electrophiles, occurs with retention of configuration. In this way, 2-methyl- and 2-allyl-4-hydroxyprolines have been prepared. When carbonyl compounds were used as electrophiles a mixture of diastereoisomers at the carbinol center was obtained.
Scheme 5.
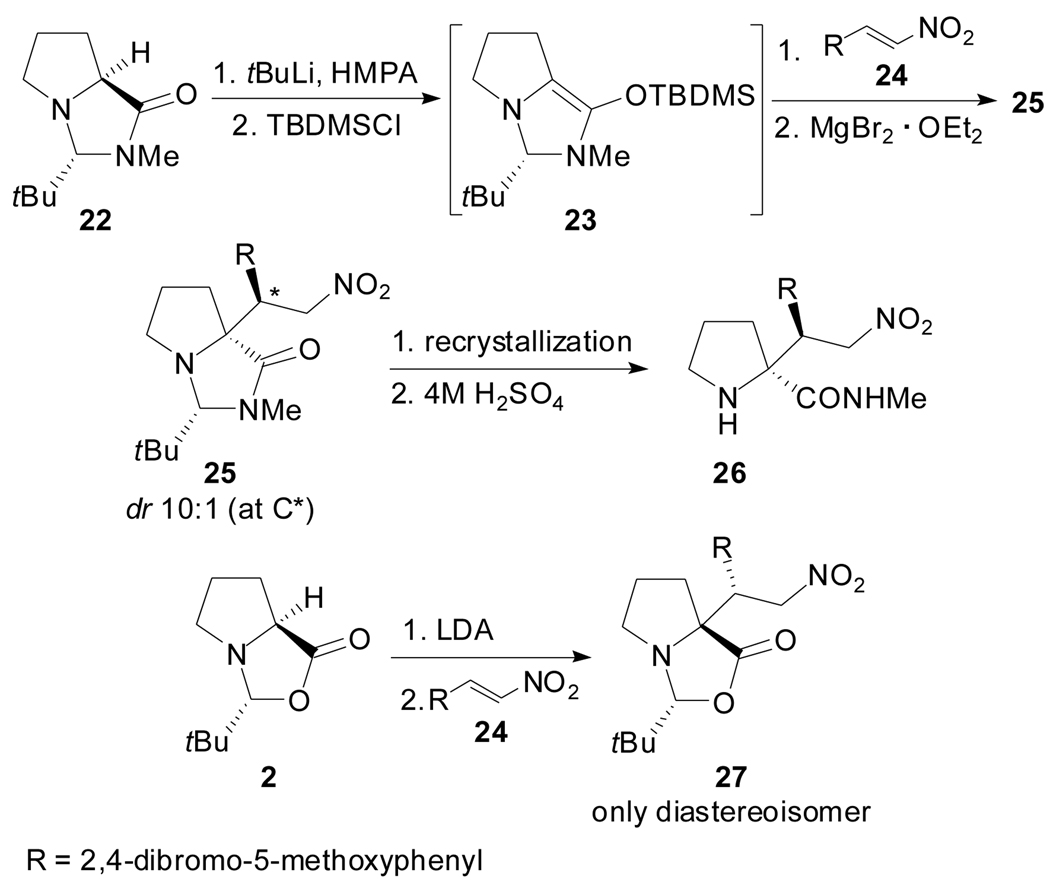
In 2002, Trauner and Hughes reported a remarkable exception to the cis rule governing the self-reproduction of chirality chemistry while aiming to the total synthesis of the marine alkaloid (−)-Amathaspiramide F (Scheme 6).[38] The N,N-acetal 22 cleanly afforded the enolate 23 by using tBuLi in the presence of HMPA. The conjugate addition to the nitro olefin 24 was possible when the enolate 23 was generated in situ and reacted in the presence of MgBr2·OEt2. A 10:1 mixture of diastereoisomers 25 was obtained, both S-configured at the newly formed quaternary center. According to the authors, the bulky tert-butyldimethylsilyl crowds the diastereoface of the double bond at the convex side of the diazabicyclo[3.3.0]octene framework, thus preventing a nucleophilic attack on that side. In contrast, the N,O-acetal 2 afforded the expected R-cis product 27 as the only diastereoisomer.
Scheme 6.
2.2 Diastereoselective Alkylations
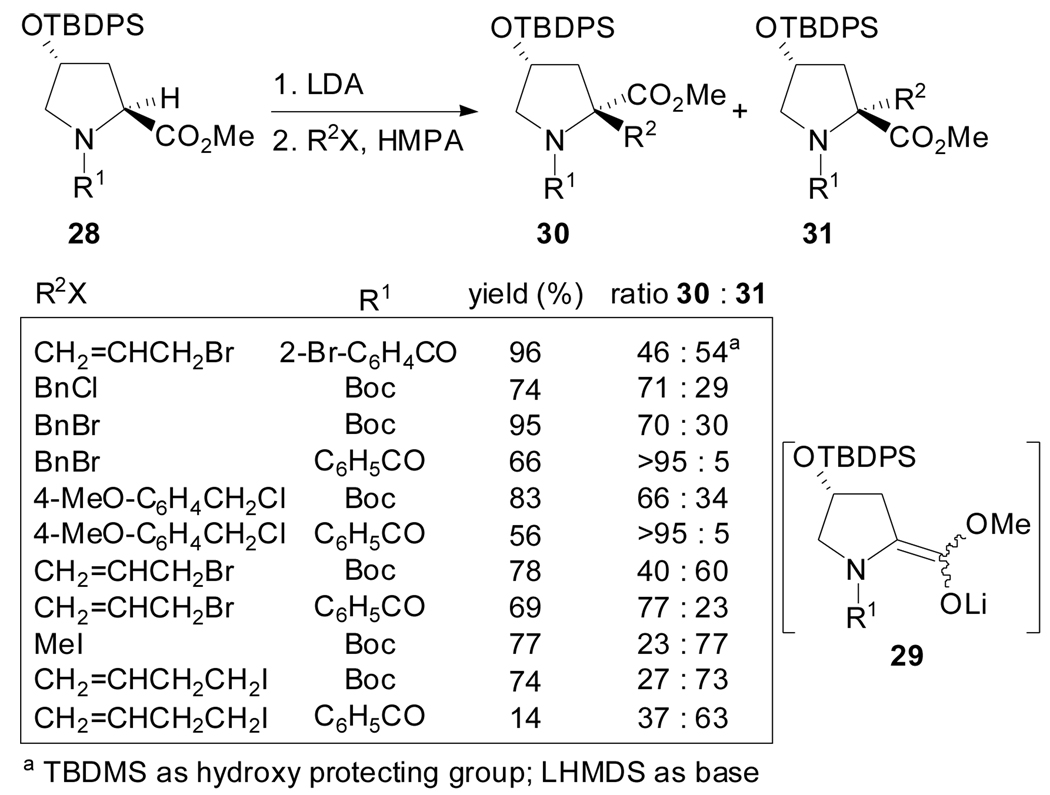
Over the last years, diverse stereoselective alkylations of substituted l- proline esters have been reported and much effort has been dedicated to rationalize the factors controlling the diastereoselectivity of the process. In this context, the stereochemical outcome of the alkylation of enolates 29 derived from N-Boc- and N-benzoyl-(2S,4R)-4-tert-butyldiphenylsilyloxyproline methyl ester has been particularly studied.[39,40] Kawahara et al. found that the diastereoselectivity of the alkylation reaction is dependent on the alkylating reagent and the N-protecting group.[40] Whereas retention of configuration was observed upon alkylation of the N-Boc derivative with allylic and homoallylic halides, the use of benzylic halides lead preferentially to products with inversion of configuration. In the case of N-benzoyl derivatives the alkylation occurred with inversion of configuration when benzylic or allylic halides were used and, in the case of benzylic halides high diastereoselectivities were observed (Scheme 7).
Scheme 7.
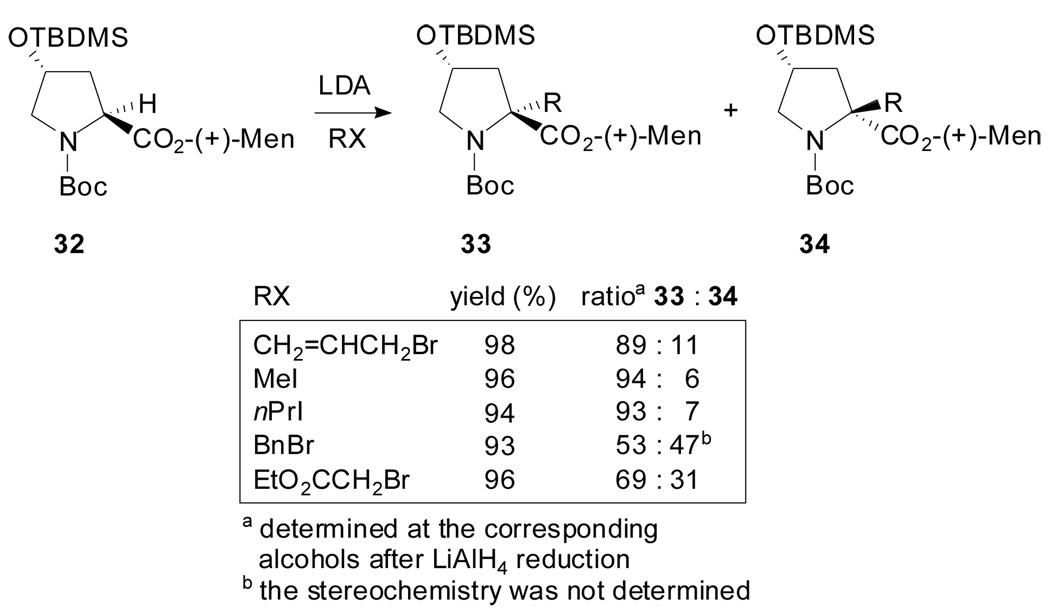
Afterwards, in 2001, Sato et al. showed that the diastereoselectivity during the alkylation of N-Boc-4-tert-butyldimethylsilyloxyproline esters can be improved when a menthyl ester derivative is engaged (Scheme 8).[41] In comparison with a methyl ester, the reaction proceeded with improved diastereoselectivities either with (+) or (−)-menthyl esters when electrophiles bearing no π-electron system were employed. According to the authors, the steric bulkiness of the ester group rather than the absolute configuration of the chiral auxiliary plays a crucial role in enhancing the selectivity.
Scheme 8.
The diastereoselective alkylations of 4-hydroxy-substituted prolines have found application towards the preparation of novel β-peptide oligomers[42] and the asymmetric synthesis of biologically active natural products, like the alkaloids (−)-velbanamine, (−)-aphanorphine[43] and (−)-TAN1251A.[44]
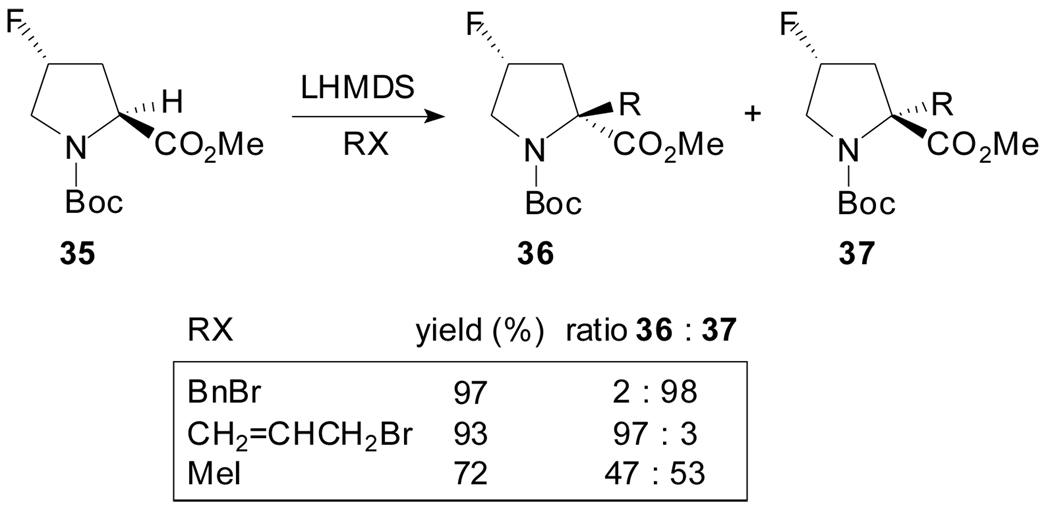
In 2006, Filosa et al. found that the alkylation of (4S)- and (4R)-fluoro-N-Boc-l- proline methyl esters proceeds also in high yield and diastereoselectivity (Scheme 9).[45] According to the authors, the stereochemical outcome of the experiments denotes a different behavior of enolates produced from 4-fluoroprolines compared with those coming from O-protected 4-hydroxyprolines.
Scheme 9.
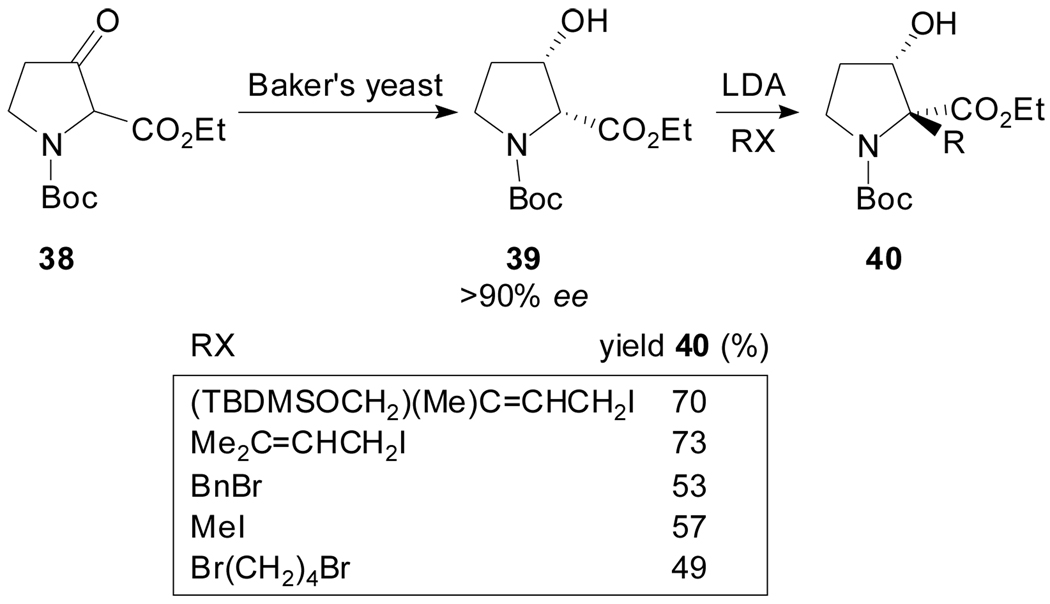
N-Boc-(2R,3S)-3-hydroxyproline ethyl ester, which is readily available from racemic 3-ketoproline 38 by Baker’s yeast reduction, has been alkylated with different electrophiles with retention of configuration and with moderate to good yields and excellent diastereoselectivity (Scheme 10).[46] This represents a useful procedure for the synthesis of α-alkyl-β-hydroxyproline derivatives 40; in fact, the β-functionalized α-prenylated proline derivative synthesized in this manner has been used as an intermediate towards the total synthesis of Paraherquamide A.[47]
Scheme 10.
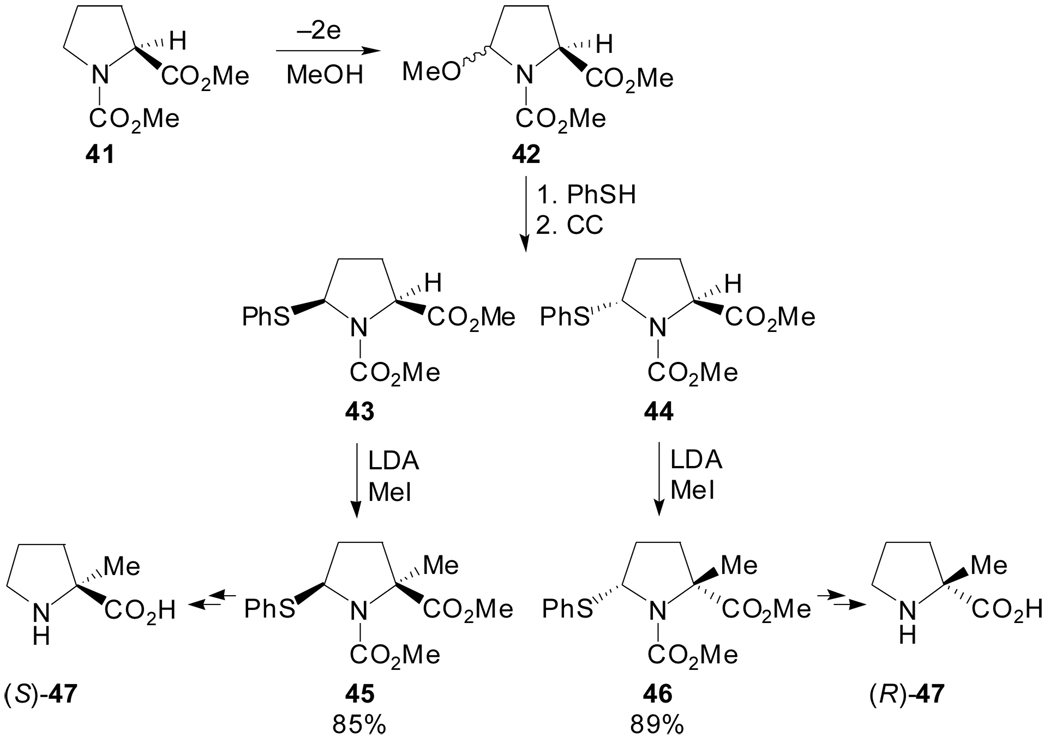
With regard to stereoselective alkylations of proline derivatives substituted at position C5, Matsumura et al. described a synthetic strategy for the preparation of both enantiomers of α-methylproline.[48] The electrochemical methoxylation of N-methoxycarbonyl-l- proline methyl ester 41, followed by the replacement of the methoxy group with a phenylthio group, afforded an almost equimolecular mixture of diastereoisomers which were easily separated by column chromatography. Subsequent α-methylation and reductive removal of the phenylthio group lead to both enantiomers of α-methylproline (Scheme 11).
Scheme 11.
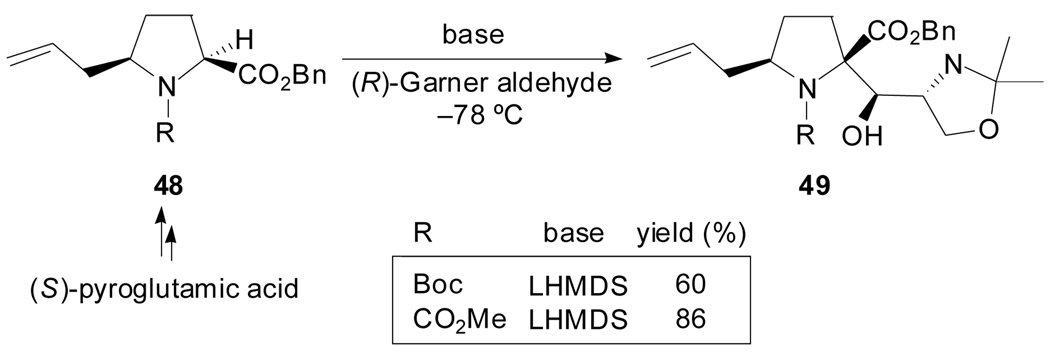
In 2001, Ma et al. reported a highly diastereoselective aldol reaction of an l- proline benzyl ester carrying an allyl substituent at position 5 with (R)-Garner aldehyde,[49] with the objective to assemble the right-hand part of Kaitocephalin which is the first naturally occurring AMPA/KA antagonist without cytotoxic effect. The allyl substituent did not seem to influence the diastereoselectivity of the aldol reaction given that the treatment of 48 with LDA provided four isomers in almost equal amounts. On the contrary, a single compound was obtained when LHMDS was used as a base (Scheme 12).
Scheme 12.
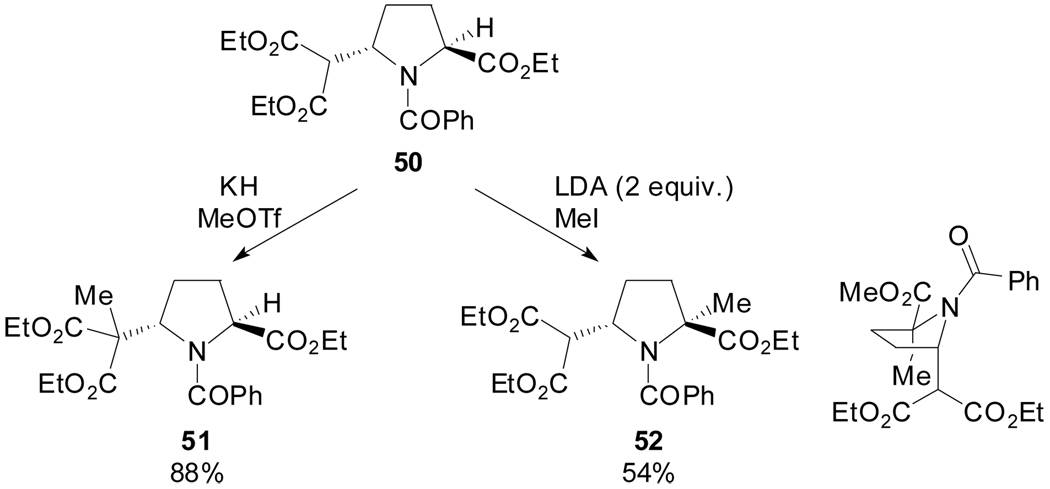
An alternative route to 2,5-disubstituted pyrrolidines based on the reduction of enamines derived from pyroglutamic acid was reported by Moloney et al.[50] They showed that the regioselective manipulation of position C2 or the malonate substituent in (2S,5S)-50 are possible, providing access to substituted pyrrolidines for a very limited number of cases. Particularly, the double deprotonation of trans-50 with excess of LDA and alkylation provided the monomethyl adduct 52 as the result of alkylation at the more reactive enolate position and in an anti-sense to the benzoyl group with a kinetic pseudo axial preference (Scheme 13).
