Abstract
Acute ethanol exposure in humans and in animal models activates the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS); the resultant increases in concentration of neuroendocrine mediators contribute to some of the immunosuppressive effects of ethanol. However, the role of these mediators in the ethanol-induced inhibition of inflammatory responses is not clear. This is complicated by the fact that most inflammatory stimuli also activate the HPA axis and SNS, and it has not been determined if ethanol plus an inflammatory stimulus increases these stress responses. Addressing this issue is the major focus of the study described herein. Complementary approaches were used, including quantitative assessment of the stress response in mice treated with polyinosinic-polycytidylic acid (poly I:C, as an inflammatory stimulus) and inhibition of the production or action of key HPA axis and SNS mediators. Treatment of mice with ethanol shortly before treatment with poly I:C yielded a significant increase in the corticosterone response as compared to the response to poly I:C alone, but the increase was small and not likely sufficient to account for the anti-inflammatory effects of ethanol. Inhibition of catecholamine and glucocorticoid production by adrenalectomy, and inhibition of catecholamine action with a sustained release antagonist (nadalol) supported this conclusion and revealed that “excess” stress responses associated with ethanol treatment is not the mechanism of suppression of pro-inflammatory cytokine production, but stress-induced corticosterone does regulate production of several of these cytokines, which has not previously been reported.
Keywords: catecholamines, corticosterone, cytokines, ethanol, poly I:C
Introduction
Acute high dose ethanol exposure inhibits innate immunity, increasing susceptibility to infection by a number of pathogens (Nelson and Kolls, 2002). Since acute, or binge drinking, is prevalent in the U.S. (Wechsler et al., 2002), insight into the mechanisms by which it dampens inflammatory responses could be useful in developing strategies to prevent the immunosuppression by ethanol and possibly other stressors. We have developed a mouse model for binge drinking to examine these mechanisms (Carson and Pruett, 1996) and have demonstrated that some of these effects are mediated by an ethanol-induced increase in glucocorticoids (corticosterone in mice) via activation of the HPA axis (Han et al., 1993; Han and Pruett, 1995; Weiss et al., 1996; Pruett et al., 2003). The SNS is also activated by acute ethanol exposure, but the role of catecholamines in immunological effects of ethanol has not been evaluated as extensively as the role of glucocorticoids. Results from one study indicate that both catecholamines and corticosterone contribute to suppression of natural killer (NK) cell activity (Wu and Pruett, 1997). Sympathetic nerves innervate lymphoid organs and most immune cells express adrenoreceptors, and it has been suggested that the sympathetic response to each immune challenge may produce different effects locally vs. systemically (Elenkov et al., 2000) in vivo. Although most studies in this field have employed cultured primary cells or cell lines, it would seem more appropriate to evaluate these effects using an in vivo exposure system.
Inflammatory stimuli have long been known to induce classical neuroendocrine stress responses, and there are indications that glucocorticoids can act as a feedback inhibitor to limit the production of pro-inflammatory cytokines induced by such stimuli (Fantuzzi et al., 1995). This complicates interpretation of previous studies in which we found that abrogating the glucocorticoid response to an inflammatory stimulus (using a glucocorticoid synthesis inhibitor and a glucocorticoid antagonist) did not seem to diminish the inhibition of pro-inflammatory cytokine production by acute administration of ethanol (Pruett et al., 2003b; Glover and Pruett, 2006). Furthermore, a role for the SNS in the ethanol-induced suppression of pro-inflammatory cytokine production or enhancement of IL-10 production was not considered in our previous studies. The study described here was conducted to address these issues. A time-course study was conducted to determine if ethanol given shortly before a potent inflammatory stimulus causes a substantially larger increase in serum corticosterone over time than the inflammatory stimulus alone. In addition, two methods were used to determine if stress-induced catecholamines play a role in the actions of ethanol in this experimental system. Adrenalectomy (to prevent the stress-induced surge in adrenal-derived epinephrine and norepinephrine) and a continuous release form of β-adrenergic antagonist (nadolol) yielded the same fundamental conclusion with regard to the role of adrenergic receptors in the effects of ethanol in this experimental system.