Scheme 13.
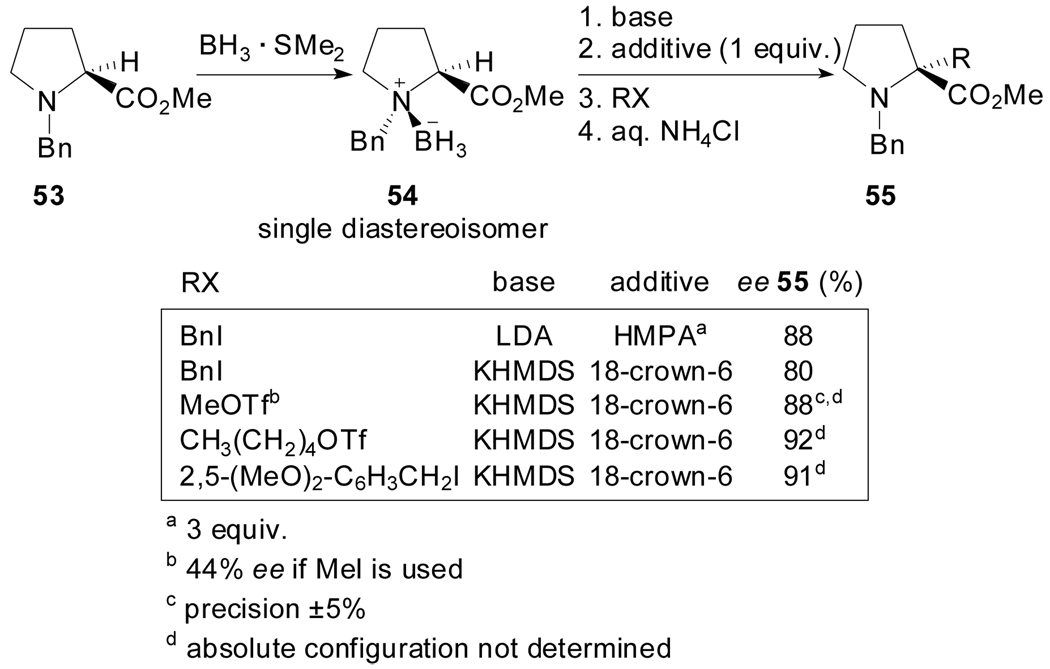
Mioskowski et al. reported the asymmetric synthesis of α-alkylproline derivatives using a method that involved successive chirality transfers via the chiral borane-amine adduct 54 which was in turn prepared as a single isomer from l-proline (Scheme 14).[51]
Scheme 14.
The optimal results were obtained by generation of the enolate by treatment with LDA followed by quenching in the presence of HMPA or, alternatively, by treatment with KHMDS and 18-crown-6 as the additive. In general, the quantity of additive had a great impact on the level of enantioselectivity. Interestingly, the absence of the crown ether caused a reversal of diastereoface selection during the alkylation of the potassium enolate (when RX= BnI) presumably due to steric reasons.
2.3 Alkylations under Phase Transfer Catalysis
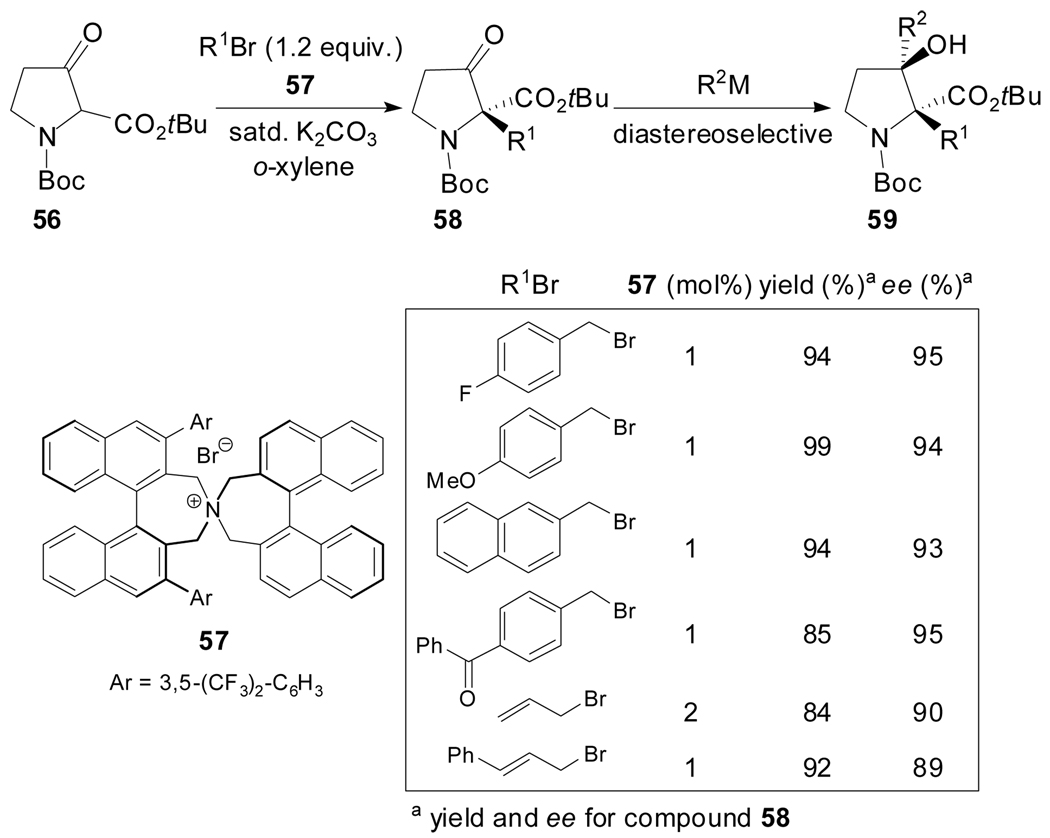
In 2005 Maruoka et al. reported an approach for the preparation of various optically active quaternary 3-oxoprolines based on the phase transfer-catalyzed highly enantioselective alkylation of the racemic α-amino-β-ketoester 56 (Scheme 15).[52] The assembly of the quaternary stereocenter was achieved by applying the C2-symmetric chiral quaternary ammonium salt 57 as the catalyst and o-xylene as the solvent since it provided a substantial enhancement of the reaction rate. The conduction of the reaction with allylic and benzylic bromides provided diverse chimeras of 3-oxoproline and phenylalanine in 89–95% ee. Moreover, selective alkylations with various Grignard reagents onto the 3-keto carbonyl group proceeded smoothly and allowed the construction of α-substituted prolines with two consecutive stereochemically defined quaternary centers.
Scheme 15.
2.4 [2+2]-Cycloaddition of Cyclic Ketenes with Imines
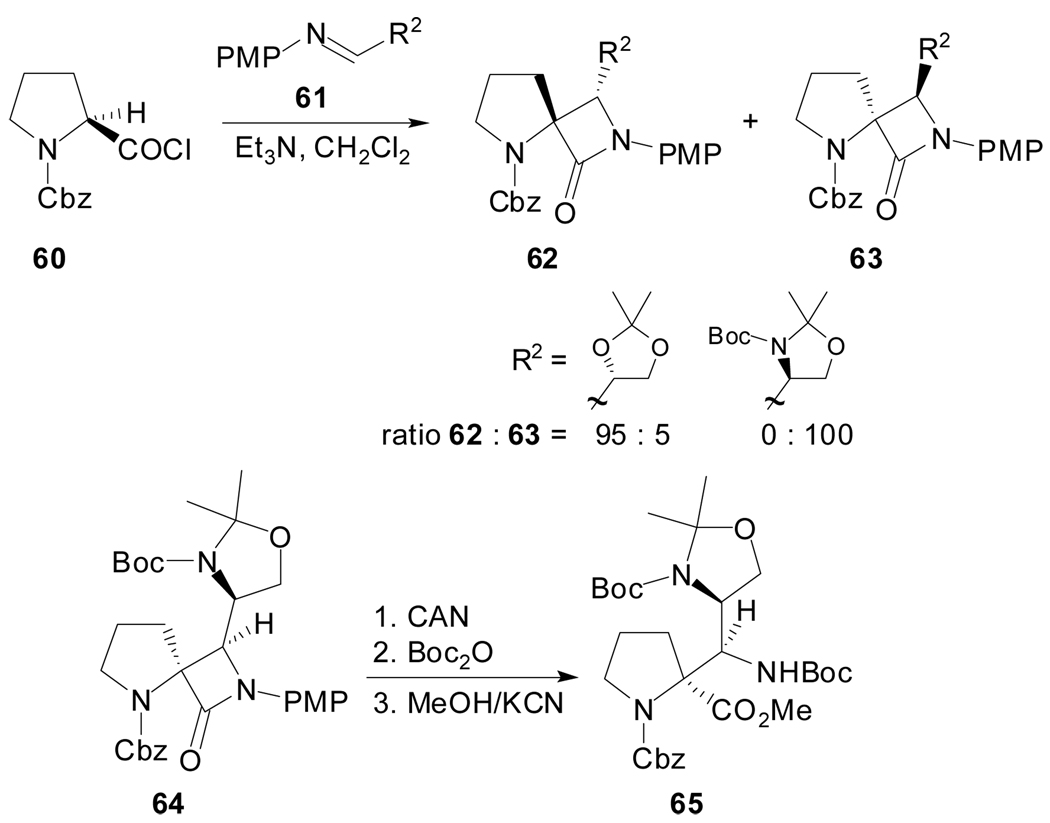
The nucleophilic ring opening of spiro β-lactams of type 62 has been described as a new methodology for the asymmetric synthesis of modified prolines. The β-lactams 62 and 63 were prepared by the [2+2]-cycloaddition of a cyclic ketene generated in situ with optically active imines (Scheme 16).[53] The [2+2]-cycloaddition reaction took place with complete stereoselectivity, thus giving the expected spiro β-lactams with a cis relative disposition of the substituent at the iminic carbon atom and the proline nitrogen and with excellent asymmetric induction. In particular, the use of 64 as starting material provided access to enantiomerically pure α-(1-aminoalkyl)-proline 65 orthogonally protected.
Scheme 16.
2.5 Ester-enolate Claisen Rearrangement of α-Vinyl-α-acyloxysilanes
The ester-enolate Claisen rearrangement is a powerful method for the enantio- and diastereoselective C–C bond formation from an original chiral ester. This method is characterized by the complete transfer of the masked chirality of the carbon attached to the acyloxy and silyl groups to the newly formed carbon center. Very recently it has been applied to an α-acyloxy-α-vinylsilane possessing a proline as the acyloxy group (Scheme 17).[54] The enolate generated from 66[55] underwent the Claisen rearrangement, through a chair-like transition state with a Z-enolate in the presence of HMPA, to give the α-substituted proline derivative 67 with transfer of the original chirality of the ester counterpart. The rearranged product provided access to chimeras of proline 69 and 72 after adequate transformations at the α-alkyl chain.
Scheme 17.
2.6 Chirality Transfer via Cyclic Ammonium Ylides
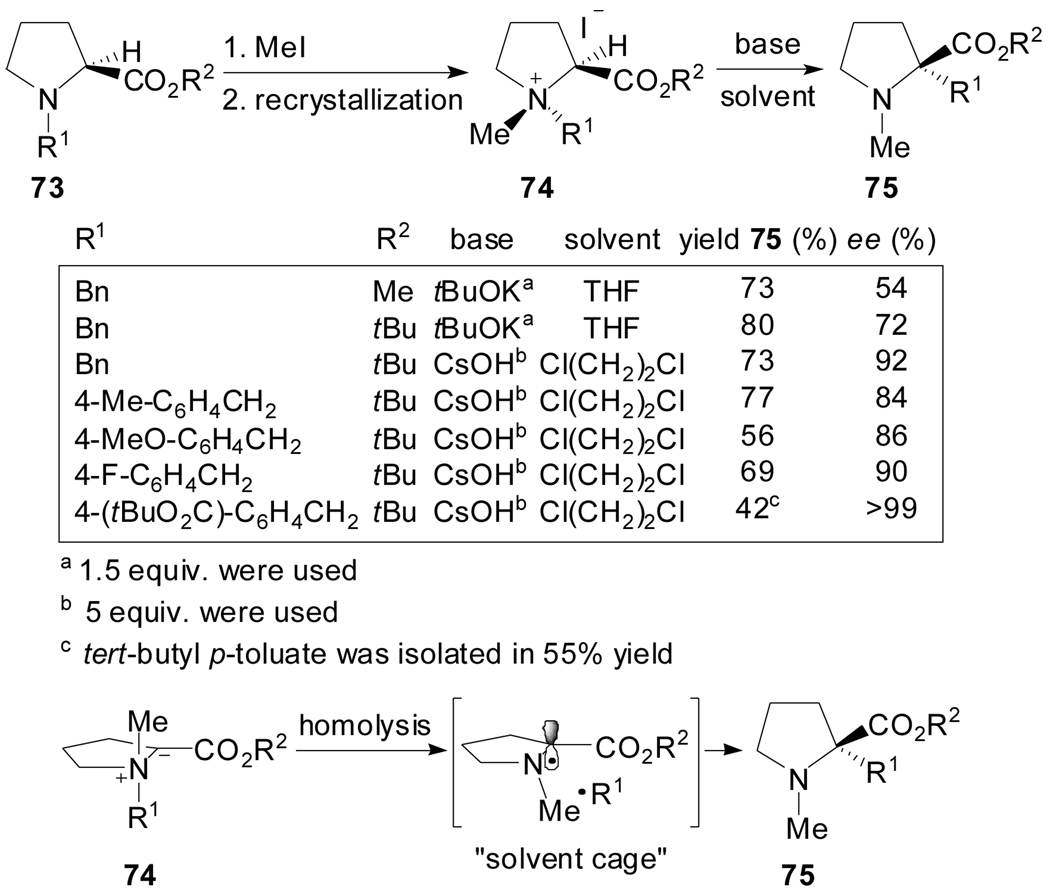
(S)-N-Benzylic proline-derived ammonium salts 74 have proven to undergo [1,2]-Stevens rearrangement[56] with a remarkably high level of the N-to-C chirality transmission, when carried out under solid-liquid biphasic conditions, to afford α-benzylic proline derivatives 75 in high enantiopurities (Scheme 18).
Scheme 18.
This rearrangement is assumed to proceed via a radical cleavage-recombination mechanism. Tayama et al. suggested that the stability of the benzylic radicals involved and the solid-liquid biphasic reaction conditions employed determine the stereochemical course of the reaction. The recombination of the radical pair initially formed from the N-ylide 74 occurs more rapidly in a solvent cage and hence more preferentially in a retentive fashion.
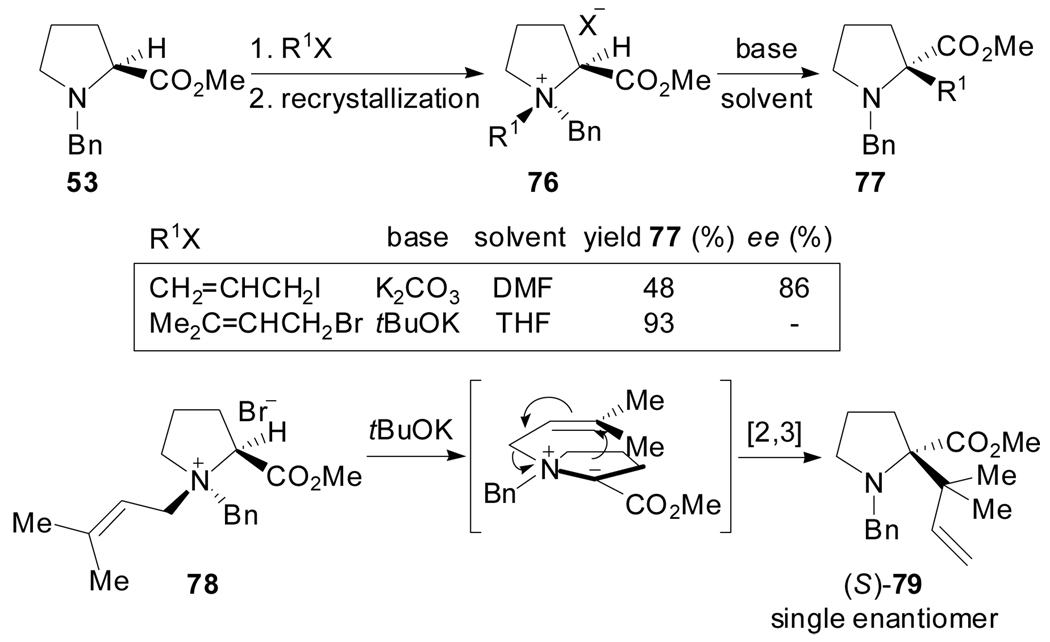
Exclusive [2,3]-shifts of an allyl and a prenyl group for substrate 76 had been earlier reported by West[57] and Coldham[58] respectively (Scheme 19). In this case, the rearrangements are stereospecific because the [2,3]-migrations are restricted to the same face, and the stereoselectivity arises from the previous N-alkylation step.
Scheme 19.
3. Syntheses with Formation of the Pyrrolidine Ring
3.1 [1,3]-Dipolar Cycloadditions: Azomethine Ylides
The 1,3-dipolar cycloaddition reaction of azomethine ylides and alkenes is a powerful method for the stereocontrolled synthesis of polysubstituted pyrrolidines and has been the subject of comprehensive accounts.[59] Hereinafter, we review the application of this methodology to the asymmetric synthesis of quaternary polysubstituted prolines, whose preparation has often been perfunctorily studied. The stereocontrol has been either achieved via a chiral auxiliary approach, based on the presence of a chiral moiety at the azomethine ylide or the dipolarophile, or alternatively employing chiral catalysts with achiral substrates. For this reason the revised approaches have been classified according to the nature of the dipolarophiles and ylides involved.
3.1.1 Chiral dipolarophiles
Grigg et al. reported complete regio- and endo-selective cycloadditions of several Ag(I) and Li(I)-metalated ylides onto (−)-menthyl acrylate (Scheme 20). The high diastereofacial selectivity in the formation of adducts 82 was explained assuming a transition state model where the isopropyl group of the menthyl moiety induces a facial shielding effect on the dipole.[60]
Scheme 20.
The racemic version of this type of procedure has recently found application for the synthesis of N-acyl polysubstituted proline inhibitors of the hepatitis virus RNA-dependent RNA polymerase, both in solution- and solid-phase. The resolution of the racemic quaternary proline derivatives was achieved by crystallization of diastereomeric salts or employing chromatographic techniques.[61]
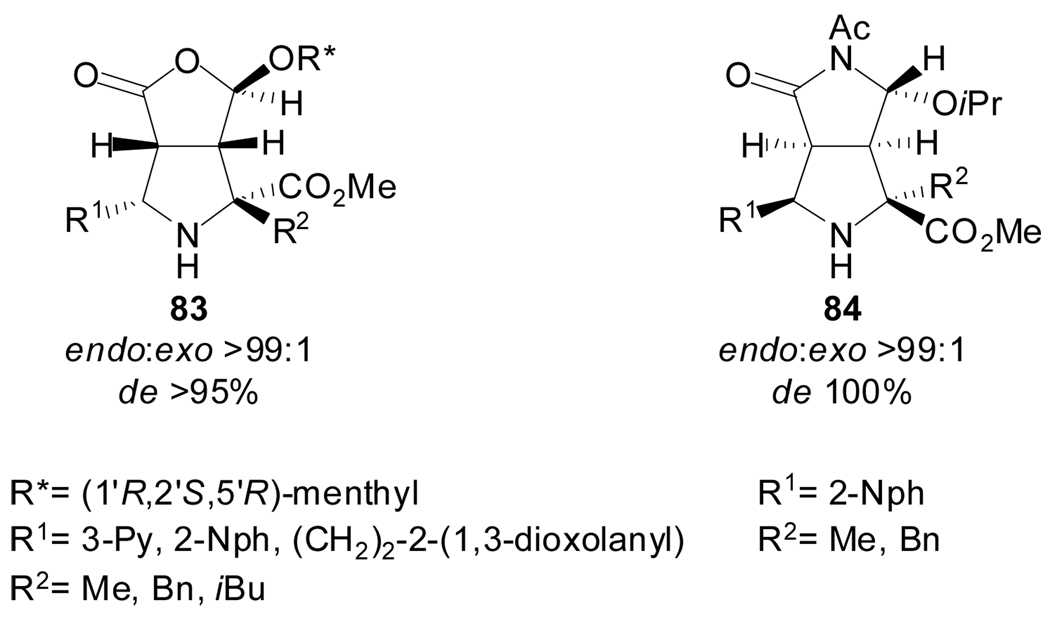
In addition, Grigg et al. reacted similar Ag(I)-metalated ylides generated both from aromatic or aliphatic imines of α-amino acids with chiral cyclic alkenes such as (5R)-(1’R,2’S,5’R-menthyloxy)-2(5H)-furanone or (5R)-N-acetyl-5-isopropoxy-2(5H)-pyrrolone. The resulting cycloadducts 83 and 84 were formed with complete regioselectivity and endo selectivity, and very high facial diastereoselectivities (Scheme 21).[62]
Scheme 21.