Materials and Methods
Mice
Female B6C3F1 mice (6-8 weeks of age) were purchased through the National Cancer Institute’s Animal Program. These mice have been used in this lab for many years due to their extensive use in studies sponsored by the National Toxicology Program. They were selected for use in the present study primarily because this will facilitate comparisons to our previous results and allow use of dosages of poly I:C and other reagents already optimized for this strain. Female mice were used in these studies because group-housed male mice exhibit aggressive behavior that causes unpredictable stress responses. Adrenalectomized (ADX), sham adrenalectomized (sham), and untreated female B6C3F1 mice (6-8 weeks of age) were purchased from Charles River Laboratories for the ADX mice experiment. All mice were allowed to acclimate for 2 weeks and were housed on a 12-hour light/dark cycle in a temperature- and humidity-controlled facility accredited by the American Association for Accreditation of Laboratory Animal Care. Animal care and use was in accord with the NIH Guide and LSUHSC policies. ADX mice were given 0.9% NaCl solution in place of their drinking water to compensate for lack of mineralocorticoids in ADX mice.
Area under the corticosterone concentration vs. time curve
Groups of mice (5 per group) included at each time point were naive (untreated), poly I:C (Sigma Chemical Co, St. Louis, MO), poly I:C + ethanol (absolute, Pharmco-AAPER, Shelbyville, KY), and ethanol plus the vehicle for poly I:C (phosphate buffered saline, Sigma-Aldrich, St. Louis, MO). Ethanol or vehicle (tissue culture grade water from Sigma-Aldrich), was administered by gavage at a dosage of 6 g/kg using a 32% (v/v) solution in water. Fifteen minutes later, poly I:C or vehicle (phosphate buffered saline) was administered at a dosage of 100 μg/mouse, intravenously in 0.1 ml. Trunk blood was collected by decapitation at 1, 2, 4, 6, 8, and 10 hours after poly I:C dosing. Each cage of 5 mice was bled within 3 minutes, and all unnecessary entry into the animal rooms was avoided to reduce handling related increases in corticosterone levels. Naive groups received no treatments and were left undisturbed until they were bled.
Experiments with adrenalectomized (ADX) mice
Adrenalectomized (ADX), sham ADX, or surgically untreated mice were used in these experiments. These mice were purchased at the same time from the same supplier. Placebo, corticosterone (5.0 mg) and/or nadolol (0.5 mg) pellets (Innovative Research of America, Sarasota, FL) were placed subcutaneously at the back of the neck while mice were under anesthesia with pentobarbital (45 mg/kg, intraperitoneally). The dosage of corticosterone pellet was selected from previous studies with ADX mice (Pruett et al., 2004), and was chosen as the best dose to match or slightly exceed the corticosterone levels induced in a stress response. Sustained release nadolol pellets from Innovative Research of America at the dosage used here have been reported in numerous articles to block sympathetic responses (Barazzone-Argiroffo et al., 2003; Kelley et al., 2002; Ghoshal et al., 1998; Dobbs et al., 1993). Administration of test reagents and collection of samples were performed the day after implantation of pellets. Mice in groups of 4 were left untreated or were treated with ethanol or vehicle (water or 0.9% saline, respectively for normal and ADX mice) 15 minutes prior to treatment with poly I:C (Invivogen, San Diego, CA) or vehicle (PBS). Our previous work indicates that this poly I:C is essentially endotoxin free (Pruett, et al., 2004). Ethanol was administered by oral gavage with a 32% v/v solution in water or in 0.9% saline, at a dosage of 6 g/kg. Poly I:C was administered intravenously at a dosage of 100 μg/mouse in 0.1 ml sterile PBS. Two hours after poly I:C administration, trunk blood was collected by decapitation. This time point was selected as optimum for most cytokines using the data from the AUC experiment (which was essentially a time course experiment).
Measurement of corticosterone, cytokines, and chemokines
Serum corticosterone concentrations were measured using a radioimmunoassay kit (Diagnostic Products Corp., Los Angeles, CA) for determination of AUC values, but due to problems with availability, an ELISA kit (Assay Designs, Ann Arbor, MI) was used subsequently. These kits yielded comparable results when the same samples were evaluated (data not shown). Cytokines and chemokines were measured using a Bioplex multiplex assay kit (Bio Rad, Hercules, CA) and Bioplex reader. Interleukin-6 concentrations were outside the upper range of the standards, so serum samples were diluted and IL-6 was measured using ELISA (BD Biosciences, San Diego, CA). Quantitation of IFN-α was accomplished using an ELISA kit (PBL Interferon Source, Piscataway, NJ).
Calculation of the area under the corticosterone concentration vs. time curve (AUC) and statistical methods
Corticosterone AUC values from 0 to 2 hours and 0 to 8 hours were calculated. The 2 hour time point was chosen because it was the peak time or shortly before the peak time for expression of most cytokines following poly I:C treatment as well as the best time point to demonstrate cytokine alterations by ethanol administration. The corticosterone value for mouse 1 from each treatment group was then plotted up to the chosen time using Prism 4.0 software (GraphPad, San Diego, CA), and the AUC was calculated using the AUC feature in this software. The remaining mice in each treatment group were plotted in the same way, so 5 individual AUC values were obtained for each treatment group. The means and statistical significance between groups were determined using analysis of variance followed by Newman-Keul’s post hoc test. Before this analysis, the mean AUC was calculated for the naive group and subtracted from the AUC value for each treatment group. Thus, the AUC values used reflect only the increase in corticosterone exposure over time, not normal circadian changes.