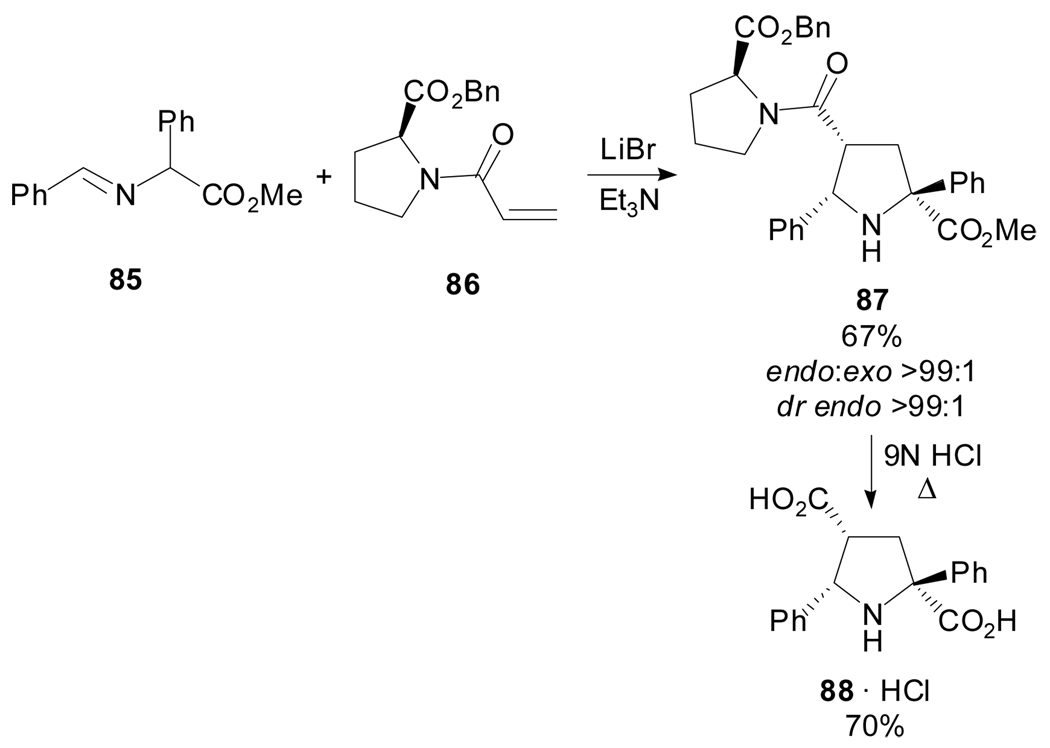
Another contribution to this type of transformation used N-acryloyl-(S)-proline esters as chiral dipolarophiles.[63] Metalated azomethine ylides, derived from aliphatic or aromatic aldehydes and aliphatic or aromatic α-amino acids, underwent highly endo-diastereoselective cycloadditions with the acrylamide of proline benzyl ester 86. An illustrative example is depicted in Scheme 22.
Scheme 22.
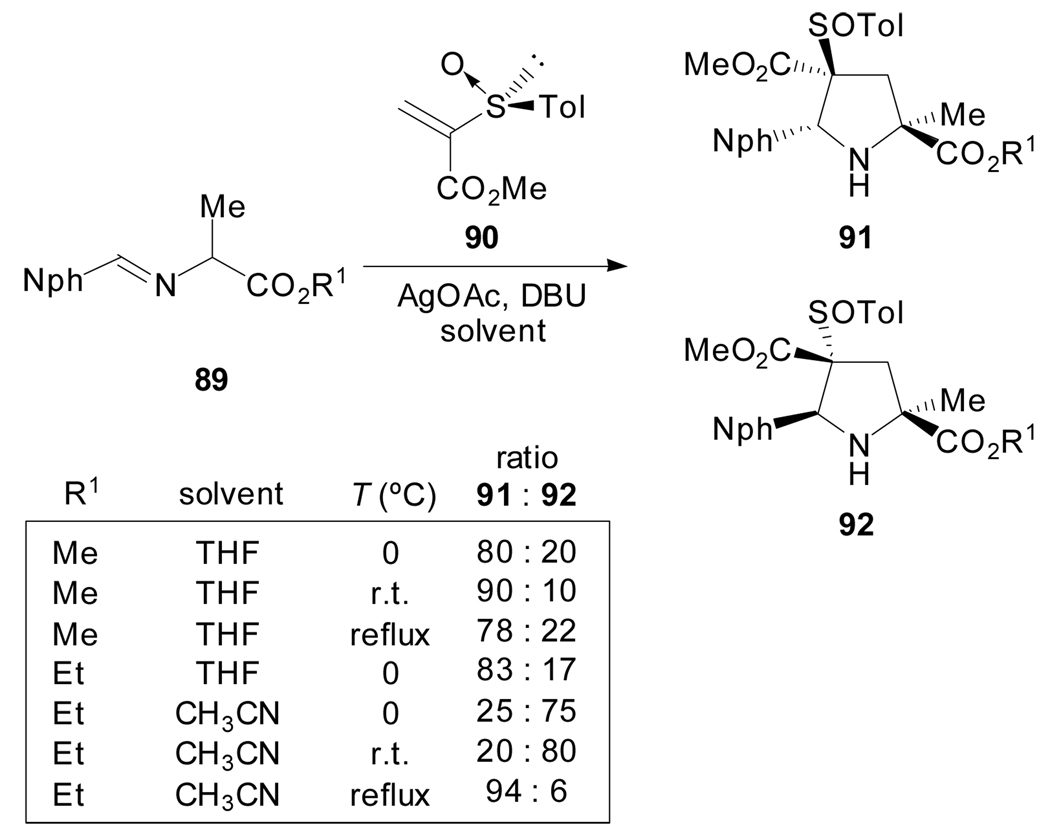
Similarly, the cycloadditions of azomethine ylides and chiral vinyl sulfoxide 90 have been reported to proceed with complete regio- and high endo- selectivity to produce 91 and 92 which are separable by column chromatography. Interestingly, the facial diastereoselectivity is, in some cases, strongly influenced by the nature of the solvent thus providing an opportunity to access prolines with trans or cis arrangements of substituents at the C2 and C5 positions (Scheme 23).[64]
Scheme 23.
Also, the [3+2] cycloaddition between the chiral E-nitroalkene 94 and the imine 93 provided 95 with complete stereocontrol (Scheme 24).[65] This polysubstituted proline belongs to a new family of inhibitors of α4β1-integrin-mediated hepatic melanoma metastasis.
Scheme 24.
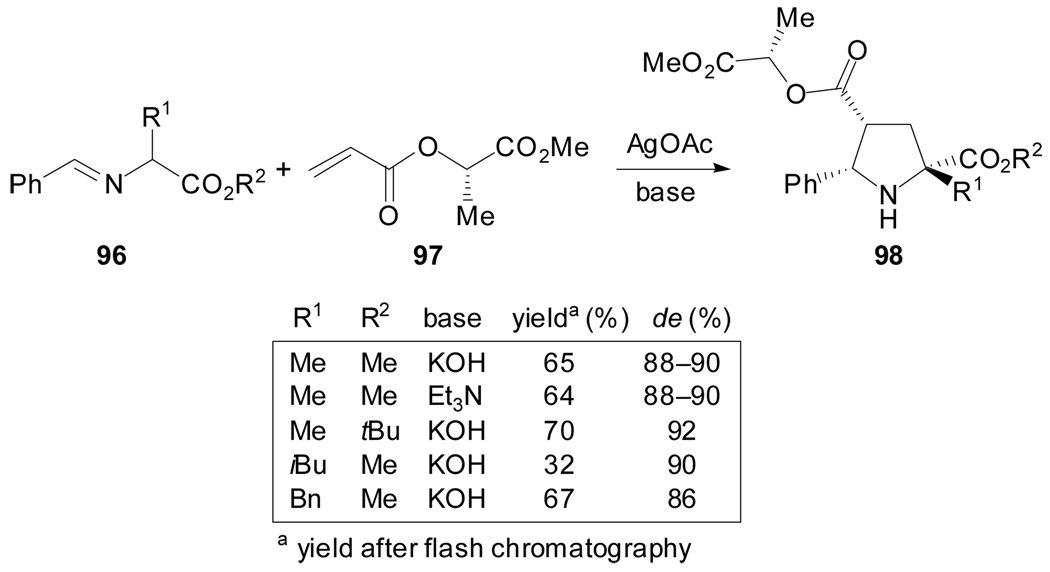
More recently, Nájera et al. reported the use of methyl (S)-lactate acrylate 97 as dipolarophile in the 1,3-dipolar cycloaddition reaction with silver azomethine ylides derived from benzaldimines of alanine, leucine and phenylalanine.[66] Enantiomerically enriched quaternary prolines resulting from the endo approach were obtained in moderate to good yields and de 86–92% (Scheme 25).
Scheme 25.
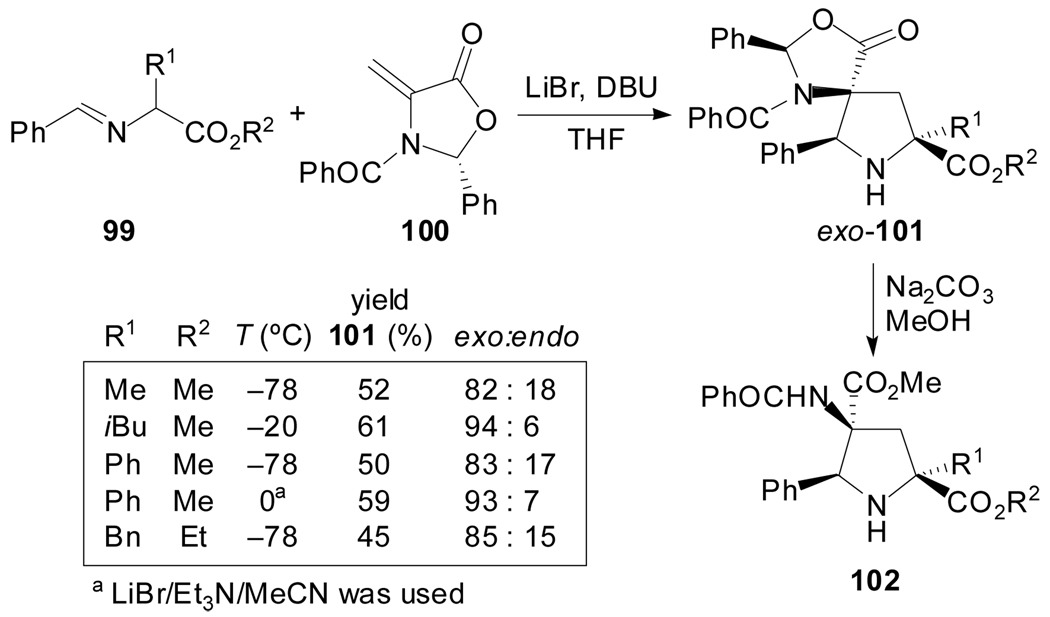
In contrast, chiral oxazolidinone 100 has been reported to undergo regioselective but exo-diastereoselective cycloaddition reactions with azomethine ylides derived from N-benzylidene α-amino acid esters 99 (Scheme 26).[67] The best diastereoselectivities were achieved working at low temperatures with LiBr/DBU/THF for the ylide generation. Some of the cycloaddition products were conveniently converted to polyfunctional prolines in high enantiomeric purity. The preference for exo-adducts was rationalized by assuming chelation between the lithium cation, the N-benzoyl carbonyl group and the ylide.
Scheme 26.
3.1.2 Chiral Azomethine Ylides
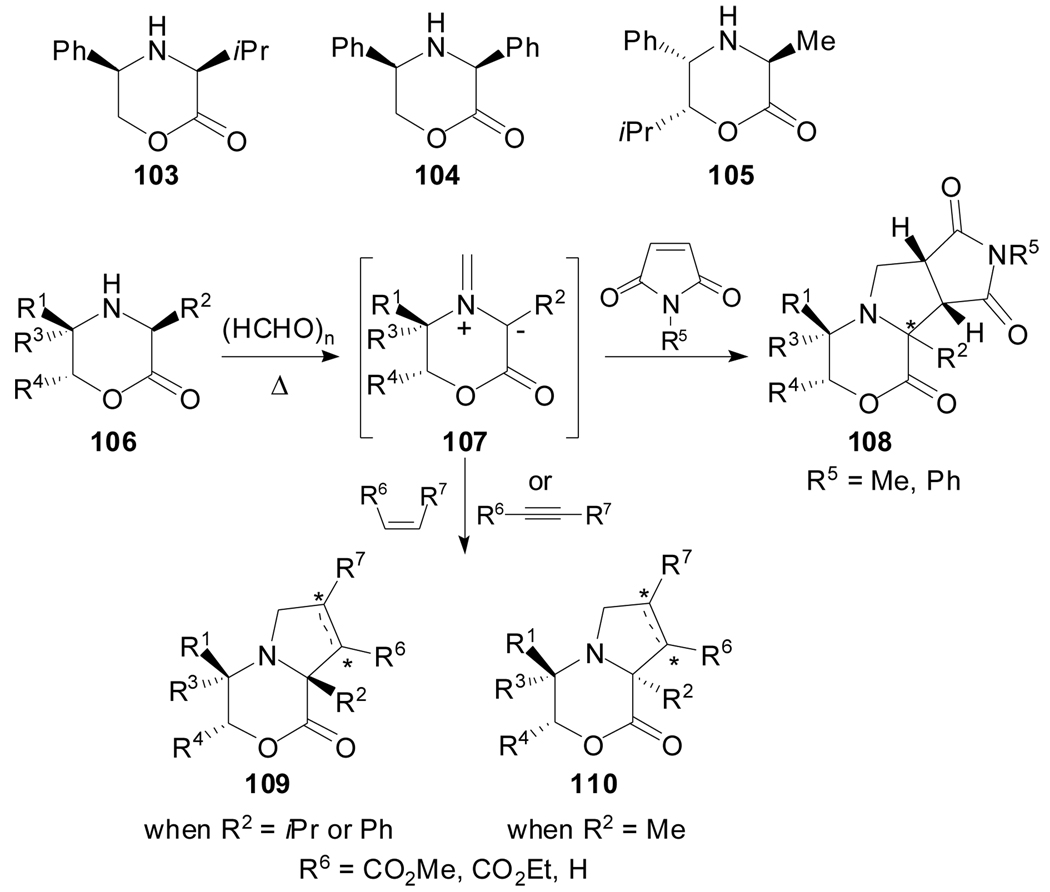
Saturated oxazin-2-ones 103,[68] 104[69] and 105[70] proved useful systems for the generation of chiral carboxy-stabilized azomethine ylides of type 107 under thermal conditions (Scheme 27). These species reacted with dipolarophiles bearing electron-withdrawing groups mainly with endo selectivity (high endo:exo ratios for compounds of type 109 and 110, but lower ratios for adducts of type 108). The removal of the morpholin-2-one templates has led in various occasions to α-alkyl or α-phenylproline derivatives. Formally, it is suggested that the α-chirality of the amino acid is “memorized” through a sequence involving chirality transfer to the cyclic template, followed by azomethine ylide generation and enantiospecific cycloaddition to regenerate the center lost during the ylide formation.
Scheme 27.
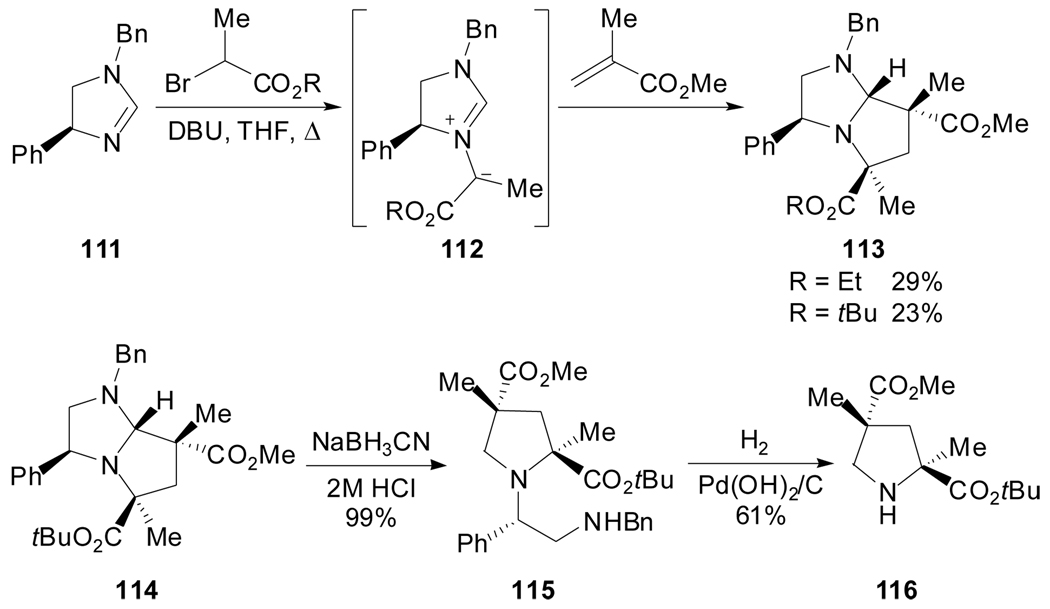
Similarly, homochiral (4R)-phenylimidazolinium ylides 112, wherein the auxiliary is conformationally restrained by virtue of a heterocyclic ring, underwent endo-selective cycloadditions to various dipolarophiles (Scheme 28).[71]
Scheme 28.
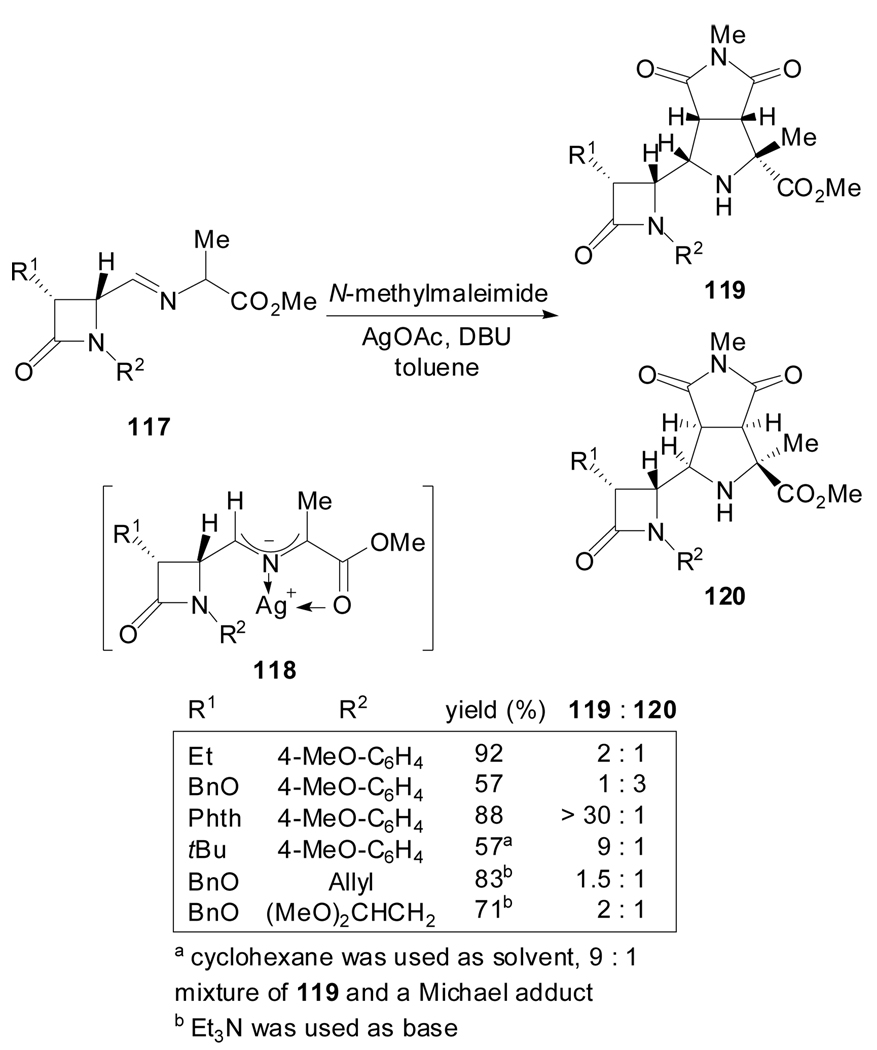
Grigg et al. described the use of N-metalated azomethine ylides containing a β-lactam ring as the chiral auxiliary[72] in cycloaddion reactions to N-methylmaleimide (Scheme 29). The variation of the substituents on the cis-β-lactam ring determined the ratio of the cycloadducts 119 and 120. The formation of only two diastereoisomers was attributed to endo-specific cycloadditions on both faces of the E,E-(syn)-dipole 118.
Scheme 29.
In addition, closely related ylides derived from 121[73] gave similar results on their reaction with α,β-unsaturated esters, and have found straightforward applications towards the synthesis of enantiopure pyrrolizidine, indolizidinone, diazatriquinane and fused tricyclic 2-azetidinone systems (Scheme 30).
Scheme 30.
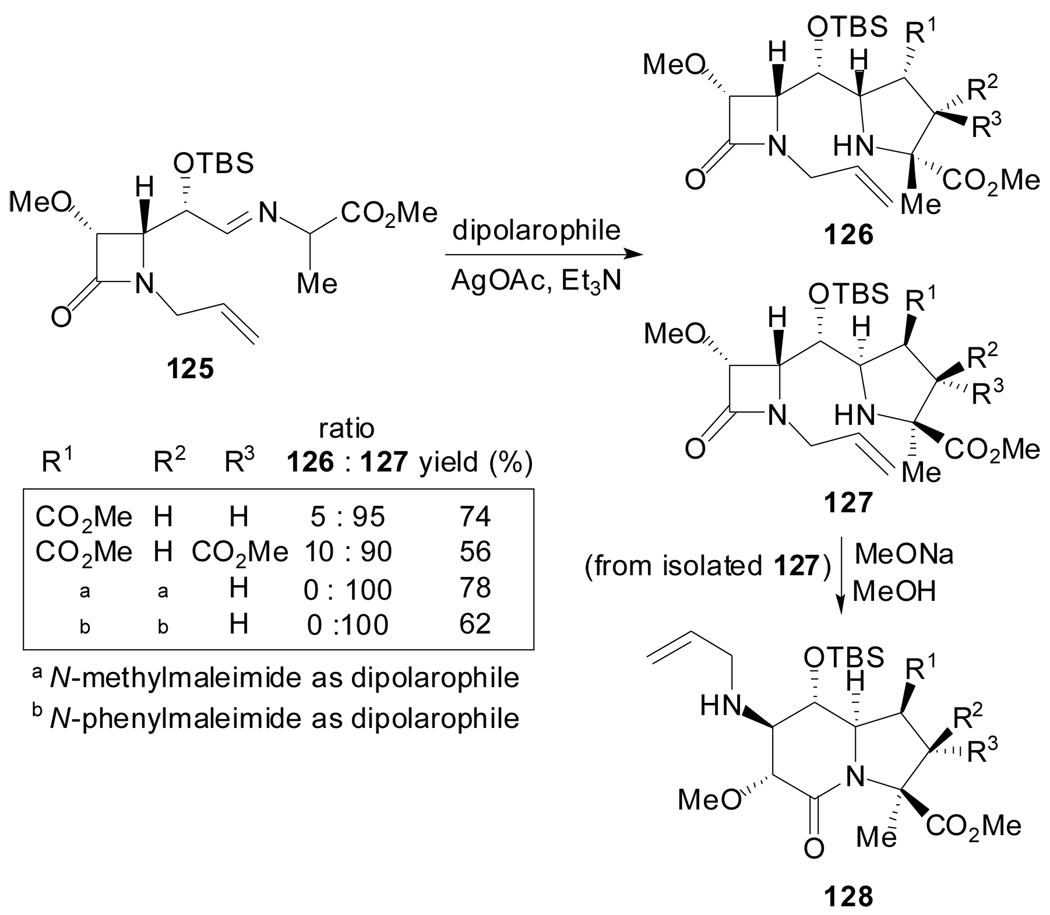
Alternatively, the intermolecular 1,3-dipolar cycloaddition reaction involving chiral ylides derived from 125,[74] provided access to indolizidinone aminoesters (Scheme 31).
Scheme 31.
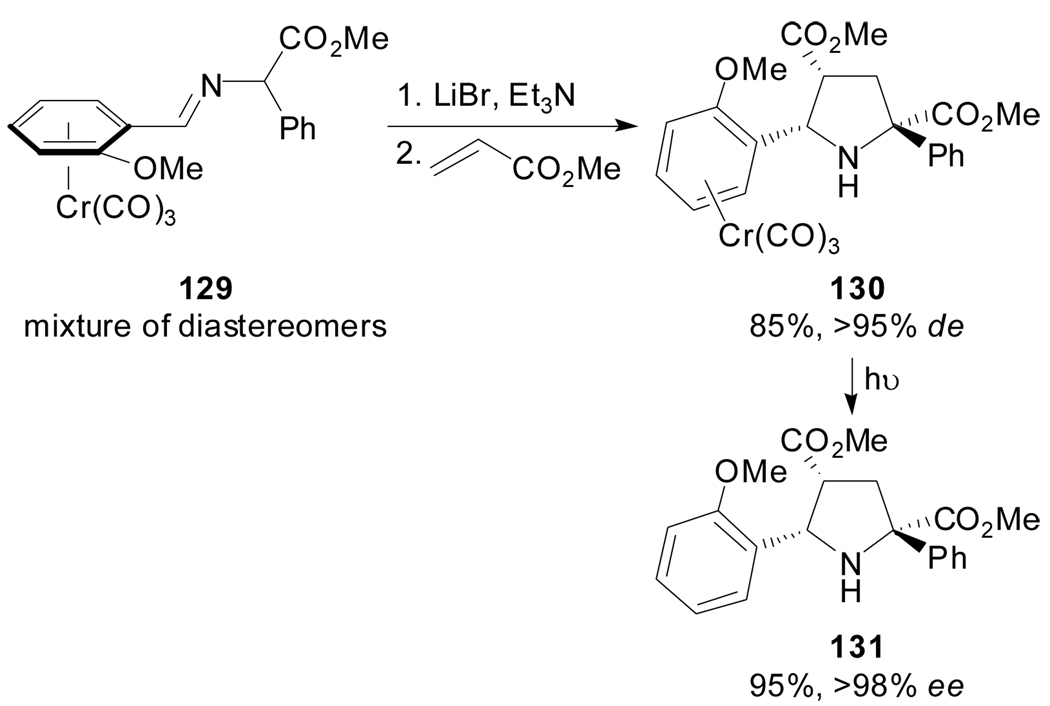
Another contribution to this type of stereocontrol used the N-lithiated azomethine ylide derived from 129 which is linked to a planar chiral arene Cr(CO)3 complex. Its cycloaddition reaction with methyl acrylate proceeded in a high diastereoselective fashion.[75] The authors rationalized the exclusive syn and endo selectivity by assuming chelation between the lithium, the imine nitrogen and the carbonyl oxygen, thus rendering the face opposite to the chromium tri-carbonyl fragment of the azomethine ylide as the one to approach the dipolarophile (Scheme 32).
Scheme 32.
3.2 [1,3]-Dipolar Cycloadditions: Nitrone Enolate Ylides
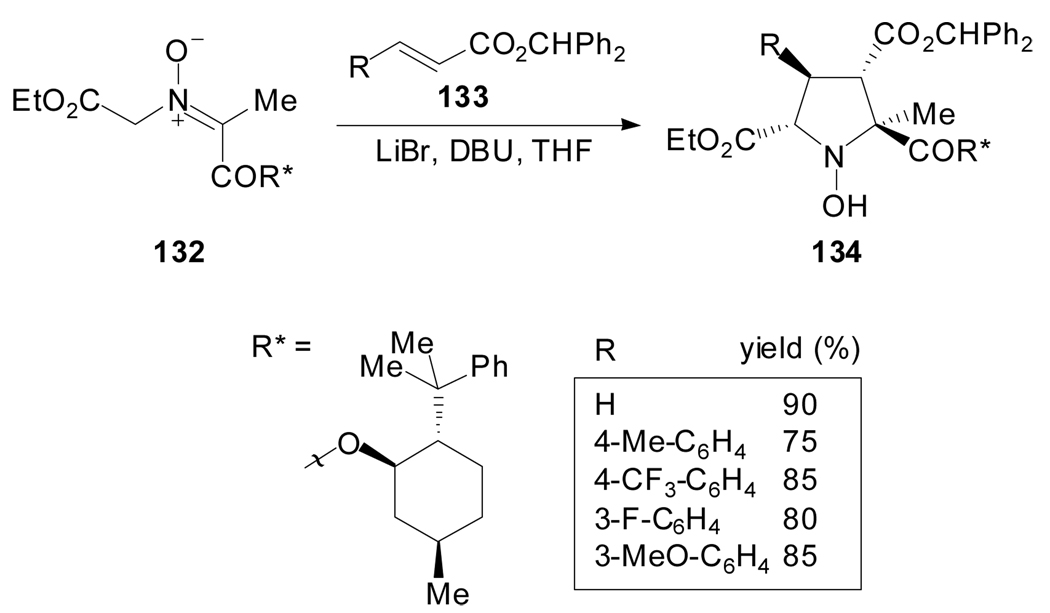
The stereoselective cycloaddition of chiral non-racemic nitrone enolate-ylides derived from glycinate esters with electron deficient alkenes affords N-hydroxy pyrrolidines which upon reduction provide polysubstituted prolines (Scheme 33). Hanessian et al. reported that, in particular, the cycloaddition of 8-phenylmenthyl ester nitrones 132 and benzhydryl cinnamates is completely stereoselective and provides N-hydroxy pyrrolidines 134 as single isomers.[76] The bulky esters on the dipole and dipolarophile appear to have a cooperative effect that ensures the high diastereoselectivity of the reaction. They also pointed out that, contrary to cyclizations of azomethine ylides, the reaction of nitrones 132 requires the presence of LiBr as an additive and no reaction takes place when AgOAc, MgBr2 or CoCl2 are employed.
Scheme 33.
3.3 Asymmetric 1,3-Dipolar Cycloadditions with Chiral Catalyst
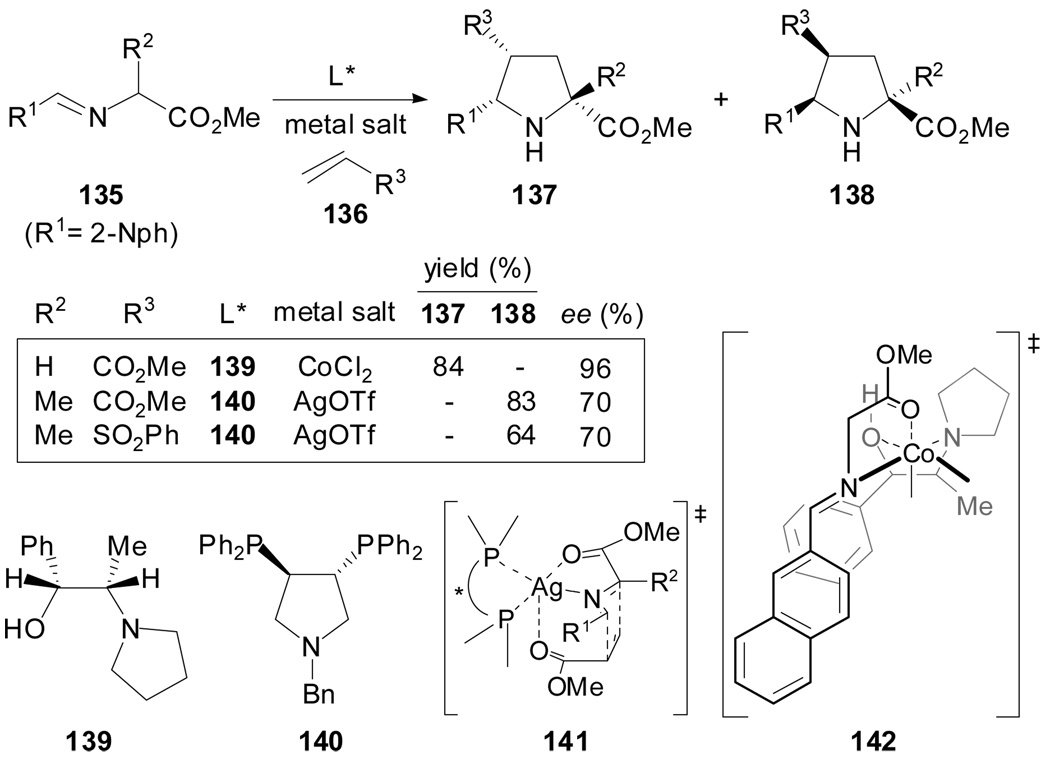
In recent years, extensive research has been devoted to achieve the catalytic asymmetric version of the 1,3-dipolar cycloaddition reactions of N-metallated azomethine ylides.[59] In 1995, Grigg et al. described that a stoichiometric amount of the catalytic system 139/CoCl2 in the reaction of the dipole generated from 135 with activated alkenes 136 gives an excellent ee of 137 or 138 (Scheme 34).[77] The total endo-selectivity displayed was explained by the transition state model 142 that shows an effective shielding of one face of the putative N-metallated dipole. In comparison, the stoichiometric catalyst formed by bisphosphane 140/AgOTf admitted a wider range of dipolarophiles to afford the other enantiomer endo-138 albeit with lower enantioselection.[78] The still high enantiomeric excesses of the resulting 2-substituted prolines could be justified by the compact transition state 141.
Scheme 34.
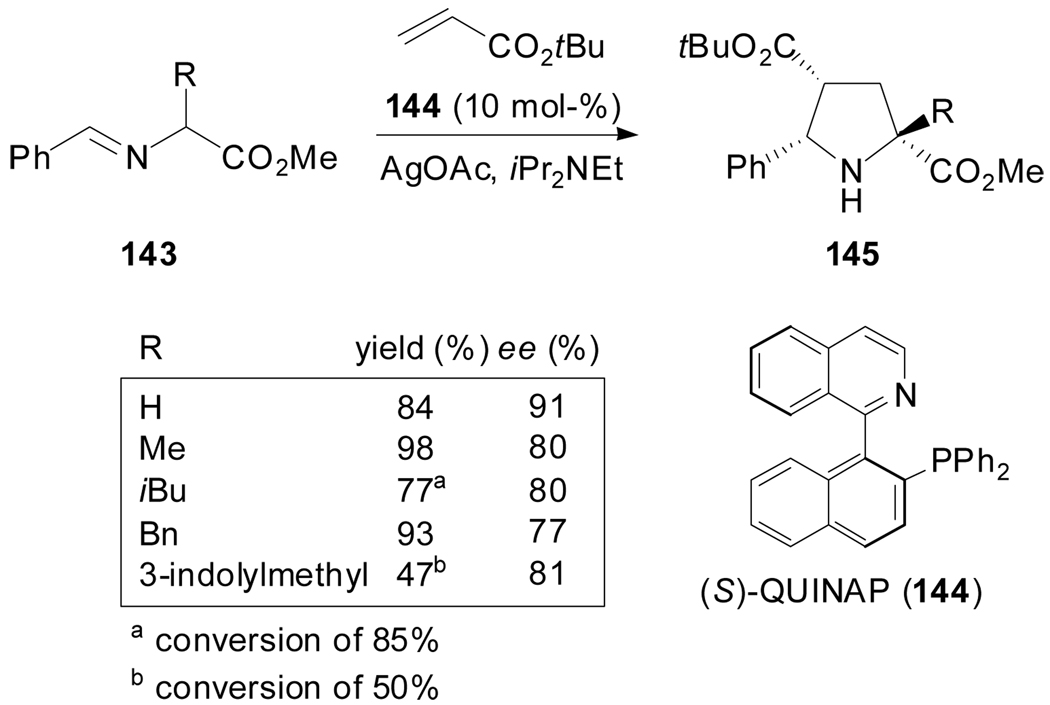
The first asymmetric catalytic 1,3-dipolar cycloaddition reaction involving a substoichiometric amount of a chiral metallic complex was described by Zhang et al. in 2002 and involved Ag(I) complexes with a variety of chiral diphosphane ligands.[79] Afterwards, Schreiber et al. performed studies on the Ag(I)-catalyzed enantioselective cycloadditions using substituted imine precursors other than glycinates,[80] applying as catalytic system AgOAc/(S)-QUINAP. Interestingly, the enantioselectivity (77–81% ee) was not significantly influenced by varying the α-substituent while the yields were very dependent on this moiety (Scheme 35).
Scheme 35.
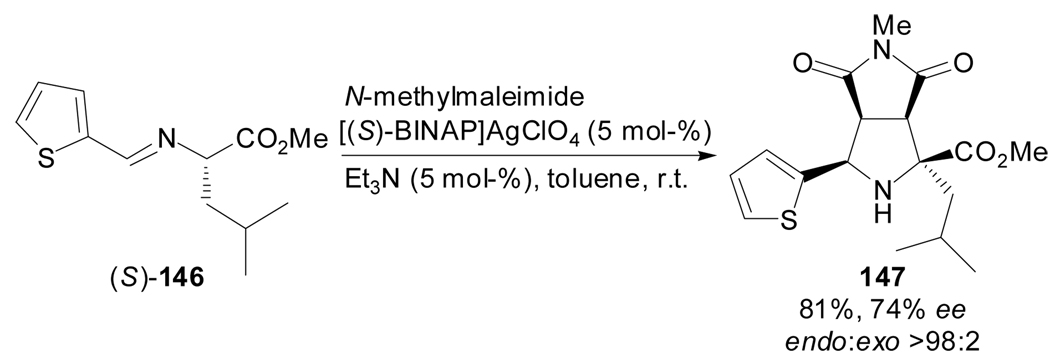
Very recently, the first enantioselective 1,3-dipolar cycloaddition reaction of amino acid derived azomethine ylides and maleimides catalyzed by stable and recyclable chiral (R)- or (S)-BINAP/AgClO4 complexes has been reported by Nájera et al.[81] The methodology, where the catalyst is recovered by simple filtration, was applied to the preparation of few quaternary polysubstituted prolines which were obtained with high endo diastereoselectivities and good enantioselectivities (Scheme 36).
Scheme 36.
Carretero et al. reported a copper-mediated catalytic system that displayed a good performance in the enantioselective 1,3-dipolar cycloaddition of azomethine ylides derived from 148 with N-phenylmaleimide.[82] The high reactivity of the combination of CuClO4 with the planar chiral P,S-ligand Fesulphos allowed a significant reduction of catalyst loading and afforded the quaternary endo-adduct as the unique reaction product (Scheme 37). This is an atypical behavior since it had been reported that for this transformation the use of Cu(II)/P,P-ligands affords high exo-selectivities and enantioselectivities.
Scheme 37.
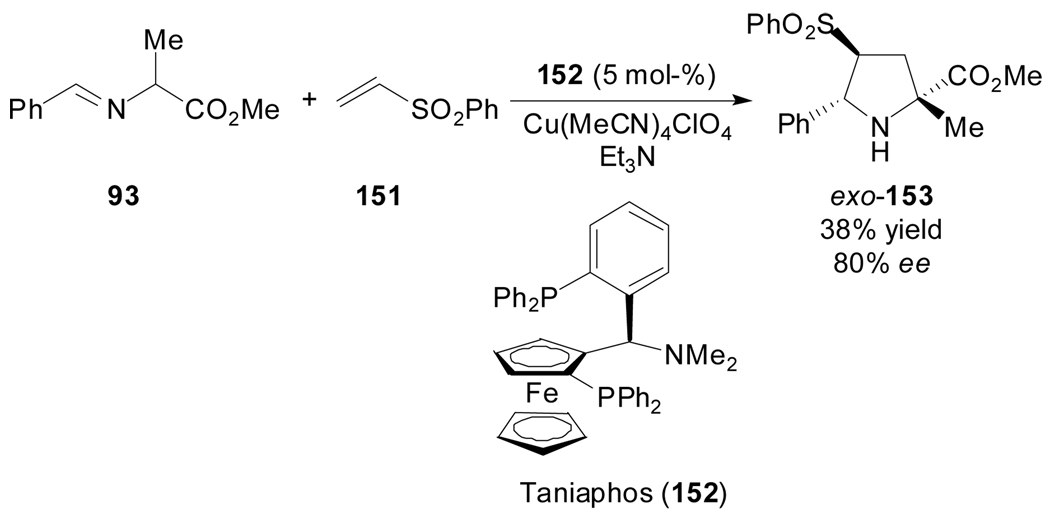
A similar copper-mediated cycloaddition employing a vinylic sulfone as dipolarophile was later reported by the same group. In this case, the combination Cu(I)-Taniaphos was optimal to achieve total exo-selectivity and good enantiocontrol (Scheme 38).[83] However, the low chemical yield reflected the sensitivity of the process to steric effects introduced by the α-substituent at the ylide.
Scheme 38.
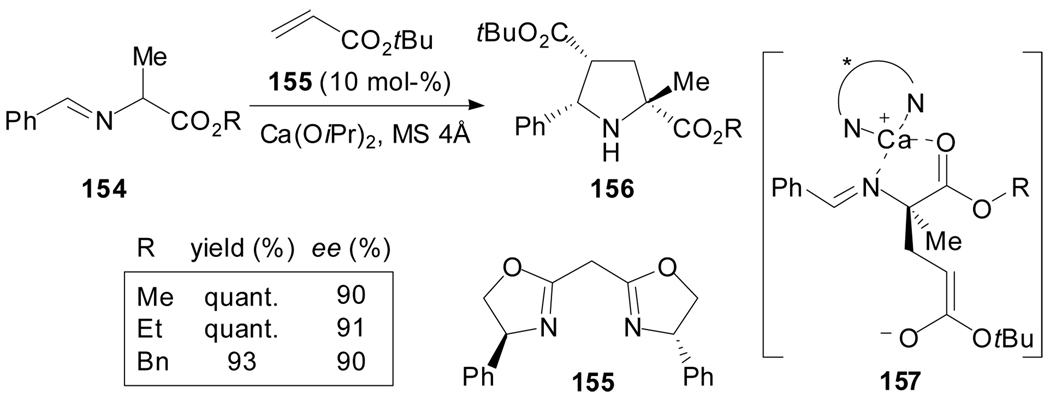
Recently, Kobayashi et al. reported the use of chiral calcium complexes as novel Brønsted base catalysts to effectively promote two types of asymmetric additions of α-amino acid derivatives with α,β-unsaturated carbonyl compounds: 1,4 additions and [2+3]-cycloaddition reactions (Scheme 39).[84] In particular, dl-alanine derivatives 154 reacted with several α,β-unsaturated carbonyl compounds to afford the corresponding proline derivatives with excellent yields and stereoselectivities. The authors proposed a plausible catalytic cycle which involves a monomeric Ca-Box complex that removes the α-proton of the derivative 154 to give a chiral calcium enolate which reacts with the α,β-unsaturated carbonyl compound to afford an initial Michael adduct 157. When the reactivity of the enolate in this adduct is high, an intramolecular cyclization occurs to afford exclusively a proline derivative.
Scheme 39.
S3.4 Intramolecular Cyclizations of Chiral Amino Acid Derivatives
3.4.1 Cyclization via C–N Bond Formation
Some of the methodologies developed for the asymmetric synthesis of α-alkylprolines are based on the construction of the pyrrolidine ring by intramolecular cyclization of a geminally disubstituted glycine equivalent with an appropriate leaving group on the side chain. In the last 20 years several types of reagents for electrophilic alkylations have been developed. Among them, bis-lactim ethers, oxazolidines, morpholinones and oxazinones have been advantageously applied.