Results
Serum corticosterone AUCs
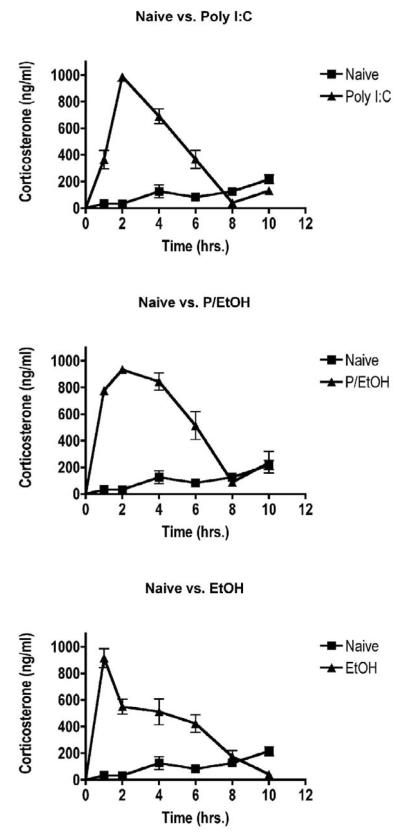
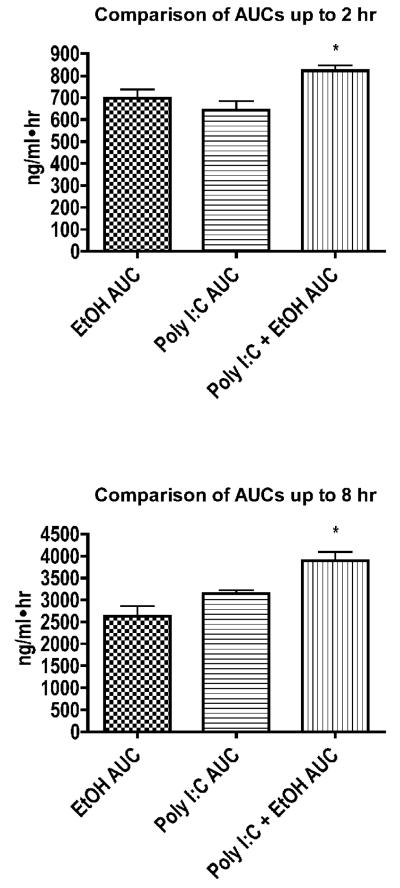
We began by determining the time course of changes in serum corticosterone concentrations in mice treated with ethanol, poly I:C, or both. As shown in Fig. 1, corticosterone rose sharply in poly I:C-treated mice within the first 1-2 hours and gradually returned to levels observed in untreated mice within 8 hours. Notably, the corticosterone concentrations in ethanol-treated groups increased more rapidly, and corticosterone concentrations in the group treated with poly I:C plus ethanol remained elevated at higher concentrations for a longer period of time than either of the other two treatment groups. Due to these differences, the area under the concentration vs. time curve (AUC) for the poly I:C + ethanol group was greater than either individual treatment when calculated for 2 hours or 8 hours (Fig. 2). However, comparison of the increase in corticosterone AUC with results from our previous studies indicates that a change in corticosterone of the magnitude shown in Fig. 2 would have a very small effect on even the most sensitive immunological parameter evaluated in our previous studies (expression of MHC class II proteins on B lymphocytes) (Pruett et al., 2003c). The percentage of MHC II high expressing B cells would be 15.0% in the group treated with poly I:C alone and 12.3% in the group treated with poly I:C + ethanol (using calculations described previously) (Pruett et al., 2003c). These studies revealed a highly significant linear correlation between MHC class II expression on B cells and area under the corticosterone concentration vs. time curve. It is unlikely that the increased exposure to corticosterone that only cases change of 2.7% in expression of MHC (which is one of the most sensitive immunological end points we have evaluated) could account for the almost complete inhibition of cytokine production observed in the present study. The other approach used in this and previous studies (blocking the action or production of corticosterone and catecholamines), reinforces the idea that the increased glucocorticoids following acute ethanol administration are not a major factor in the immunosuppressive effects of ethanol on cytokine production (Glover and Pruett, 2006).