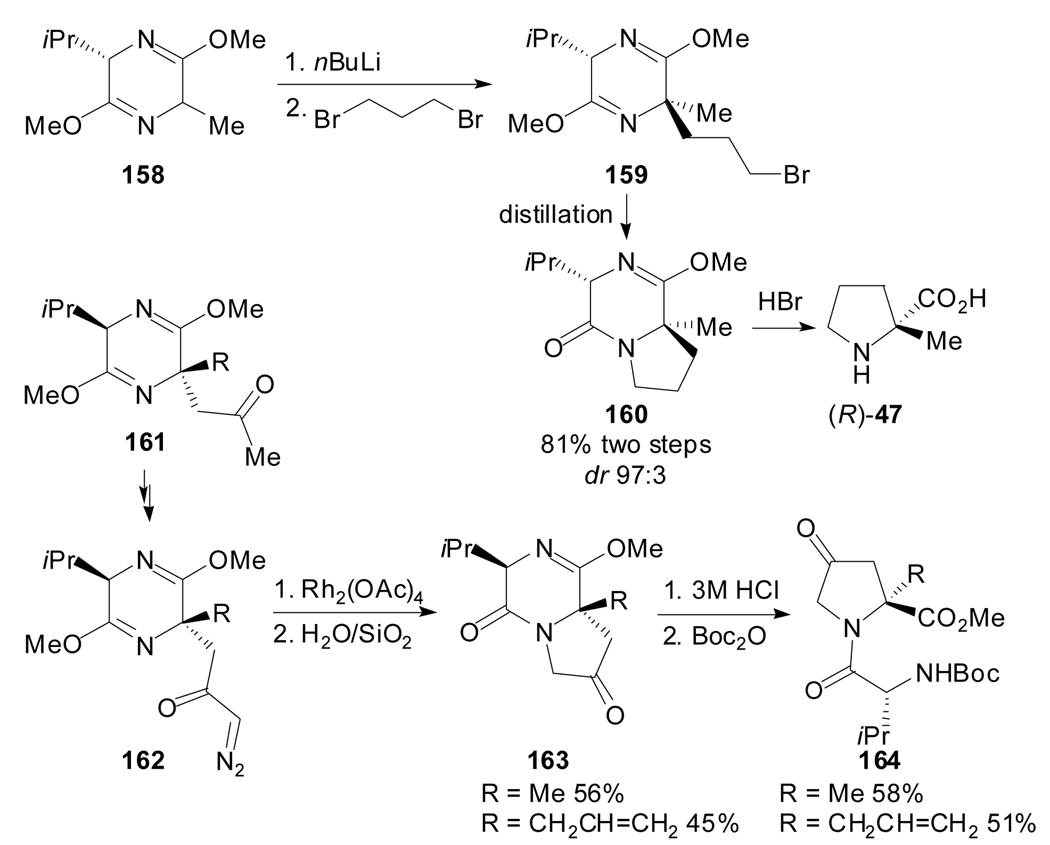
In 1987, Schöllkopf’s methodology, initially developed for the synthesis of acyclic quaternary amino acids, was applied to the synthesis of α-alkylproline derivatives using suitable dielectrophiles. Thus, the commercially available bis-lactim ether of cyclo-(L-Val-Ala) 158 was alkylated with 1,3-dibromopropane to provide the corresponding dialkylated bis-lactim ether 159, which upon cyclization by heating afforded the (R)-2-methylproline precursor 160 (Scheme 40).[85] Later, Undheim et al. showed an alternative annulation procedure by rhodium(II)-catalysed carbenoid insertions in diazoketone substrates 162.[86] The insertion reaction occurred with complete chemoselectivity at the adjacent annular nitrogen in preference to C–C double bond additions or C–H insertions, and no racemization was observed neither in the carbenoid reaction nor the subsequent hydrolysis.
Scheme 40.
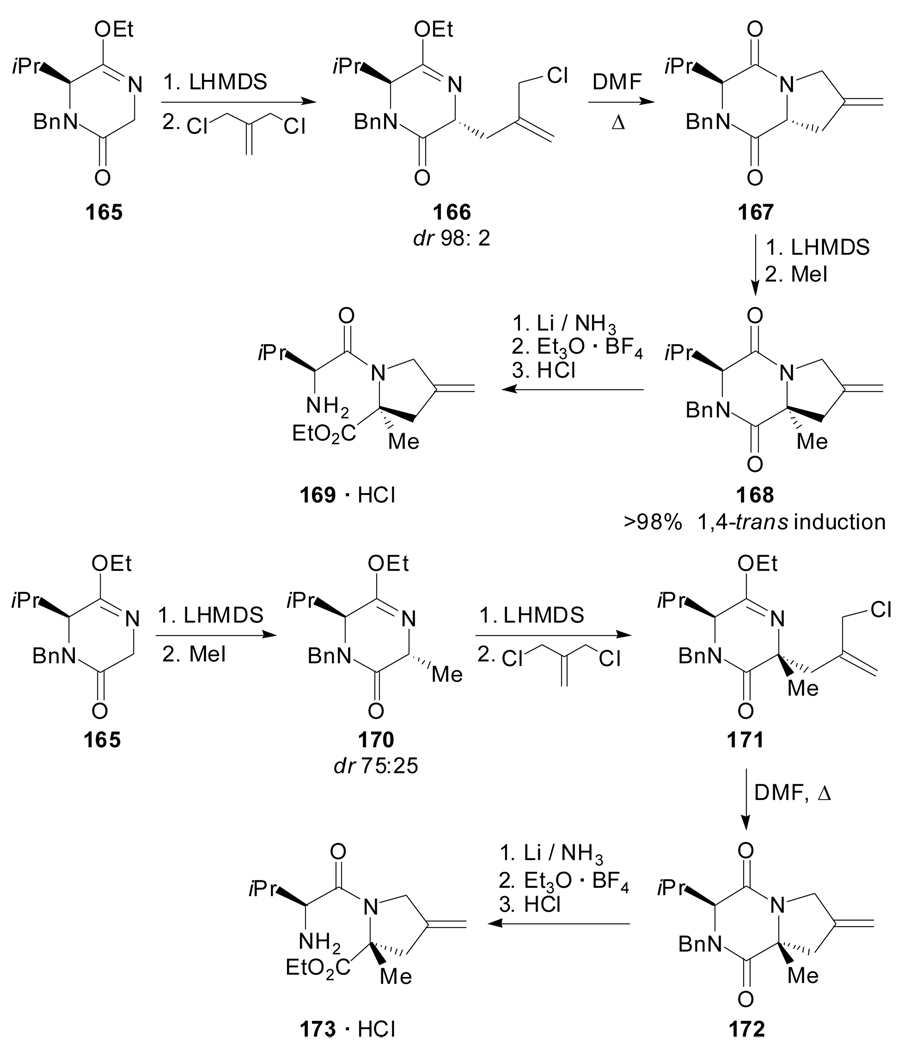
More recently, Porzi and Sandri described the use of the mono-lactim ether 165, derived from l-valine, as the chiral synthon for stereoselective alkylations.[87] The approach allowed the preparation of pseudo-peptides 169 and 173, which only differ in the configuration of one stereogenic center. This was accomplished on the basis of the trans-induction in the alkylation reaction of the chiral synthon 165, and changing the sequence of the electrophiles employed (Scheme 41).
Scheme 41.
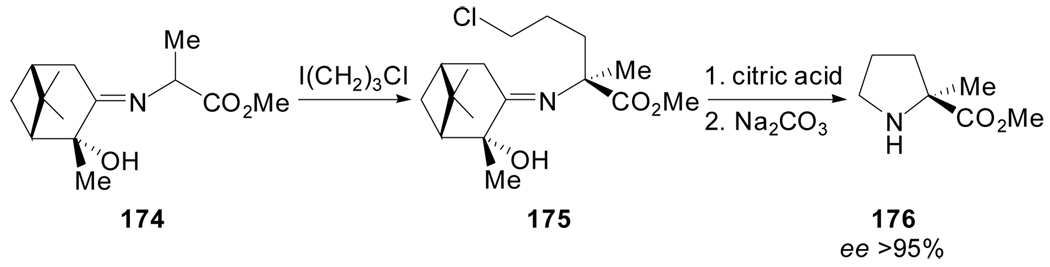
Viallefont et al. described the synthesis of (S)-α-methylproline methyl ester 176 using a similar strategy that takes on the highly diastereoselective alkylation of a Schiff base derived from alanine and enantiomerically pure (2R,3R,5R)-2-hydroxypinan-3-one.[88] The treatment of 174 with 1-chloro-3-iodopropane, followed by hydrolytic cleavage of the auxiliary and cyclization afforded 176 in 95% ee (Scheme 42).
Scheme 42.
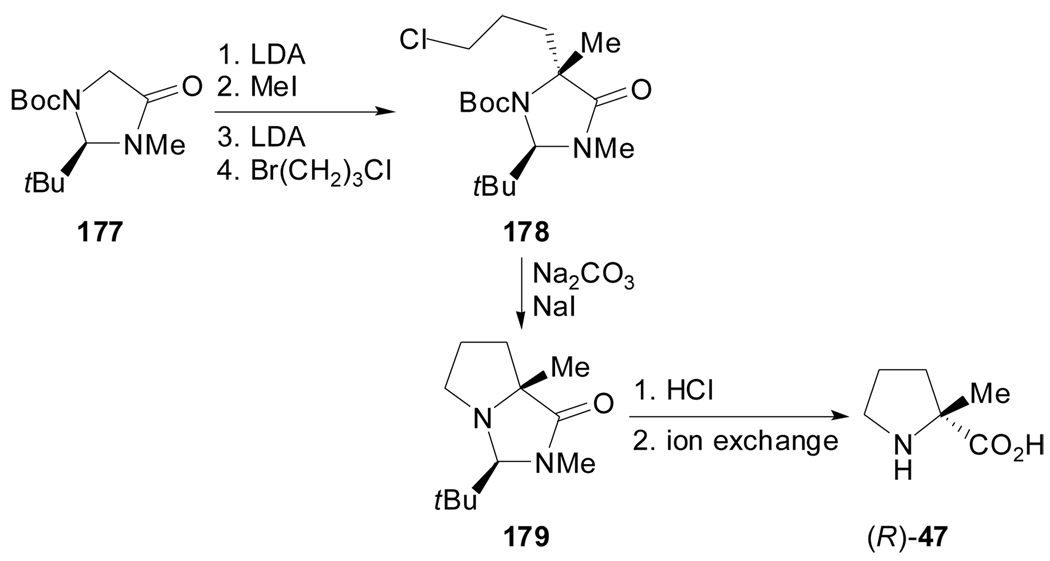
Alternatively, Seebach et al. reported the use of an imidazolidinone as the enantiomerically pure glycine derivative. The alkylation of tert-butyl (R)-2-tert-butyl-3-methyl-4-oxo-imidazolidinecarboxylate 177, first with methyl iodide and then with 1-bromo-3-chloropropane, afforded the intermediate 178 that provided (R)-2-methylproline after cyclization and hydrolysis (Scheme 43).[89]
Scheme 43.
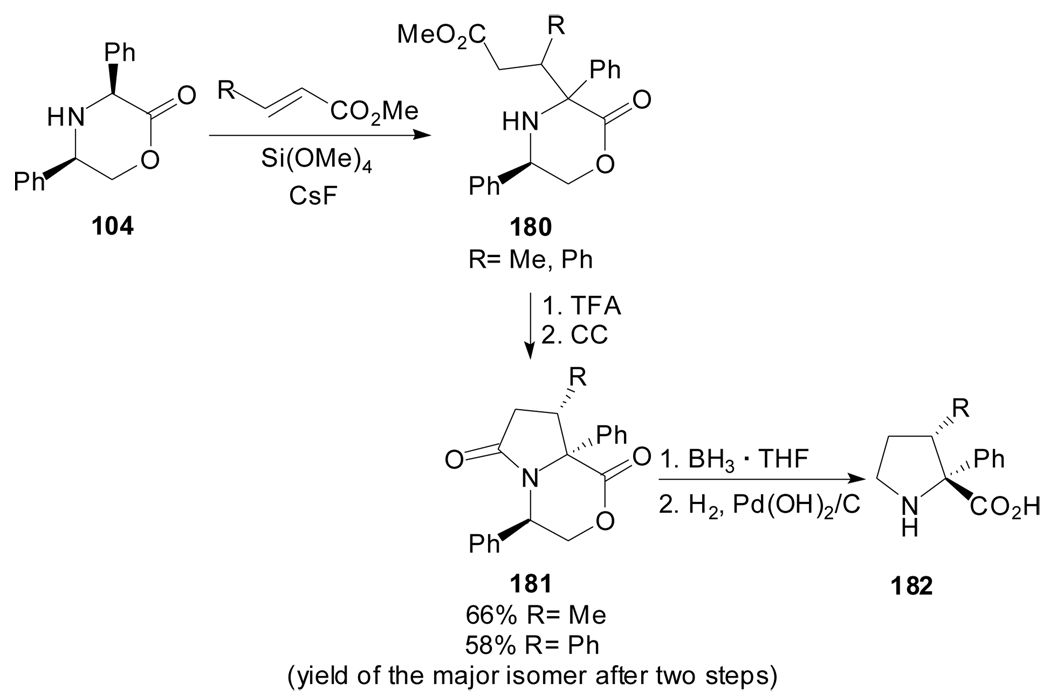
(R)-phenylglycinol-based morpholinones and oxazolidines have also served as chiral heterocyclic precursors for the construction of the pyrrolidine ring in a similar manner.[90,91] Harwood et al. reported that (3S,5R)-diphenylmorpholin-2-one 104 undergoes 1,4-addition reactions with acrylates to give a mixture of products from which the major compound can be isolated after lactamization.[90] Chemoselective reduction of the lactam ring and hydrogenolysis of the chiral auxiliary afforded enantiomerically pure 3-substituted 2-phenylprolines 182 (Scheme 44).
Scheme 44.
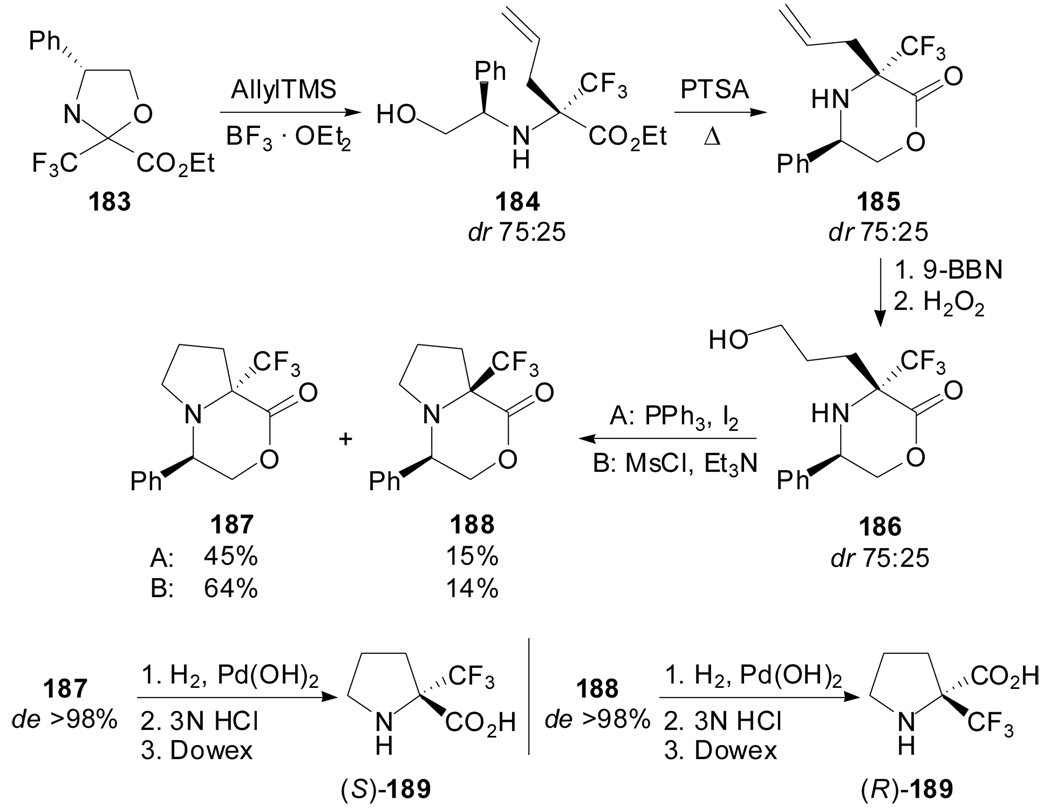
Brigaud et al. described the use of the (R)-phenylglycinol-based oxazolidine 183 as chiral intermediate for the introduction of a fluorinated group at the α-position of proline (Scheme 45).[91] The strategy involved the allylation reaction of the oxazolidine 183, initially obtained as a 75:25 diastereomeric mixture from ethyl trifluoropyruvate and (R)-phenylglycinol under PPTS catalysis. The resulting diastereoisomeric allylic aminoesters 184 were converted into the morpholinones 185, which were subjected to hydroboration and cyclization by means of iodine substitution or mesylate activation of the hydroxyl group. The two bicyclic diastereomers, which can be separated by column chromatograpy, furnished the corresponding enantiopure α-trifluoromethylprolines upon removal of the chiral auxiliary by hydrogenolysis.
Scheme 45.
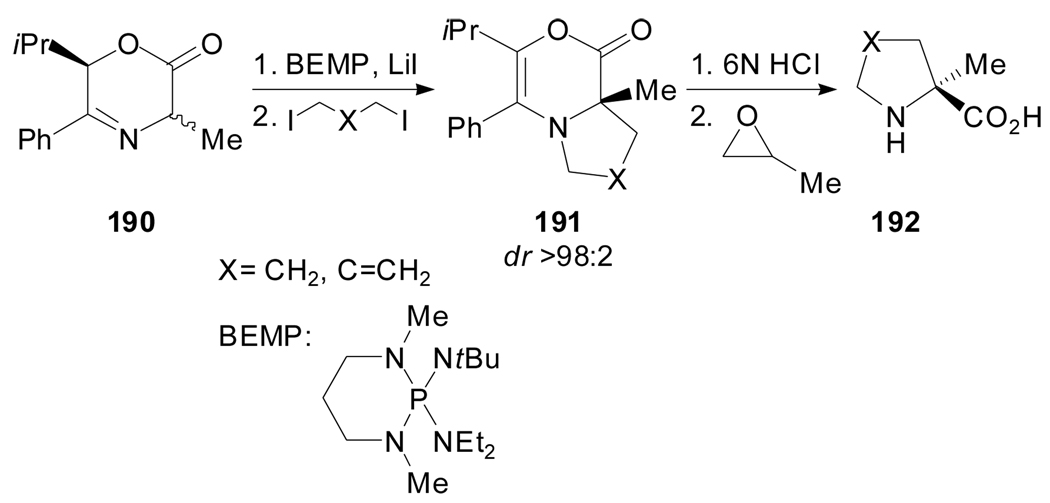
Nájera et al. proposed the use of oxazinone 190, an easily enolizable chiral alanine template with cyclic structure, for the alkylation with dielectrophiles (Scheme 46).[92] Chiral 3,6-dihydro-2H-1,4-oxazin-2-one 190 was diastereoselectively dialkylated with 1,3-diiodopropanes using 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,2,3-diazaphosphorine (BEMP) as a base in the presence of lithium iodide. Subsequent acid hydrolysis and treatment with propylene oxide to release the free amino acid afforded (S)-α-methylproline derivatives in 99% ee.
Scheme 46.
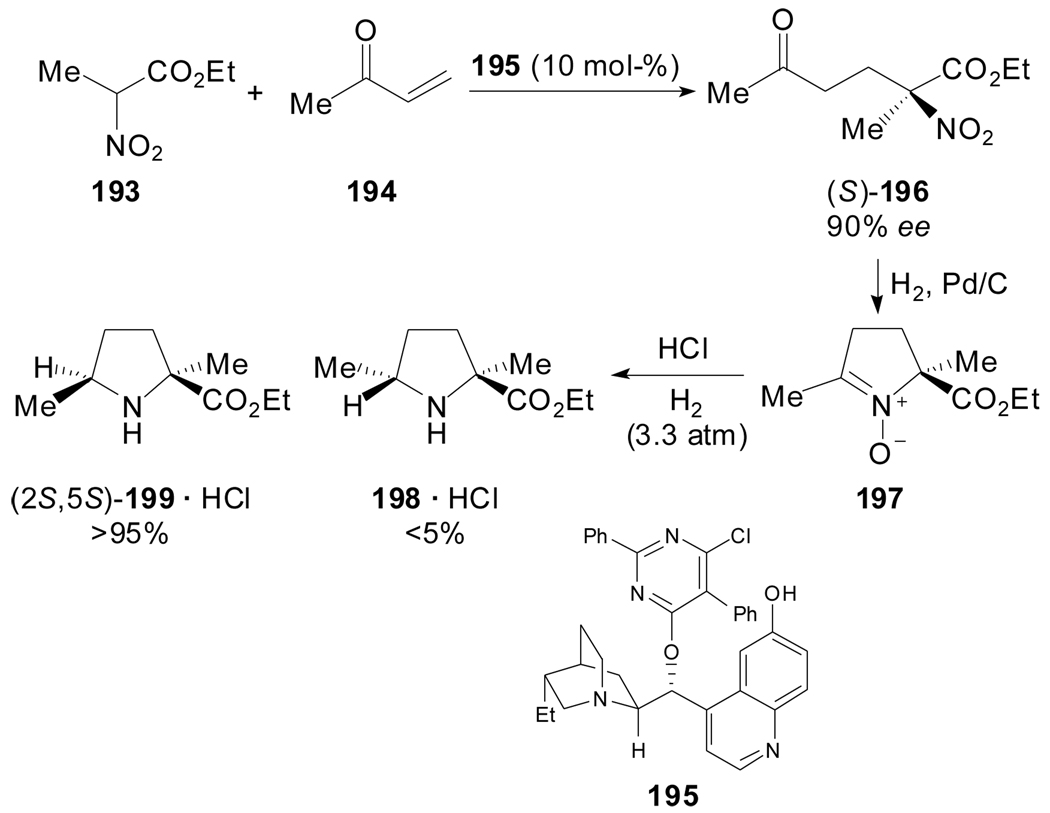
In 2006, Snider et al. reported the synthesis of (−)-cis-2,5-dimethylproline ethyl ester 199 as key intermediate towards the total synthesis of (+)-NP25302, a cell-cell adhesion inhibitor structurally related to the antitumoral Jenamidines A1/A2 (Scheme 47). The efficient and stereospecific preparation[93] of 199 started with the enantioselective Michael reaction of ethyl 2-nitropropionate 193 and methyl vinyl ketone 194 using the modified hydroquinone 195 as catalyst. The construction of the pyrrolidine ring was achieved by reductive cyclization of the chiral nitro ketone followed by hydrogenation of 197 in a one pot procedure. Hydrogenation of 196 (1 atm) over 10% Pd/C with Na2SO4 in EtOH gave the nitrone 197 which afforded the quaternary proline 199 (dr >20:1), when the hydrogenation was carried over at 3.3 atm, after addition of HCl.
Scheme 47.