Figure 1. Corticosterone time course for calculation of the area under the concentration vs. time curve.
Mice were treated with ethanol (6 g/kg by gavage) or vehicle (water) 15 minutes prior to poly I:C (100 μg/mouse, intravenously). Naive groups received no treatment. Blood was collected from different groups (n = 5) at 1, 2, 4, 6, 8, and 10 hours after poly I:C dosing. Corticosterone levels were measured in serum by radioimmunoassay. Values shown are means ± SEM.
Figure 2. Comparison of areas under the corticosterone concentration vs. time curve (AUCs).
Corticosterone concentrations from Figure 1 were used to calculate corticosterone AUCs (as described in Materials and Methods) A, up to 2 hours and B, up to 8 hours and these AUCs were compared using analysis of variance followed by Neuman-Keul’s post hoc test. Values shown are mean AUCs ± SEM (n = 5). The * indicates significant differences (p < 0.05).
Serum cytokines in AUC experiment
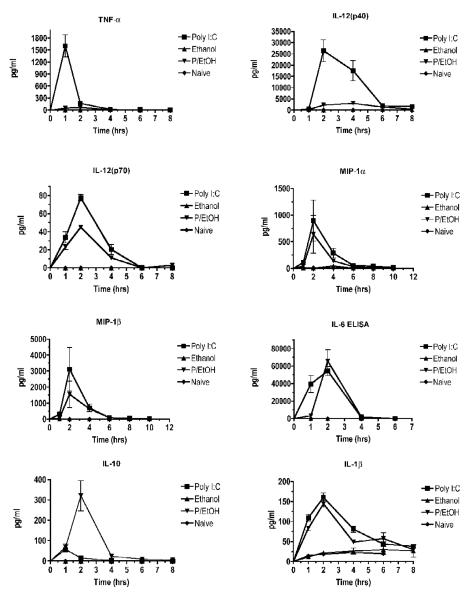
In the experiment shown in Fig. 3, we examined the effects of ethanol alone or in combination with poly I:C on levels of cytokines and chemokines in vivo. Ethanol treatment alone did not cause an increase in any of the cytokines or chemokines tested. As expected, the pro-inflammatory cytokines TNF-α, IL-12 (p40), and IL-12 (p70) were increased by poly I:C treatment, and this increase was at least partially abrogated by ethanol. Of the cytokines tested, the production of IL-1β, IL-6, and IL-10 were not inhibited by ethanol. Chemokines tested were MIP-1α, MIP-1β, and KC. Of these, MIP-1α and MIP-1β were not significantly inhibited by ethanol. KC results in both the poly I:C and poly I:C + ethanol groups were above the range of the standard curve at 1 and 2 hours, later dropping down to naive levels by 8 hours (data not shown). Thus, it is not clear if there was a difference, but a large quantity of KC was produced in both the presence and absence of ethanol.
Figure 3. Cytokine time courses.
Serum collected from mice used in the experiment shown in Figure 1 was used to measure cytokine concentrations for different treatment groups at a range of times after stimulation with poly I:C. Cytokine concentrations (except for IL-6 and IFN-α) were measured using Bioplex multiplex assays. IL-6 and IFN-α were measured by ELISA. Values shown are means ± SEM (n = 5), and values that do not share any lowercase letters above the bar are significantly different (p < 0.05, by ANOVA and Newman-Keul’s post hoc test).