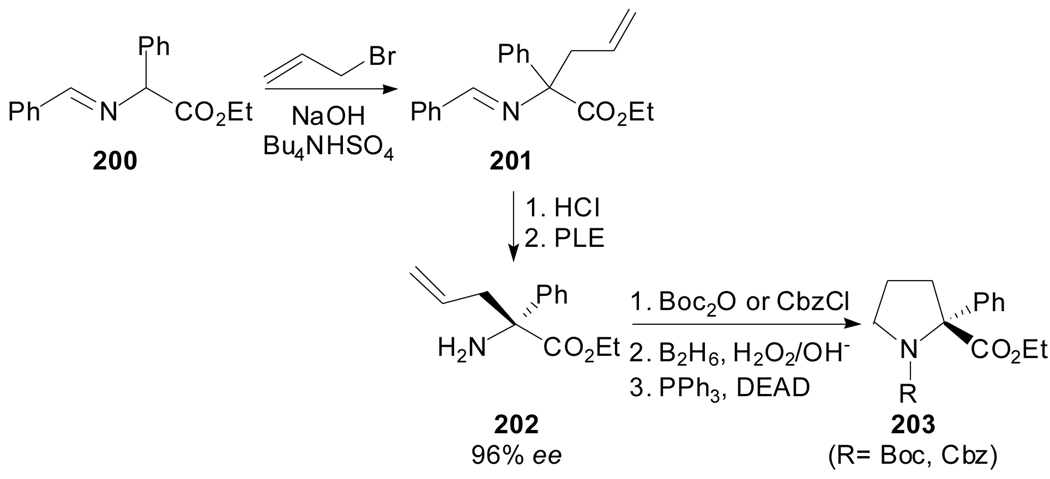
A convenient synthesis of protected (R)-α-phenylproline derivatives, based on the construction of the pyrrolidine ring via the Mitsunobu reaction was developed[94] by Tourwé et al. The N-benzylidene phenylglycine ethyl ester 200 was allylated under phase-transfer catalysis conditions, and the resulting product was enzymatically resolved using pig liver esterase (Scheme 48). Protection, hydroboration, oxidation and subsequent ring closure using the Mitsunobu protocol gave enantiomerically pure (R)-α-phenylproline.
Scheme 48.
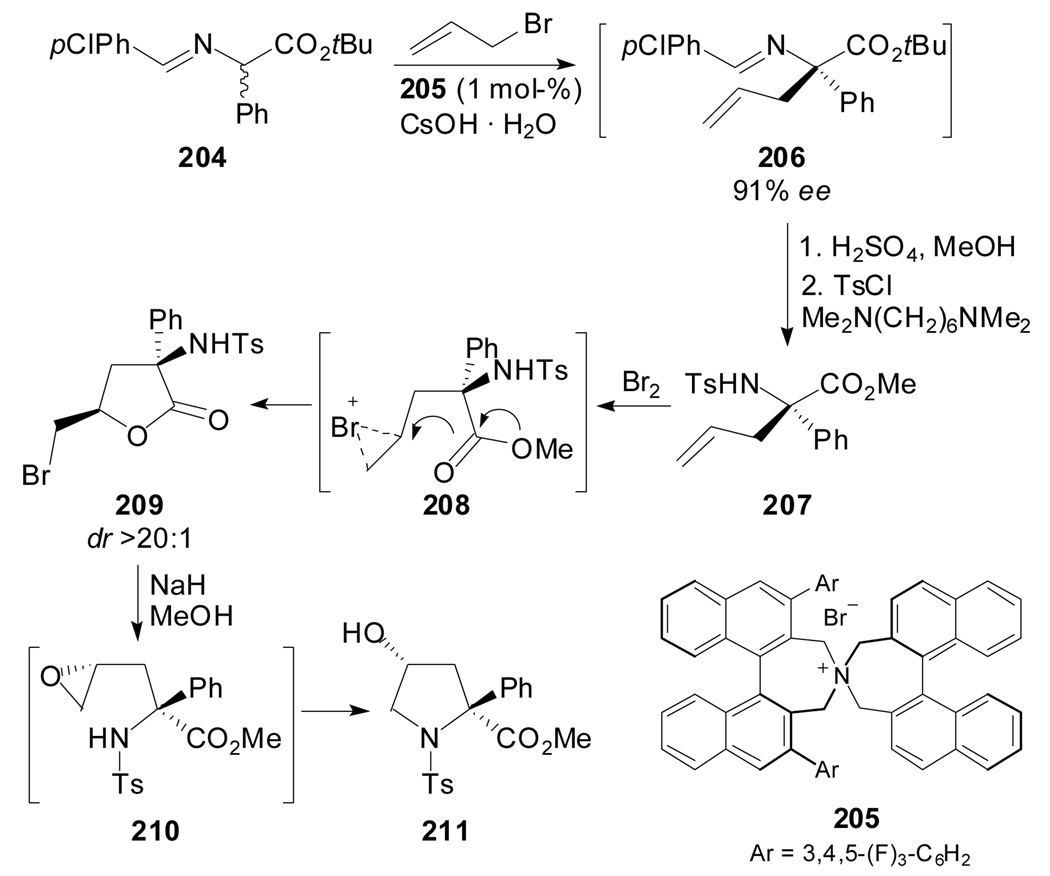
A highly stereoselective synthesis of (2S,4R)-N-tosyl-4-hydroxy-2-phenylproline methyl ester was achieved by developing a chiral synthesis of the 2-allyl-2-phenylglycine derivative 207 followed by a stereoselective bromine-mediated cyclization (Scheme 49).[95] Maeda et al. reported the generation of (S)-α-allyl phenylglycine methyl ester 207 by catalytic asymmetric alkylation of 204 in the presence of (S,S)-205 and subsequent hydrolysis, transesterification and tosylation of the aldimine intermediate. The bromolactonization of 207 afforded the γ-lactone 209 in high diastereoselectivity. A NaOMe-mediated opening of the γ-lactone ring, followed by an intramolecular attack of the tosylamide nitrogen on the epoxide terminus of 210 furnished the desired 4-hydroxy-2-phenylproline derivative.
Scheme 49.
3.4.2 Cyclization via C–C Bond Formation: Memory of Chirality
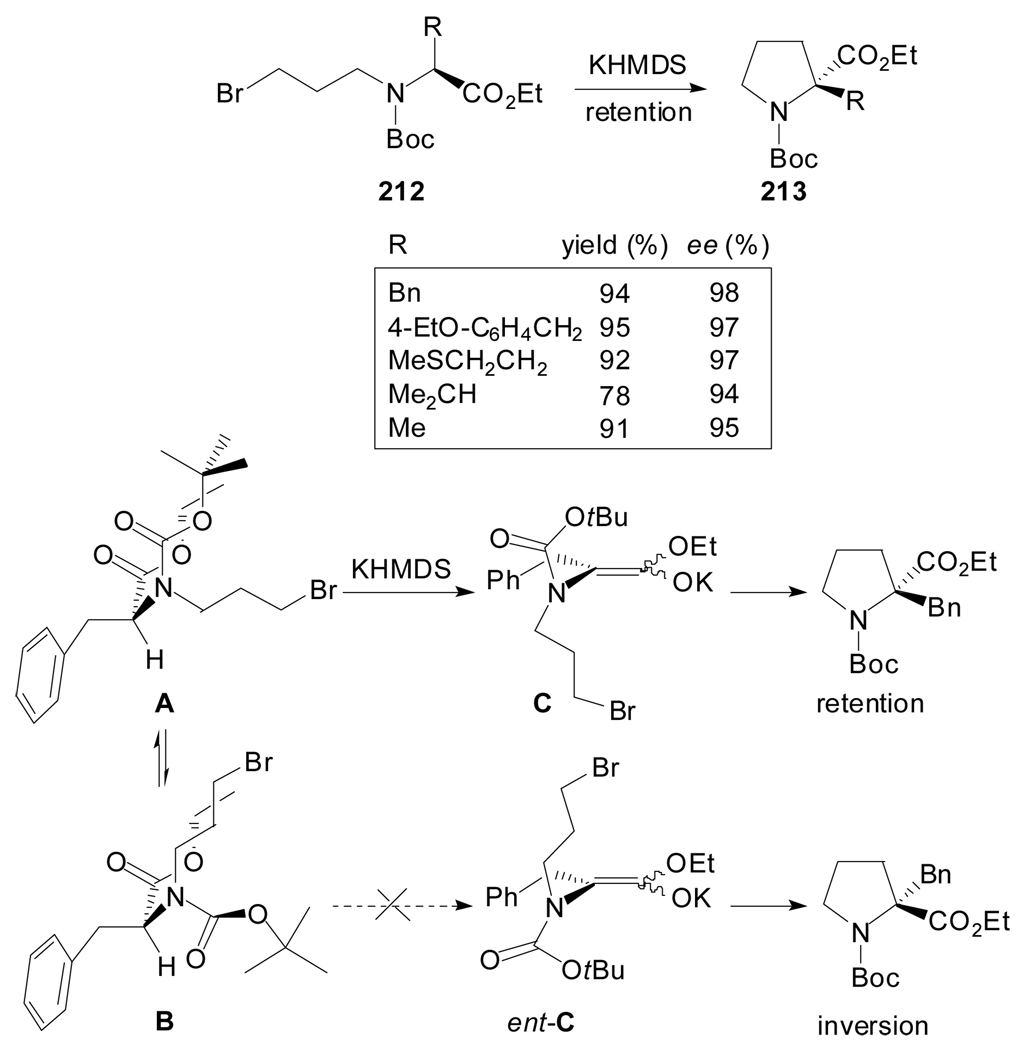
Kawabata et al. reported an efficient protocol that affords α-quaternary prolines in high enantiomeric purities by application of a memory of chirality strategy[96] which relies on a retentive deprotonation/alkylation procedure at the α-carbon of appropriate chiral amino esters (Scheme 50).[97] Thus, N-Boc-N-ω-bromoalkyl-α-amino acid derivatives were designed as substrates for this asymmetric intramolecular cyclizations. Their treatment with KHMDS in DMF at −60 °C gave diverse α-quaternary proline derivatives 213 of high enantiomeric purity. The key insights of this method are the preservation of the chirality of the starting amino ester in the form of transient conformational chirality of the reactive enolate intermediate and the high stereospecificity during the subsequent cyclization. The choice of the protecting group on the nitrogen has proven to be critical for the generation of the chiral non-racemic enolate intermediate.
Scheme 50.
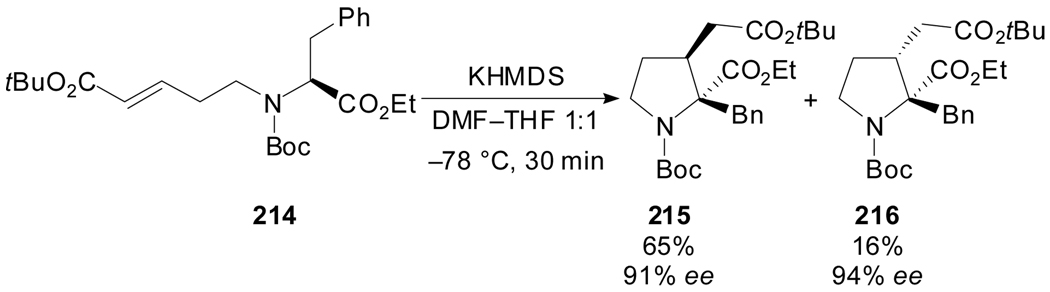
The asymmetric construction of α-quaternary prolines with contiguous tertiary stereocenters[98] was achieved by the intramolecular conjugate addition of an enolate with a chiral C–N axis according to the strategy illustrated in Scheme 51. The intramolecular cyclization, upon treatment with KHMDS, took place with retention of configuration at the α carbon and a relative trans-stereochemistry between the two ester groups.
Scheme 51.
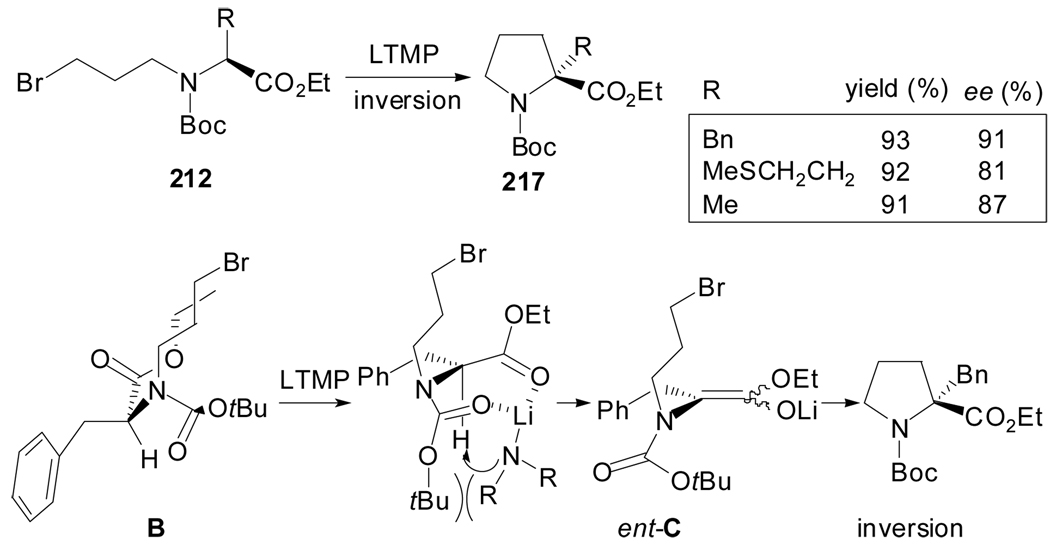
More recently, conditions for the enantiodivergent intramolecular cyclization of N-Boc-N-ω-bromoalkyl-α-amino acid derivatives 212 have been reported (Scheme 52).[99] Their treatment with lithium amide bases in poor coordinative solvents, like THF or toluene, enforces chelation of the carbonyl groups with the lithium cation prior deprotonation. The transient conformational chirality of the resulting reactive enolate derives, upon cyclization, in α-quaternary prolines with inversion of configuration.
Scheme 52.
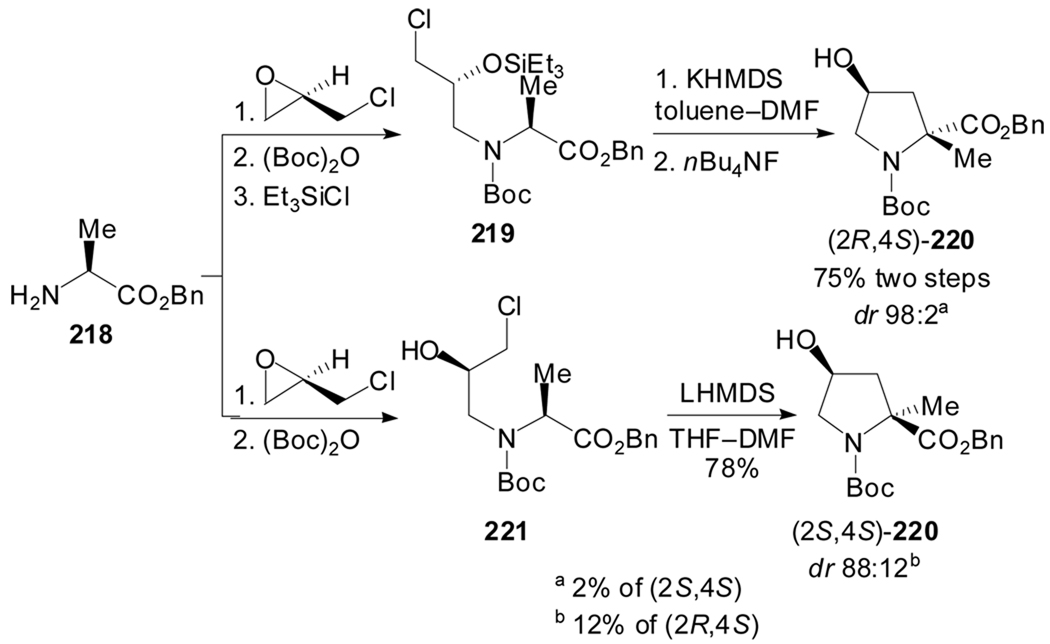
Kolaczkowski et al. applied this methodology to the stereoselective synthesis of 4-hydroxy-α-methylprolines.[100] Their approach builds on the observation that epichlorhydrin reacts stereospecifically with carboxy-protected alanine to give the corresponding chiral chlorhydrins. Installation of protecting groups in the (2S,4R)-chlorhydrin, and cyclization upon standard memory of chirality methodology (KHMDS, DMF, −60 °C) led to the selective formation of the trans 4-hydroxy-α-methylproline (Scheme 53). Interestingly, the omission of the hydroxyl protecting group in the (2S,4R)-chlorhydrin led to the cis diastereoisomer upon deprotonation with LHMDS.
Scheme 53.
In 1999, a remarkable example of a photochemical chiral-memory effect furnishing α-methylproline derivatives was described by Giese et al.[101] The stereospecific singlet Norrish-Yang photocyclization of the l-alanine derivative 222 in the presence of the triplet quencher naphthalene yielded isomer 224 with the cis configuration as the major product (92% ee). The attacked sp3-hybridized chiral center in 222 is transferred into the product 224 with retention, although it had been converted into a prochiral sp2-hybridized radical center in the diradical intermediate 223 (Scheme 54). Ab initio calculations suggest that the conformational freedom of the diradical intermediate is restrained with a specific hydrogen-bond network involving three centers.[102]
Scheme 54.
3.4.3 Cyclization via C–C Bond Formation: Ring Closing Metathesis
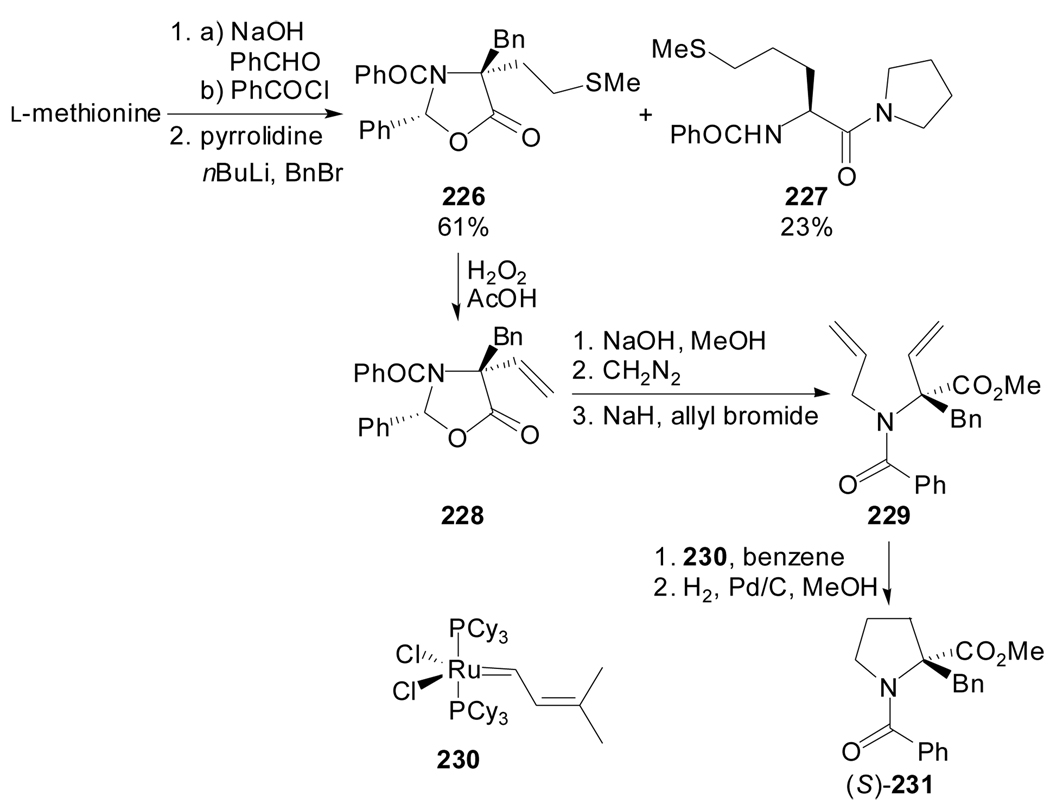
The synthesis of a phenylalanine mimic constrained in a proline-like conformation by ring closing metathesis was described by Abell et al. in 2000 (Scheme 55).[103] They envisaged the synthesis of α-benzylproline 231 by metathesis reaction of diene 229, itself prepared by α-benzylation of a l-methionine derived oxazolidinone, followed by elimination, ring hydrolysis and N-allylation reaction. Besides the Grubbs’ RCM chemistry, the synthetic sequence utilises Seebach’s oxazolidinone chemistry to introduce the α-benzyl group with control of the absolute configuration and a methionine side chain to provide a masked vinyl group.
Scheme 55.
4. Resolution procedures
In 1977, Overberger and Jon reported the resolution of 2-methylproline.[104] The l-enantiomer was obtained from the fractional crystallization of the quinine salt of (−)-N-carbobenzoxy-2-methylproline and the d-enantiomer from the collected filtrates. The indirect and direct analytical separation of enantiomers of α-substituted proline analogues has been studied by high-performance liquid chromatography. In this sense, the chiral derivatizating agent (S)-N-(4-nitrophenoxycarbonyl)phenylalanine methoxyethyl ester has been successfully applied for the indirect analytical HPLC resolution of enantiomers of α-alkyl, α-allyl and α-benzylproline analogues.[105] Direct HPLC methods which rely on the use of a d-penicillamine based chiral ligand exchange column,[106] or a quinine-derived chiral anion-exchanger stationary phase[107] have also been reported by Péter et al. More recently, Zhao and Pritts developed a direct HPLC method using a commercially available polysaccharide type of chiral stationary phase (Chiralpak AD-H) and conducted the analytical separation of Boc-2-methylproline, Boc-2-methylproline benzyl ester and Boc-2-methyl-4-hydroxyproline benzyl ester with baseline or near baseline resolution under optimized conditions.[108]
5. Miscellaneous
5.1 Ruthenium Catalyzed Oxidation
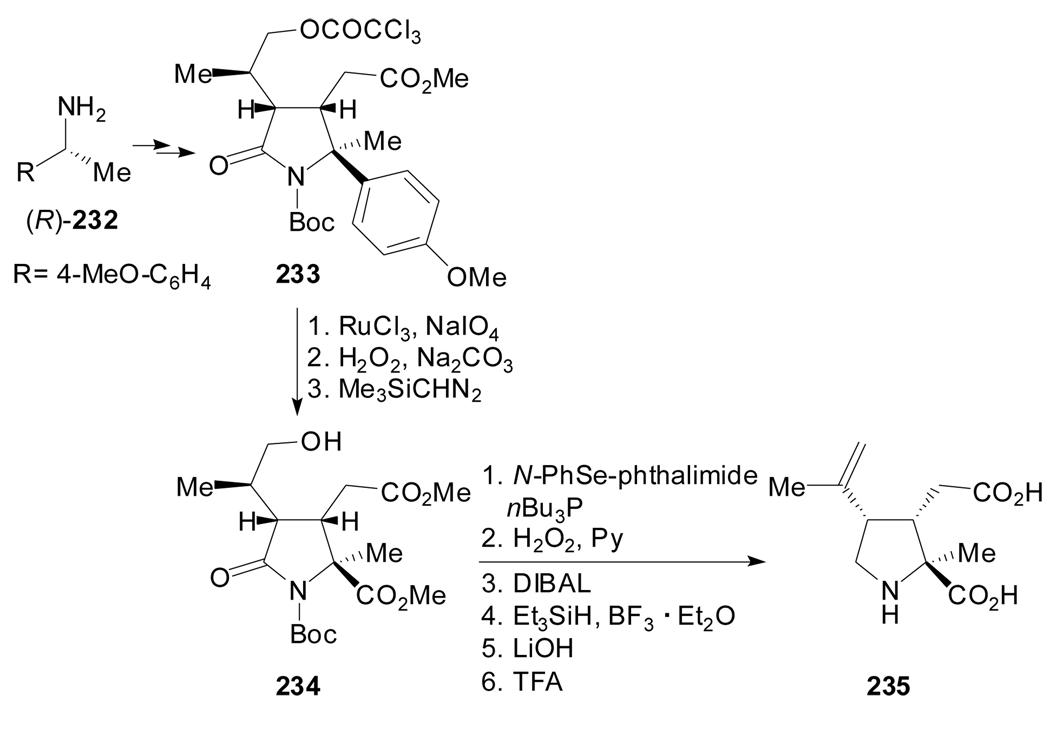
Clayden et al. described the asymmetric synthesis of the α-methyl analogue of (−)-kainic acid 235via a procedure that involves the oxidative degradation of 233 as the way to introduce the carboxyl substituent at the α carbon of 234 (Scheme 56).[109] Despite the susceptibility of the p-methoxyphenyl group to oxidation with Ru(VIII) this stage of the synthesis stopped short of completion and required further hydrogen peroxide treatment to oxidise any α-ketoacid to the carboxylic acid.
Scheme 56.
5.2 Nucleophilic Addition to a Cyclic Nitrone
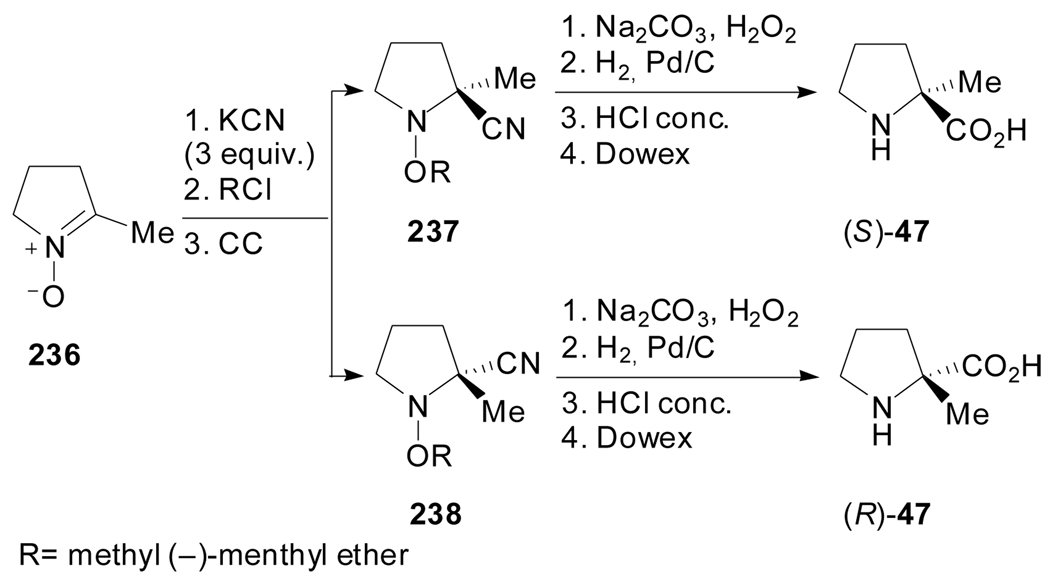
Shatzmiller et al. reported the preparation of optically pure chloromethyl (−)-menthyl ether and its application to the asymmetric synthesis of α-methyl proline (Scheme 57).[110] The treatment of 3,4-dihydro-5-methyl-2H-pyrrol-1-oxide 236 with KCN followed by addition of chloromethyl (−)-menthy1 ether afforded a mixture of diastereomeric amino nitriles 237 and 238 which were separable by column chromatography. The hydrolysis of the chiral nitriles, followed by cleavage of the N–O bonds and hydrolysis of the resulting amides into carboxylic acids provided access to both enantiomers of α-methylproline.
Scheme 57.
5.3 Duhamel Ring Contraction
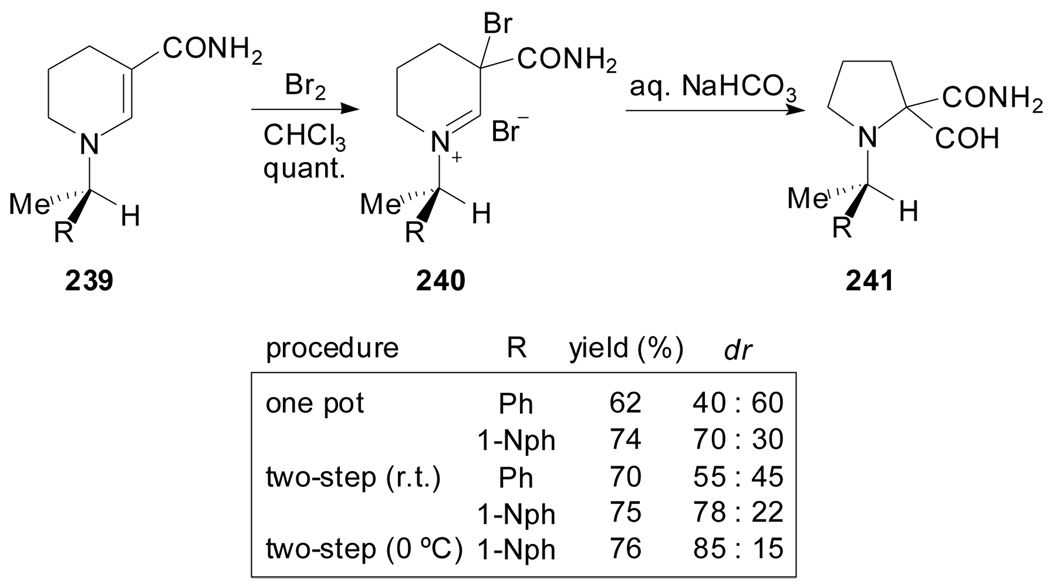
An approach to various proline chimeras bearing a quaternary α-stereogenic center via Duhamel ring contraction of enamines was described by Plaquevent et al.[111] A “removable chiral auxiliary” strategy to induce diastereoselectivity during the ring contraction process was employed (Scheme 58). The chiral enamines 239 were prepared by hydrogenation of the corresponding pyridinium salts, and were converted to iminiun bromides 240. The best diastereoselectivities were achieved at 0 °C when crowding the enamine (R= 1-naphtyl). According to the authors, the inversion of diastereoselectivity in the two step procedure with respect to the one pot, when R= Ph, suggests the competition of two mechanisms.
Scheme 58.
6. Conclusions
In this review we have collected available methods for the stereoselective and asymmetric synthesis of α-substituted prolines. Although a number of strategies have been widely and effectively applied many other approaches have been established for the construction of the fully substituted stereocenter of proline analogues. In this context, some methods are appropriate for the synthesis of proline including α-alkyl, α-phenyl or α-benzyl substituents, whereas others are more suited for the preparation of polysubstituted quaternary prolines. The recent emergence of asymmetric catalytic approaches for the construction of quaternary α,α-disubstituted amino acids has also delivered noteworthy contributions in the area of proline analogues. The selected methods described in this microreview should encourage further investigations focusing on the refinement of efficient methods and the development of straightforward catalytic processes attractive for scalable purposes.
Acknowledgments
We are thankful to Dr. A. I. Jiménez for helpful discussions and assistance. Financial support from the Ministerio de Educación y Ciencia–FEDER (project CTQ2007-62245) and Gobierno de Aragón (project PIP206/2005 and research group E40) is gratefully acknowledged. This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract number N01-CO-12400. The content of this publication does not necessarily reflect the view of the policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government. This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- Ac
acetyl
- 9-BBN
9-borabicyclo[3.3.1]nonane
- BEMP
2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,2,3-diazaphosphorine
- BINAP
2,20-bis(diphenylphosphanyl)-1,10-binaphthyl
- Bn
benzyl
- Boc
tert-butoxycarbonyl
- CAN
cerium ammonium nitrate
- Cbz
benzyloxycarbonyl
- CC
column chromatography
- DBU
1,8-diazabicyclo[5.4.0]undec-7-ene
- DEAD
diethyl azodicarboxylate
- DIBAL
diisobuylaluminum hydride
- DME
dimethoxyethane
- DMF
N,N-dimethylformamide
- HMPA
hexamethylphosphorous triamide
- KHMDS
potassium bis(trimethylsilyl)amide
- LDA
lithium diisopropylamide
- LHMDS
lithium bis(trimethylsilyl)amide
- LTMP
lithium 2,2,6,6-tetramethylpiperidide
- m-CPBA
m-chloroperoxybenzoic acid
- Men
menthyl
- Mes
methanesulfonyl
- MS
molecular sieves
- Nph
naphthyl
- Pht
phthaloyl
- PLE
pig liver esterase
- PMP
p-methoxyphenyl
- PPTS
pyridinium p-toluenesulfonate
- PTSA
p-toluenesulfonic acid
- Py
pyridyl
- QUINAP
1-(2-diphenylphosphanyl-1-naphthyl)isoquinoline
- TBAF
tetra-n-butylammonium fluoride
- TBDMS
tert-butyldimethylsilyl
- TBDPS
tert-butyldiphenylsilyl
- tBuTMG
2-tert-butyl-1,1,3,3-tetramethylguanidine
- TFA
trifluoroacetic acid
- Tf
trifluoromethanesulfonyl
- TMS
trimethylsilyl
- Tol
p-tolyl
- Ts
p-toluenesulfonyl
Biographies

M. Isabel Calaza received her Ph. D. in Chemistry from the University of Santiago (Spain) in 2000 under the guidance of Prof. F. J. Sardina and Dr. M. R. Paleo. After a postdoctoral stay in the laboratories of Prof. P. Knochel at the Ludwig Maximilians University of Munich (Germany) and an industrial appointment at AstraZeneca R&D Mölndal (Sweden) she moved to the University of Zaragoza (Spain) in 2007, as a research scientist. Her research interests include design and synthesis of non-natural amino acids, their structural study and their applications in the field of catalysis.

Carlos Cativiela is Full Professor of Organic Chemistry at the University of Zaragoza since 1996. His scientific activity started in the field of asymmetric synthesis oriented to the preparation of amino acids. He has developed different methodologies for the synthesis of a wide variety of non-natural constrained amino acids in enantiomerically pure form either by enantio-/diastereoselective syntheses or by chromatographic resolution procedures. Current research interests also involve the incorporation of such non-natural amino acids into peptides of structural or biological interest. He is author of more than 320 papers and 10 reviews.
References
- 1.Kastin AJ, editor. Handbook of Biologically Active Peptides. London: Academic Press, Elsevier Inc; 2006. [Google Scholar]
- 2.Adessi C, Soto C. Curr. Med. Chem. 2002;9:963–978. doi: 10.2174/0929867024606731. [DOI] [PubMed] [Google Scholar]
- 3.See for example: Cowell SM, Lee YS, Cain JP, Hruby VJ. Curr. Med. Chem. 2004;11:2785–2798. doi: 10.2174/0929867043364270. Toniolo C, Crisma M, Formaggio F, Peggion C. Biopolymers (Pept. Sci.) 2001;60:396–419. doi: 10.1002/1097-0282(2001)60:6<396::AID-BIP10184>3.0.CO;2-7. Hruby VJ, Li G, Haskell-Luevano C, Shenderovich M. Biopolymers (Pept. Sci.) 1997;43:219–266. doi: 10.1002/(SICI)1097-0282(1997)43:3<219::AID-BIP3>3.0.CO;2-Y.
- 4.a) Karoyan P, Sagan S, Lequin O, Quancard J, Lavielle S, Chassaing G. Substituted Prolines: Syntheses and Applications in Structure-Activity Relationship Studies of Biologically Active Peptides. In: Attanasi OA, Spinelli D, editors. Targets in Heterocyclic Systems. Chemistry and Properties. vol. 8. Cambridge: Royal Society of Chemistry; 2005. pp. 216–273. [Google Scholar]; b) Cativiela C, Díaz-de-Villegas MD. Tetrahedron: Asymmetry. 2000;11:645–732. [Google Scholar]; c) Mauger AB. J. Nat. Prod. 1996;59:1205–1211. doi: 10.1021/np9603479. [DOI] [PubMed] [Google Scholar]
- 5.a) Reiersen H, Rees AR. Trends Biochem. Sci. 2001;26:679–684. doi: 10.1016/s0968-0004(01)01957-0. [DOI] [PubMed] [Google Scholar]; b) Vanhoof G, Goossens F, de Meester I, Hendriks D, Scharpé S. FASEB J. 1995;9:736–744. [PubMed] [Google Scholar]; c) MacArthur MW, Thornton JM. J. Mol. Biol. 1991;218:397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- 6.a) Baures PW, Ojala WH, Gleason WB, Johnson RL. J. Pept. Res. 1997;50:1–13. doi: 10.1111/j.1399-3011.1997.tb00614.x. [DOI] [PubMed] [Google Scholar]; b) Flippen-Anderson JL, Gilardi R, Karle IL, Frey MH, Opella SJ, Gierasch LM, Goodman M, Madison V, Delaney NG. J. Am. Chem. Soc. 1983;105:6609–6614. [Google Scholar]; c) Delaney NG, Madison V. J. Am. Chem. Soc. 1982;104:6635–6641. [Google Scholar]
- 7.a) Coleman DR, IV, Ren Z, Mandal PK, Cameron AG, Dyer GA, Muranjan S, Campbell M, Chen X, McMurray JS. J. Med. Chem. 2005;48:6661–6670. doi: 10.1021/jm050513m. [DOI] [PubMed] [Google Scholar]; b) Doherty GA, Yang GX, Borges E, Tong S, McCauley ED, Treonz KM, Van Riper G, Pacholok S, Si Q, Koo GC, Shah K, Mumford RA, Hagmann WK. Bioorg. Med. Chem. Lett. 2003;13:1891–1895. doi: 10.1016/s0960-894x(03)00308-1. and references therein; [DOI] [PubMed] [Google Scholar]; c) Kopka IE, Lin LS, Mumford RA, Lanza T, Jr, Magriotis PA, Young D, DeLaszlo SE, MacCoss M, Mills SG, Van Riper G, McCauley E, Lyons K, Vincent S, Egger LA, Kidambi U, Stearns R, Colletti A, Teffera Y, Tong S, Owens K, Levorse D, Schmidt JA, Hagmann WK. Bioorg. Med. Chem. Lett. 2002;12:2415–2418. doi: 10.1016/s0960-894x(02)00460-2. and references therein; [DOI] [PubMed] [Google Scholar]; d) Nanzer AP, Torda AE, Bisang C, Weber C, Robinson JA, van Gunsteren WF. J. Mol. Biol. 1997;267:1012–1025. doi: 10.1006/jmbi.1997.0911. [DOI] [PubMed] [Google Scholar]; e) Bisang C, Weber C, Inglis J, Schiffer CA, van Gunsteren WF, Jelesarov I, Bosshard HR, Robinson JA. J. Am. Chem. Soc. 1995;117:7904–7915. [Google Scholar]; f) Welsh JH, Zerbe O, von Philipsborn W, Robinson JA. FEBS Lett. 1992;297:216–220. doi: 10.1016/0014-5793(92)80541-n. [DOI] [PubMed] [Google Scholar]; g) Hinds MG, Welsh JH, Brennand DM, Fisher J, Glennie MJ, Richards NGJ, Turner DL, Robinson JA. J. Med. Chem. 1991;34:1777–1789. doi: 10.1021/jm00110a005. [DOI] [PubMed] [Google Scholar]
- 8.For examples on proline chimeras see: Sugase K, Horikawa M, Sugiyama M, Ishiguro M. J. Med. Chem. 2004;47:489–492. doi: 10.1021/jm030232j. Quancard J, Karoyan P, Lequin O, Wenger E, Aubry A, Lavielle S, Chassaing G. Tetrahedron Lett. 2004;45:623–625. Paradisi MP, Mollica A, Cacciatore I, Di Stefano A, Pinnen F, Caccuri AM, Ricci G, Duprè S, Spirito A, Lucente G. Bioorg. Med. Chem. 2003;11:1677–1683. doi: 10.1016/s0968-0896(03)00041-5. Damour D, Pulicani J-P, Vuilhorgne M, Mignani S. Synlett. 1999:786–788. and references therein.
- 9.a) Alonso de Diego SA, Muñoz P, González-Muñiz R, Herranz R, Martín-Martínez M, Cenarruzabeitia E, Frechilla D, del Río J, Jimeno ML, García-López MT. Bioorg. Med. Chem. Lett. 2005;15:2279–2283. doi: 10.1016/j.bmcl.2005.03.015. [DOI] [PubMed] [Google Scholar]; b) Kobayashi S, Chikushi A, Tougu S, Imura Y, Nishida M, Yano Y, Matsuzaki K. Biochemistry. 2004;43:15610–15616. doi: 10.1021/bi048206q. [DOI] [PubMed] [Google Scholar]; c) Sem DS, Baker BL, Victoria EJ, Jones DS, Marquis D, Yu L, Parks J, Coutts SM. Biochemistry. 1998;37:16069–16081. doi: 10.1021/bi9807207. [DOI] [PubMed] [Google Scholar]; d) Jones DS, Gamino CA, Randow ME, Victoria EJ, Yu L, Coutts SM. Tetrahedron Lett. 1998;39:6107–6110. [Google Scholar]; e) Alig L, Edenhofer A, Hadváry P, Hürzeler M, Knopp D, Müller M, Steiner B, Trzeciak A, Weller T. J. Med. Chem. 1992;35:4393–4407. doi: 10.1021/jm00101a017. [DOI] [PubMed] [Google Scholar]; f) Thaisrivongs S, Pals DT, Lawson JA, Turner SR, Harris DW. J. Med. Chem. 1987;30:536–541. doi: 10.1021/jm00386a016. [DOI] [PubMed] [Google Scholar]; g) Pinnock RD, Woodruff GN, Turnbull MJ. Neuropharmacology. 1983;22:687–696. doi: 10.1016/0028-3908(83)90091-6. [DOI] [PubMed] [Google Scholar]
- 10.See for example; Haque TS, Ewing WR, Mapelli C, Lee VG, Sulsky RB, Riexinger DJ, Martinez RL, Zhu Y, Ruan Z. 2007082264. PCT Int. Appl., WO. 2007 Gluckman PD, Guan J, Woodnorth M-A, Brimble MA. 2007004641. US Pat. Appl., US. 2007 Ewing WR, Mapelli C, Riexinger DJ, Lee VG, Sulsky RB, Zhu Y. 2006127948. PCT Int. Appl., WO. 2006 Bo YY, Chakrabarti PP, Chen N, Liao H, Norman MH, Stec M, Tamayo N, Wang X. 2006183745. US Pat. Appl., US. 2006 Chao B, Deckwerth TL, Furth PS, Linton SD, Spada AP, Ullman BR, Weinhouse MI. 2006017295. PCT Int. Appl., WO. 2006 Brimble MA, Harris PWR, Sieg F. 2006127702. PCT Int. Appl., WO. 2006
- 11.a) Kay BK, Williamson MP, Sudol M. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]; b) Williamson MP. Biochem. J. 1994;297:249–260. doi: 10.1042/bj2970249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kee KS, Seetharama JDS. Curr. Pharm. Des. 2003;9:1209–1224. doi: 10.2174/1381612033454900. [DOI] [PubMed] [Google Scholar]
- 13.Dalko PI, editor. Enantioselective Organocatalysis. Weinheim: Wiley-VCH; 2007. [Google Scholar]
- 14.a) Cativiela C, Díaz-de-Villegas MD. Tetrahedron: Asymmetry. 2007;18:569–623. [Google Scholar]; b) Cativiela C, Díaz-de-Villegas MD. Tetrahedron: Asymmetry. 1998;9:3517–3599. [Google Scholar]
- 15.a) Vogt H, Bräse S. Org. Biomol. Chem. 2007;5:406–430. doi: 10.1039/b611091f. [DOI] [PubMed] [Google Scholar]; b) Tanaka M. Chem. Pharm. Bull. 2007;55:349–358. doi: 10.1248/cpb.55.349. [DOI] [PubMed] [Google Scholar]; c) Ohfune Y, Shinada T. Eur. J. Org. Chem. 2005:5127–5143. doi: 10.1021/jo0477244. [DOI] [PubMed] [Google Scholar]; d) Nájera C. Synlett. 2002:1388–1403. [Google Scholar]
- 16.Seebach D, Naef R. Helv. Chim. Acta. 1981;64:2704–2708. [Google Scholar]
- 17.Seebach D, Boes M, Naef R, Schweizer WB. J. Am. Chem. Soc. 1983;105:5390–5398. [Google Scholar]
- 18.Beck AK, Blank S, Job K, Seebach D, Sommerfeld T. Org. Synth. 1995;72:62–73. [Google Scholar]
- 19.Seebach D, Sting AR, Hoffmann M. Angew. Chem., Int. Ed. Engl. 1996;35:2708–2748. [Google Scholar]
- 20.Papillon JPN, Taylor RJK. Org. Lett. 2000;2:1987–1990. doi: 10.1021/ol0058792. [DOI] [PubMed] [Google Scholar]
- 21.Tanimori S, Sunami T, Fukubayashi K, Kirihata M. Tetrahedron. 2005;61:2481–2492. [Google Scholar]
- 22.Genin MJ, Baures PW, Johnson RL. Tetrahedron Lett. 1994;35:4967–4968. [Google Scholar]
- 23.Wang H, Germanas JP. Synlett. 1999:33–36. [Google Scholar]
- 24.Harris PWR, Brimble MA, Muir VJ, Lai MYH, Trotter NS, Callis DJ. Tetrahedron. 2005;61:10018–10035. [Google Scholar]
- 25.Pettersen D, Ahlberg P. Tetrahedron: Asymmetry. 2005;16:2075–2080. [Google Scholar]
- 26.Pisaneschi F, Cordero FM, Lumini M, Brandi A. Synlett. 2007:2882–2884. [Google Scholar]
- 27.Artman GD, III, Grubbs AW, Williams RM. J. Am. Chem. Soc. 2007;129:6336–6342. doi: 10.1021/ja070259i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amedjkouh M, Ahlberg P. Tetrahedron: Asymmetry. 2002;13:2229–2234. [Google Scholar]
- 29.a) Bittermann H, Böckler F, Einsiedel J, Gmeiner P. Chem. Eur. J. 2006;12:6315–6322. doi: 10.1002/chem.200600432. [DOI] [PubMed] [Google Scholar]; b) Khalil EM, Ojala WH, Pradhan A, Nair VD, Gleason WB, Mishra RK, Johnson RL. J. Med. Chem. 1999;42:628–637. doi: 10.1021/jm980525q. [DOI] [PubMed] [Google Scholar]; c) Genin MJ, Johnson RL. J. Am. Chem. Soc. 1992;114:8778–8783. [Google Scholar]
- 30.a) Hoffmann T, Laning H, Waibel R, Gmeiner P. Angew. Chem., Int. Ed. 2001;40:3361–3364. doi: 10.1002/1521-3773(20010917)40:18<3361::aid-anie3361>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]; b) Gramberg D, Weber C, Beeli R, Inglis J, Bruns C, Robinson JA. Helv. Chim. Acta. 1995;78:1588–1606. [Google Scholar]; c) Dumas J-P, Germanas JP. Tetrahedron Lett. 1994;35:1493–1496. [Google Scholar]; d) Gramberg D, Robinson JA. Tetrahedron Lett. 1994;35:861–864. [Google Scholar]
- 31.a) Hinds MG, Welsh JH, Brennand DM, Fisher J, Glennie MJ, Richards NGJ, Turner DL, Robinson JA. J. Med. Chem. 1991;34:1777–1789. doi: 10.1021/jm00110a005. [DOI] [PubMed] [Google Scholar]; b) Hinds MG, Richards NGJ, Robinson JA. J. Chem. Soc., Chem. Commun. 1988:1447–1449. [Google Scholar]
- 32.a) Alonso de Diego SA, Gutiérrez-Rodríguez M, Pérez de Vega MJ, González-Muñiz R, Herranz R, Martín-Martínez M, Cenarruzabeitia E, Frechilla D, del Río J, Jimeno ML, García-López MT. Bioorg. Med. Chem. Lett. 2006;16:3396–3400. doi: 10.1016/j.bmcl.2006.04.033. [DOI] [PubMed] [Google Scholar]; b) Bittermann H, Gmeiner P. J. Org. Chem. 2006;71:97–102. doi: 10.1021/jo0517287. [DOI] [PubMed] [Google Scholar]; c) Fernández MM, Diez A, Rubiralta M, Montenegro E, Casamitjana N, Kogan MJ, Giralt E. J. Org. Chem. 2002;67:7587–7599. doi: 10.1021/jo025999i. [DOI] [PubMed] [Google Scholar]
- 33.For the use of α-allyl proline in natural product synthesis see: Baran PS, Hafensteiner BD, Ambhaikar NB, Guerrero CA, Gallagher JD. J. Am. Chem. Soc. 2006;128:8678–8693. doi: 10.1021/ja061660s. Baran PS, Guerrero CA, Ambhaikar NB, Hafensteiner BD. Angew. Chem., Int. Ed. 2005;44:606–609. doi: 10.1002/anie.200461864. Tanimori S, Fukubayashi K, Kirihata M. Tetrahedron Lett. 2001;42:4013–4016.