Effects of adrenalectomy, corticosterone replacement, and beta blocker on corticosterone, cytokine, and chemokine concentrations following treatment with poly I:C with or without ethanol
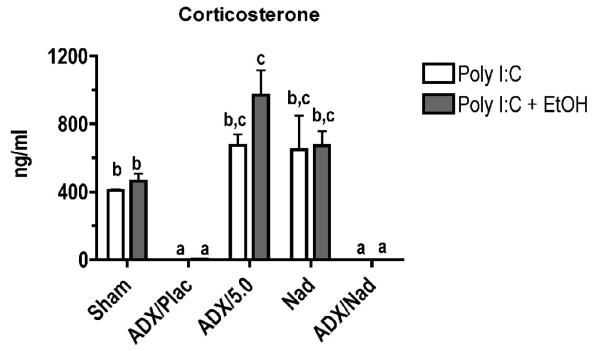
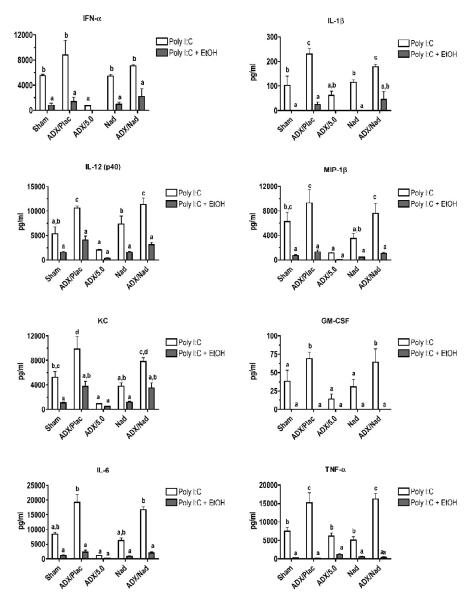
The strategy in this experiment was to evaluate the role of corticosterone (by adding corticosterone back to ADX mice with sustained release pellets), systemic catecholamines (which would not increase as normal in ADX mice), and peripheral catecholamines (the effects of which on β2-adrenergic receptors are blocked by nadolol). As expected, ADX mice without corticosterone replacement pellets produced virtually no corticosterone when treated with poly I:C or poly I:C + ethanol, whereas sham ADX, non-ADX mice with nadolol pellets, and ADX mice with corticosterone replacement pellets all had elevated corticosterone levels (Fig. 4A). It should be noted that all corticosterone concentrations here (except those in ADX mice) are greater than the 50-250 ng/ml (depending on time of day) corticosterone concentrations we have reported in many previous studies using this experimental system. This was expected based on reports that most inflammatory stimuli as well as ethanol are stressors.
Figure 4. Effects of ADX on corticosterone and cytokine concentrations.
Placebo, corticosterone (5.0 mg) and/or nadolol (0.5 mg) pellets were placed subcutaneously in ADX, sham, or untreated mice. One day later, mice were treated with ethanol (6 g/kg by gavage) or appropriate vehicle (water or 0.9% NaCl) 15 minutes prior to poly I:C (100 μg/mouse, intravenously) or vehicle (PBS). Blood was collected 2 hours after poly I:C administration. A) Serum corticosterone levels were measured by enzyme immunoassay. B and C) Cytokine levels (except for IL-6 and IFN-α) were measured using Bioplex multiplex assays. IL-6 and IFN-α were measured by ELISA. Values shown are means ± SEM. Results were analyzed by ANOVA followed by the Newman-Keuls post hoc test. The lowercase letters above the bars indicate the results of that analysis; groups that do not share any letter in common are significantly different (p < 0.05).
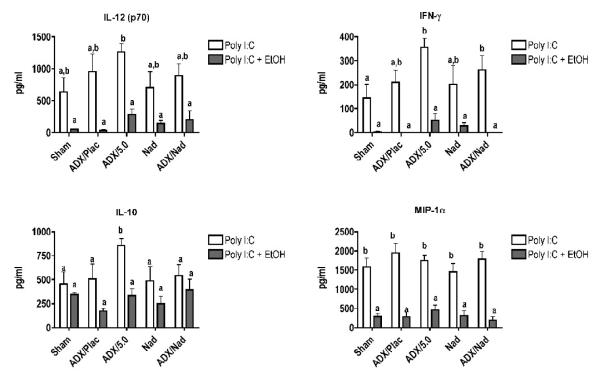
All cytokines and chemokines tested were increased by poly I:C treatment, and they could be categorized by groups according to the association between corticosterone levels and changes in cytokine concentration. In the first group, cytokine/chemokine production (Fig. 4B) was inversely related to corticosterone concentrations (Fig. 4A). This association was particularly evident for IL-6. The production of IL-6 was enhanced substantially in the ADX groups. This also occurred in ethanol treated groups, but comparison of ADX + poly I:C group and the ADX + poly I:C + ethanol group indicates that inhibition of IL-6 production by ethanol is proportionally similar to inhibition of IL-6 in the absence of elevated corticosterone or catecholamine concentrations (in ADX mice). The results for all of the cytokines and chemokines in this first group and in our previous study demonstrate that the stress response to inflammatory stimulus markedly regulates the production of many cytokines, perhaps to prevent adverse effects of excessive cytokine concentrations. For most of these cytokines, it has been previously reported that glucocorticoids can inhibit their production. The second group, consisting of IL-10, IL-12 (p70), IFN-γ, and MIP-1α, did not demonstrate any apparent correlation between corticosterone and cytokine levels (Fig. 4C). Acute ethanol treatment inhibited pro-inflammatory cytokine and chemokine production regardless of corticosterone concentration, even in ADX groups. Groups implanted with nadolol pellets did not demonstrate significant differences from their matching treatment groups (nadolol vs. sham, ADX + Nad + poly I:C vs. ADX + Placebo + poly I:C). This suggests that catecholamines acting through β2-adrenergic receptors are not involved in regulating cytokine production in response to poly I:C. In addition, ethanol had the same effect on cytokine production with or without nadolol treatment for all cytokines and chemokines, indicating that catecholamines acting through β2-adrenergic receptors are not involved in modulation of cytokine and chemokine production by ethanol. In addition, ethanol in ADX mice with corticosterone replacement yielded the same or greater suppression of cytokines/chemokines than observed in Sham ADX mice. If systemic epinephrine played a major role in this suppression, corticosterone alone should not have been sufficient to overcome the lack of both corticosterone and systemic epinephrine response in ADX mice. This is consistent with the results from mice treated with nadolol pellets in suggesting that catecholamines are not a major mediator of cytokine/chemokines production in this experimental system.