- 34.a) Harris PWR, Brimble MA. Org. Biomol. Chem. 2006;4:2696–2709. doi: 10.1039/b605293b. [DOI] [PubMed] [Google Scholar]; b) Tong Y, Olczak J, Zabrocki J, Gershengorn MC, Marshall GR, Moeller KD. Tetrahedron. 2000;56:9791–9800. [Google Scholar]
- 35.a) Watanabe H, Okue M, Kobayashi H, Kitahara T. Tetrahedron Lett. 2002;43:861–864. [Google Scholar]; b) Usse S, Guillaumet G, Viaud M-C. J. Org. Chem. 2000;65:914–917. doi: 10.1021/jo991089y. [DOI] [PubMed] [Google Scholar]
- 36.Vartak AP, Young VG, Johnson RL. Org. Lett. 2005;7:35–38. doi: 10.1021/ol047958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber T, Seebach D. Helv. Chim. Acta. 1985;68:155–161. [Google Scholar]
- 38.Hughes CC, Trauner D. Angew. Chem., Int. Ed. 2002;41:4556–4559. doi: 10.1002/1521-3773(20021202)41:23<4556::AID-ANIE4556>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 39.Sato T, Kugo Y, Nakaumi E, Ishibashi H, Ikeda M. J. Chem. Soc. Perkin Trans. 1995;1:1801–1808. [Google Scholar]
- 40.Nagumo S, Mizukami M, Akutsu N, Nishida A, Kawahara N. Tetrahedron Lett. 1999;40:3209–3212. [Google Scholar]
- 41.Sato T, Kawasaki S, Oda N, Yagi S, El Bialy SAA, Uenishi J, Yamauchi M, Ikeda M. J. Chem. Soc. Perkin Trans. 2001;1:2623–2631. [Google Scholar]
- 42.Huck BR, Gellman SH. J. Org. Chem. 2005;70:3353–3362. doi: 10.1021/jo048639z. [DOI] [PubMed] [Google Scholar]
- 43.a) Cossy J, Mirguet O, Pardo DG. Synlett. 2001:1575–1577. [Google Scholar]; b) Tamura O, Yanagimachi T, Ishibashi H. Tetrahedron: Asymmetry. 2003;14:3033–3041. [Google Scholar]
- 44.Nagumo S, Matoba A, Ishii Y, Yamaguchi S, Akutsu N, Nishijima H, Nishida A, Kawahara N. Tetrahedron. 2002;58:9871–9877. [Google Scholar]
- 45.Filosa R, Holder C, Auberson YP. Tetrahedron Lett. 2006;47:8929–8932. [Google Scholar]
- 46.Williams RM, Cao J. Tetrahedron Lett. 1996;37:5441–5444. [Google Scholar]
- 47.a) Williams RM, Cao J, Tsujishima H. Angew. Chem., Int. Ed. 2000;39:2540–2544. doi: 10.1002/1521-3773(20000717)39:14<2540::aid-anie2540>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]; b) Williams RM, Cao J, Tsujishima H, COx RJ. J. Am. Chem. Soc. 2003;125:12172–12178. doi: 10.1021/ja036713+. [DOI] [PubMed] [Google Scholar]
- 48.Matsumura Y, Kinoshita T, Yanagihara Y, Kanemoto N, Watanabe M. Tetrahedron Lett. 1996;37:8395–8398. [Google Scholar]
- 49.Ma D, Yang J. J. Am. Chem. Soc. 2001;123:9706–9707. doi: 10.1021/ja016403w. [DOI] [PubMed] [Google Scholar]
- 50.a) Hussaini SR, Moloney MG. Org. Biomol. Chem. 2006;4:2600–2615. doi: 10.1039/b604183c. [DOI] [PubMed] [Google Scholar]; b) Hussaini SR, Moloney MG. Org. Biomol. Chem. 2003;1:1838–1841. doi: 10.1039/b303789d. [DOI] [PubMed] [Google Scholar]
- 51.Ferey V, Vedrenne P, Toupet L, Le Gall T, Mioskowski C. J. Org. Chem. 1996;61:7244–7245. doi: 10.1021/jo961432o. [DOI] [PubMed] [Google Scholar]
- 52.Ooi T, Miki T, Maruoka K. Org. Lett. 2005;7:191–193. doi: 10.1021/ol047921p. [DOI] [PubMed] [Google Scholar]
- 53.a) Macías A, Ramallal AM, Alonso E, del Pozo C, González J. J. Org. Chem. 2006;71:7721–7730. doi: 10.1021/jo061189l. [DOI] [PubMed] [Google Scholar]; b) Macías A, Alonso E, del Pozo C, Venturini A, González J. J. Org. Chem. 2004;69:7004–7012. doi: 10.1021/jo040163w. [DOI] [PubMed] [Google Scholar]
- 54.Sakaguchi K, Yamamoto M, Watanabe Y, Ohfune Y. Tetrahedron Lett. 2007;48:4821–4824. [Google Scholar]
- 55.Sakaguchi K, Fujita M, Suzuki H, Higashino M, Ohfune Y. Tetrahedron Lett. 2000;41:6589–6592. [Google Scholar]
- 56.Tayama E, Nanbara S, Nakai T. Chem. Lett. 2006;35:478–479. [Google Scholar]
- 57.Glaeske KW, West FG. Org. Lett. 1999;1:31–33. [Google Scholar]
- 58.Arboré APA, Cane-Honeysett DJ, Coldham I, Middleton ML. Synlett. 2000:236–238. [Google Scholar]
- 59.a) Pandey G, Banerjee P, Gadre SR. Chem. Rev. 2006;106:4484–4517. doi: 10.1021/cr050011g. [DOI] [PubMed] [Google Scholar]; b) Nájera C, Sansano JM. Angew. Chem., Int. Ed. 2005;44:6272–6276. doi: 10.1002/anie.200501074. [DOI] [PubMed] [Google Scholar]; c) Husinec S, Savic V. Tetrahedron: Asymmetry. 2005;16:2047–2061. [Google Scholar]
- 60.a) Grigg R, Thornton-Pett M, Yoganathan G. Tetrahedron. 1999;55:1763–1780. [Google Scholar]; b) Barr DA, Dorrity MJ, Grigg R, Hargreaves S, Malone JF, Montgomery J, Redpath J, Stevenson P, Thronton-Pett M. Tetrahedron. 1995;51:273–294. [Google Scholar]; c) Barr DA, Dorrity MJ, Grigg R, Malone JF, Montgomery J, Rajviroongit S, Stevenson P. Tetrahedron Lett. 1990;31:6569–6572. [Google Scholar]
- 61.a) Slater MJ, Amphlett EM, Andrews DM, Bravi G, Burton G, Cheasty AG, Corfield JA, Ellis MR, Fenwick RH, Fernandes S, Guidetti R, Haigh D, Hartley CD, Howes PD, Jackson DL, Jarvest RL, Lovegrove VLH, Medhurst KJ, Parry NR, Price H, Shah P, Singh OMP, Stocker R, Thommes P, Wilkinson C, Wonacott A. J. Med. Chem. 2007;50:897–900. doi: 10.1021/jm061207r. [DOI] [PubMed] [Google Scholar]; b) Burton G, Ku TW, Carr TJ, Kiesow T, Sarisky RT, Lin-Goerke J, Hofmann GA, Slater MJ, Haigh D, Dhanak D, Johnson VK, Parry NR, Thommes P. Bioorg. Med. Chem. Lett. 2007;17:1930–1933. doi: 10.1016/j.bmcl.2007.01.034. [DOI] [PubMed] [Google Scholar]; c) Burton G, Ku TW, Carr TJ, Kiesow T, Sarisky RT, Lin-Goerke J, Baker A, Earnshaw DL, Hofmann GA, Keenan RM, Dhanak D. Bioorg. Med. Chem. Lett. 2005;15:1553–1556. doi: 10.1016/j.bmcl.2005.01.076. [DOI] [PubMed] [Google Scholar]
- 62.a) Cooper DM, Grigg R, Hargreaves S, Kennewell P, Redpath J. Tetrahedron. 1995;51:7791–7808. [Google Scholar]; b) Rispens MT, Keller E, de Lange B, Zijlstra RWJ, Feringa BL. Tetrahedron: Asymmetry. 1994;5:607–624. [Google Scholar]
- 63.Waldmann H, Bläser E, Jansen M, Letschert H-P. Chem. Eur. J. 1995;1:150–154. [Google Scholar]
- 64.García-Ruano JL, Tito A, Peromingo MT. J. Org. Chem. 2002;67:981–987. doi: 10.1021/jo010797s. [DOI] [PubMed] [Google Scholar]
- 65.Zubia A, Mendoza L, Vivanco S, Aldaba E, Carrascal T, Lecea B, Arrieta A, Zimmerman T, Vidal-Vanaclocha F, Cossío FP. Angew. Chem., Int. Ed. 2005;44:2903–2907. doi: 10.1002/anie.200462497. [DOI] [PubMed] [Google Scholar]
- 66.Nájera C, de M, Retamosa G, Sansano JM. Tetrahedron: Asymmetry. 2006;17:1985–1989. [Google Scholar]
- 67.Pyne SG, Safaei-G J, Koller F. Tetrahedron Lett. 1995;36:2511–2514. [Google Scholar]
- 68.Anslow AS, Harwood LM, Phillips H, Watkin D. Tetrahedron: Asymmetry. 1991;2:997–1000. [Google Scholar]
- 69.Anslow AS, Harwood LM, Lilley IA. Tetrahedron: Asymmetry. 1995;6:2465–2468. [Google Scholar]
- 70.Chinchilla R, Falvello LR, Galindo N, Nájera C. Eur. J. Org. Chem. 2001:3133–3140. doi: 10.1021/jo991736l. [DOI] [PubMed] [Google Scholar]
- 71.a) Jones RCF, Howard KJ, Snaith JS. Tetrahedron Lett. 1996;37:1707–1710. [Google Scholar]; b) Jones RCF, Howard KJ, Snaith JS. Tetrahedron Lett. 1996;37:1711–1714. [Google Scholar]
- 72.Grigg R, Thornton-Pett M, Xu J, Xu L-H. Tetrahedron. 1999;55:13841–13866. [Google Scholar]
- 73.a) Alcaide B, Almendros P, Alonso JM, Redondo MC. J. Org. Chem. 2003;68:1426–1432. doi: 10.1021/jo026112l. [DOI] [PubMed] [Google Scholar]; b) Alcaide B, Almendros P, Alonso JM, Aly MF. J. Org. Chem. 2001;66:1351–1358. doi: 10.1021/jo005686s. [DOI] [PubMed] [Google Scholar]; c) Alcaide B, Almendros P, Alonso JM, Aly MF, Redondo MC. Synlett. 2001:773–776. [Google Scholar]; d) Alcaide B, Almendros P, Alonso JM, Aly MF. Chem. Commun. 2000:485–486. [Google Scholar]
- 74.Alcaide B, Almendros P, Redondo MC, Ruiz MP. J. Org. Chem. 2005;70:8890–8894. doi: 10.1021/jo051402y. [DOI] [PubMed] [Google Scholar]
- 75.Schnell B, Bernardinelli G, Kündig EP. Synlett. 1999:348–350. [Google Scholar]
- 76.Hanessian S, Bayrakdarian M. Tetrahedron Lett. 2002;43:967–971. [Google Scholar]
- 77.Allway P, Grigg R. Tetrahedron Lett. 1991;32:5817–5820. [Google Scholar]
- 78.Grigg R. Tetrahedron: Asymmetry. 1995;6:2475–2486. [Google Scholar]
- 79.Longmire JM, Wang B, Zhang X. J. Am. Chem. Soc. 2002;124:13400–13401. doi: 10.1021/ja025969x. [DOI] [PubMed] [Google Scholar]
- 80.Chen C, Li X, Schreiber SL. J. Am. Chem. Soc. 2003;125:10174–10175. doi: 10.1021/ja036558z. [DOI] [PubMed] [Google Scholar]
- 81.Nájera C, de M, Retamosa G, Sansano JM. Org. Lett. 2007;9:4025–4028. doi: 10.1021/ol701577k. [DOI] [PubMed] [Google Scholar]
- 82.Cabrera S, Gómez-Arrayás R, Carretero JC. J. Am. Chem. Soc. 2005;127:16394–16395. doi: 10.1021/ja0552186. [DOI] [PubMed] [Google Scholar]
- 83.a) Llamas T, Gómez-Arrayás R, Carretero JC. Synlett. 2007:950–956. [Google Scholar]; b) Llamas T, Gómez-Arrayás R, Carretero JC. Org. Lett. 2006;8:1795–1798. doi: 10.1021/ol060314c. [DOI] [PubMed] [Google Scholar]
- 84.Saito S, Tsubogo T, Kobayashi S. J. Am. Chem. Soc. 2007;129:5364–5365. doi: 10.1021/ja0709730. [DOI] [PubMed] [Google Scholar]
- 85.Schöllkopf U, Hinrichs R, Lonsky R. Angew. Chem., Int. Ed. Engl. 1987;26:143–145. [Google Scholar]
- 86.Andrei M, Efskind J, Viljugrein T, Romming C, Undheim K. Tetrahedron: Asymmetry. 2004;15:1301–1313. [Google Scholar]
- 87.Balducci D, Grandi A, Porzi G, Sandri S. Tetrahedron: Asymmetry. 2005;16:1453–1462. [Google Scholar]
- 88.a) El Achquar A, Boumzebra M, Roumestant M-L, Viallefont P. Tetrahedron. 1988;44:5319–5332. [Google Scholar]; b) Bajgrowicz J, El Achquar A, Roumestant M-L, Pigière C, Viallefont P. Heterocycles. 1986;24:2165–2167. [Google Scholar]
- 89.Seebach D, Dziadulewicz E, Behrendt L, Cantoreggi S, Fitzi R. Liebigs Ann. Chem. 1989:1215–1232. [Google Scholar]
- 90.Harwood LM, Hamblett G, Jiménez-Díaz AI, Watkin DJ. Synlett. 1997:935–938. [Google Scholar]
- 91.Chaume G, Van Severen M-C, Marinkovic S, Brigaud T. Org. Lett. 2006;8:6123–6126. doi: 10.1021/ol062593+. [DOI] [PubMed] [Google Scholar]
- 92.a) Abellán T, Chinchilla R, Galindo N, Nájera C, Sansano JM. J. Heterocyclic Chem. 2000;37:467–479. [Google Scholar]; b) Chinchilla R, Galindo N, Nájera C. Synthesis. 1999:704–717. [Google Scholar]; c) Chinchilla R, Galindo N, Nájera C. Tetrahedron: Asymmetry. 1998;9:2769–2772. [Google Scholar]
- 93.Duvall JR, Wu F, Snider BB. J. Org. Chem. 2006;71:8579–8590. doi: 10.1021/jo061650+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Betsbrugge J, Tourwé D, Kaptein B, Kierkels H, Broxterman R. Tetrahedron. 1997;53:9233–9240. [Google Scholar]
- 95.a) Ikunaka M. Org. Process Res. Dev. 2007;11:495–502. [Google Scholar]; b) Maeda K, Miller RA, Szumigala RH, Jr, Shafiee A, Karady S, Armstrong JD., III Tetrahedron Lett. 2005;46:1545–1549. [Google Scholar]
- 96.Zhao H, Hsu DC, Carlier PR. Synthesis. 2005:1–16. [Google Scholar]
- 97.Kawabata T, Kawakami S, Majumdar S. J. Am. Chem. Soc. 2003;125:13012–13013. doi: 10.1021/ja0378299. [DOI] [PubMed] [Google Scholar]
- 98.Kawabata T, Majumdar S, Tsubaki K, Monguchi D. Org. Biomol. Chem. 2005;3:1609–1611. doi: 10.1039/b416535g. [DOI] [PubMed] [Google Scholar]
- 99.Kawabata T, Matsuda S, Kawakami S, Monguchi D, Moriyama K. J. Am. Chem. Soc. 2006;128:15394–15395. doi: 10.1021/ja0670761. [DOI] [PubMed] [Google Scholar]
- 100.Kolaczkowski L, Barnes DM. Org. Lett. 2007;9:3029–3032. doi: 10.1021/ol071023m. [DOI] [PubMed] [Google Scholar]
- 101.Giese B, Wettstein P, Stähelin C, Barbosa F, Neuburger M, Zehnder M, Wessig P. Angew. Chem., Int. Ed. 1999;38:2586–2587. [PubMed] [Google Scholar]
- 102.Sinicropi A, Barbosa F, Basosi R, Giese B, Olivucci M. Angew. Chem., Int. Ed. 2005;44:2390–2393. doi: 10.1002/anie.200461898. [DOI] [PubMed] [Google Scholar]
- 103.Gardiner J, Abell AD. Org. Biomol. Chem. 2004;2:2365–2370. doi: 10.1039/B406450J. [DOI] [PubMed] [Google Scholar]
- 104.Overberger CG, Jon YS. J. Polym. Sci. Polym. Chem. Ed. 1977;15:1413–1421. [Google Scholar]
- 105.a) Péter A, Török G, Vékes E, Van Betsbrugge J, Tourwé D. J. Liq. Chromatogr. Relat. Technol. 2004;27:17–29. [Google Scholar]; b) Péter A, Vékes E, Tóth G, Tourwé D, Borremans F. J. Chromatogr. A. 2002;948:283–294. doi: 10.1016/s0021-9673(01)01475-3. [DOI] [PubMed] [Google Scholar]; c) Péter A, Vékes E, Armstrong DW, Tourwé D. Chromatographia. 2002;56:41–47. [Google Scholar]
- 106.Ilisz I, Tourwé D, Armstrong DW, Péter A. Chirality. 2006;18:539–543. doi: 10.1002/chir.20257. [DOI] [PubMed] [Google Scholar]
- 107.Péter A, Vékes E, Árki A, Tourwé D, Lindner W. J. Sep. Sci. 2003;26:1125–1132. [Google Scholar]
- 108.Zhao Y, Pritts WA. J. Chromatogr. A. 2007;1156:228–235. doi: 10.1016/j.chroma.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 109.Clayden J, Knowles FE, Menet CJ. Tetrahedron Lett. 2003;44:3397–3400. [Google Scholar]
- 110.Shatzmiller S, Dolithzky B-Z, Bahar E. Liebigs Ann. Chem. 1991:375–379. [Google Scholar]
- 111.Trancard D, Tout J-B, Giard T, Chichaoui I, Cahard D, Plaquevent J-C. Tetrahedron Lett. 2000;41:3843–3847. [Google Scholar]