Results for IL-10 demonstrated no significant effects of ethanol and an influence of corticosterone only in the ADX group with a corticosterone pellet. This differed somewhat from results in our previous studies, in which ethanol increased IL-10 production in poly I:C-treated mice. However, those earlier studies were performed using a poly I:C preparation that was subsequently found to be contaminated with a significant amount of endotoxin (Pruett et al., 2004). In a recent comparison study in which both the contaminated preparation and an essentially endotoxin-free poly I:C preparation was used, the pattern of change of cytokine production caused by ethanol was similar. In contrast for IL-10, ethanol induced a significant increase when administered with the endotoxin-contaminated poly I:C, but not with the endotoxin-free poly I:C used in our current study (data not shown). Results for IL-1β, IL-12, KC, GM-CSF, IL-6, and TNF-α indicate that loss of the corticosterone and/or systemic epinephrine response in ADX mice caused an increase in poly I:C-induced production of these molecules. Thus, stress mediators are involved in normal regulation of responses of these cytokines and serve to limit excessive production of these cytokines and chemokines.
Discussion
Acute ethanol exposure has been shown to be immunosuppressive, due at least in part to its ability to create a neuroendocrine stress response. This response includes the activation of the hypothalamic-pituitary-adrenal axis, which in mice, primarily culminates in elevated corticosterone concentrations (Carson and Pruett, 1996; Rivier et al., 1984). The results reported here demonstrate that acute ethanol exposure augments the poly I:C-induced corticosterone response, whereas it attenuates the poly I:C-induced pro-inflammatory cytokine production. The increase in corticosterone levels following acute ethanol administration occurs more rapidly than the increase following poly I:C, which, interestingly, coincides with the optimal inhibition by ethanol of the early poly I:C-induced cytokines TNF-α and IL-6. These results at first seem to suggest that corticosterone is a major factor involved in the suppression of cytokines following acute ethanol exposure. However, this is not consistent with the results from ADX mice, since the suppression of cytokine/chemokine production due to ethanol occurs regardless of corticosterone levels, as demonstrated by comparing sham and ADX/placebo mice.
The adrenal gland is the site of both glucocorticoid and catecholamine synthesis. The adrenal cortex, derived embryologically from mesodermal mesenchyme, secretes steroid hormones, including glucocorticoids, whereas the adrenal medulla, derived from neural crest cells, secretes catecholamines, including epinephrine and norepinephrine (Ross et al., 2003). In a stress response, the adrenal medulla releases epinephrine and norepinephrine in approximately a 4:1 ratio (Elenkov et al., 2000). The primary source of norepinephrine in a stress response is from sympathetic nerve endings (Kvetnansky et al., 1979). Therefore, adrenalectomized mice should have a strongly diminished corticosterone and epinephrine response, with only a slightly diminished norepinephrine response to stress. Virtually all lymphoid organs are innervated by sympathetic nerve endings, and all lymphoid cells except Th2 cells express β-adrenergic receptors (Sanders et al., 1997). Expression of α-adrenergic receptors on lymphoid cells is an area of debate, but they are likely not expressed on lymphoid cells, except in certain pathologic conditions, or in certain populations, such as alveolar macrophages (Sanders et al., 1997; Elenkov et al., 2000). Therefore, a non-specific β-blocker such as nadolol should effectively block sympathetic stimulation of cells of the innate immune system.
Nadolol pellets placed in both ADX and non-ADX mice and corticosterone replacement pellets placed in ADX mice provide several lines of evidence that catecholamines do not play a major role in regulation of TLR3-induced cytokine responses or in the inhibition of cytokine production by ethanol. Mice in the ADX groups that received a corticosterone pellet had lower poly I:C-induced concentrations of cytokines than other groups, consistent with a corticosterone concentration greater than measured in all other groups (Fig. 4A). This indicates the importance of corticosterone in suppression of cytokine concentration in this system and suggests that the lower, stress-induced level of corticosterone is sufficient to regulate most cytokines to some degree. The decrease in corticosterone in ADX mice is associated with an increase in poly I:C-induced cytokine concentrations. Two lines of evidence indicate that loss of epinephrine/norepinephrine in ADX mice does not contribute substantially to this effect. First, blocking the action of epinephrine/norepinephrine through β-adrenergic receptors using nadolol did not abrogate the effect of ADX. Second, in our previous study (Glover and Pruett, 2006), a glucocorticoid antagonist (RU 486) and a glucocortioid synthesis inhibitor (aminoglutethimide) yielded similar effects on poly I:C-induced cytokine responses as ADX. However, these agents do not affect catecholamines, so the increased concentration of some cytokines caused by these agents or by ADX must be due to loss of corticosterone, not catecholamines. To our knowledge, this is the first report indicating that TLR3 mediated cytokine production is not substantially regulated by catecholamines acting via β-adrenergic receptors. Cytokine induction mediated by TLR4 has been reported to be regulated by catecholamines (Brown et al., 1991; Ignatowski et al., 1996; Hasko et al., 1998b; Elenkov et al., 1995; Meltzer et al., 2004). A difference between TLR3 and TLR4 in this regard is not particularly surprising, because TLR3 lacks a major branch of the signal transduction pathway activated by TLR4, and this part of the pathway could be the target that allows regulation by catecholamines.
Results from ADX mice indicate that modulation of IL-10 production does not appear to be mediated by either arm of the neuroendocrine stress response nor by ethanol. It is not surprising that IL-10 does not follow the same pattern of response as the other cytokines and chemokines tested (i.e., IL-1β, IL-6, IFN-α, GM-CSF, TNF-α, MIP-1β, and KC) because these are generally considered pro-inflammatory under most conditions, whereas IL-10 is not. Interestingly, IL-12 (p70), IFN-γ, and MIP-1α, however, are considered pro-inflammatory, and yet do not follow the same pattern of inhibition at elevated corticosterone levels as most others tested (note the ADX group with 5 mg corticosterone pellet in Fig. 4C). The molecular basis for this pattern of effects is not known. In fact, mechanisms of inhibition of relevant signaling pathways (e.g., NF-κB) identified by investigators using pharmacological dosages of synthetic glucocorticoids were not identified in mouse T lymphocytes exposed to stress-inducible concentrations of corticosterone (Pruett et al.,, 2003). Additional research is needed to characterize the mechanisms that are important in regulation of cytokine production by stress-inducible concentrations of glucocorticoids.
Both the HPA axis and the SNS play a role in immunoregulation associated with stressors. Since the two systems are so often induced together in vivo, use of in vitro and even ex vivo studies cannot adequately model the complexities of interactions in vivo. In addition, it has been demonstrated that not only viral and bacterial components, but also several cytokines, when injected systemically can increase either corticosterone, norepinephrine, or both differentially (summarized in Dunn, 2006). All of these factors make it difficult to separate the effects due to HPA versus SNS activation on immunosuppression in a whole animal. Adrenalectomy, corticosterone replacement, and a peripheral β-blocker were used in this study to examine the role of glucocorticoids and catecholamines in the ethanol-induced immunosuppression in mice stimulated with poly I:C. Although the HPA axis did appear to mediate suppression of proinflammatory cytokines in mice treated with poly I:C alone, it was not the primary mediator of the suppression of cytokine production caused by acute ethanol administration. Future studies in our lab will focus on the activation of transcription factors involved in TLR3 signaling and the effects of the stress response of acute ethanol administration on their activation.
The most commonly used synthetic agonist for TLR3 is polyinosinic polycytidylic acid (poly I:C), and this analog of double stranded RNA activates both murine and human cells. However, there is some evidence of differences in the regulation of TLR3 expression in these species (Heinz et al., 2003) and in the cell types that can be effectively activated to produce cytokines (Lundberg et al., 2007). However, protection from some viral infections (e.g., Herpes simplex) is clearly mediated by TLR3 in humans as well as mice (Ashkar et al., 2004; Bustamante et al., 2008), and type I interferons are important products of TLR3 activation in both species (Svensson et al., 2007; Matsumoto and Seya, 2008), suggesting that similar signaling pathways are induced and similar regulatory pathways can reasonably be expected. Although there are caveats, it would seem that the basic findings in this mouse model will probably be applicable in humans.
Acknowledgments
This work was supported by a grant from the National Institute on Alcoholism and Alcohol Abuse (grant number R01 AA009505).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ader R, Cohen N, Felten D. Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet. 1995;345:99–103. doi: 10.1016/s0140-6736(95)90066-7. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Ashkar AA, Yao XD, Gill N, Sajic D, Patrick AJ, Rosenthal KL. Toll-like receptor (TLR)-3, but not TLR4, agonist protects against genital herpes infection in the absence of inflammation seen with CpG DNA. Journal Infect Dis. 2004;190:1841–1849. doi: 10.1086/425079. [DOI] [PubMed] [Google Scholar]
- Barazzone-Argiroffo C, Pagano A, Juge C, Metrailler I, Rochat A, Vesin C, Donati Y. Glucocorticoids aggravate hyperoxia-induced lung injury through decreased nuclear factor-kappa B activity. Am J Physiol Lung Cell Mol Physiol. 2003;284:L197–204. doi: 10.1152/ajplung.00239.2002. [DOI] [PubMed] [Google Scholar]
- Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A. The cytokine-HPA axis feed-back circuit. Z Rheumatol. 2000;59(Suppl 2):II/26-30. doi: 10.1007/s003930070014. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, Rey AD. Physiology of psychoneuroimmunology: a personal view. Brain Behav Immun. 2007;21:34–44. doi: 10.1016/j.bbi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Brown R, Li Z, Vriend CY, Nirula R, Janz L, Falk J, Nance DM, Dyck DG, Greenberg AH. Suppression of splenic macrophage interleukin-1 secretion following intracerebroventricular injection of interleukin-1 beta: evidence for pituitary-adrenal and sympathetic control. Cell Immunol. 1991;132:84–93. doi: 10.1016/0008-8749(91)90008-y. [DOI] [PubMed] [Google Scholar]
- Bustamante J, Boisson-Dupuis S, Jouanguy E, Picard C, Puel A, Abel L, Casanova JL. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr Opinion Immunol. 2008;20:39–48. doi: 10.1016/j.coi.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res. 1996;20:132–138. doi: 10.1111/j.1530-0277.1996.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biology of interleukin 1. Faseb J. 1988;2:108–115. [PubMed] [Google Scholar]
- Dobbs CM, Vasquez M, Glaser R, Sheridan JF. Mechanisms of stress-induced modulation of viral pathogenesis and immunity. J Neuroimmunol. 1993;48:151–160. doi: 10.1016/0165-5728(93)90187-4. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Hasko G, Kovacs KJ, Vizi ES. Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha production by selective alpha- and beta-adrenergic drugs in mice. J Neuroimmunol. 1995;61:123–131. doi: 10.1016/0165-5728(95)00080-l. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Fantuzzi G, Di Santo E, Sacco S, Benigni F, Ghezzi P. Role of the hypothalamus-pituitary-adrenal axis in the regulation of TNF production in mice. Effect of stress and inhibition of endogenous glucocorticoids. J Immunol. 1995;155:3552–3555. [PubMed] [Google Scholar]
- Fahey TJ, 3rd, Tracey KJ, Tekamp-Olson P, Cousens LS, Jones WG, Shires GT, Cerami A, Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992;148:2764–2769. [PubMed] [Google Scholar]
- Ghoshal K, Wang Y, Sheridan JF, Jacob ST. Metallothionein induction in response to restraint stress. Transcriptional control, adaptation to stress, and role of glucocorticoid. J Biol Chem. 1998;273:27904–27910. doi: 10.1074/jbc.273.43.27904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover M, Pruett SB. Role of corticosterone in immunosuppressive effects of acute ethanol exposure on Toll-like receptor mediated cytokine production. J Neuroimmune Pharmacol. 2006;1:435–442. doi: 10.1007/s11481-006-9037-z. [DOI] [PubMed] [Google Scholar]
- Han YC, Lin TL, Pruett SB. Thymic atrophy caused by ethanol in a mouse model for binge drinking: involvement of endogenous glucocorticoids. Toxicol Appl Pharmacol. 1993;123:16–25. doi: 10.1006/taap.1993.1216. [DOI] [PubMed] [Google Scholar]
- Han YC, Pruett SB. Mechanisms of ethanol-induced suppression of a primary antibody response in a mouse model for binge drinking. J Pharmacol Exp Ther. 1995;275:950–957. [PubMed] [Google Scholar]
- Hasko G, Szabo C. Regulation of cytokine and chemokine production by transmitters and co-transmitters of the autonomic nervous system. Biochem Pharmacol. 1998a;56:1079–1087. doi: 10.1016/s0006-2952(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Hasko G, Szabo C, Nemeth ZH, Salzman AL, Vizi ES. Stimulation of beta-adrenoceptors inhibits endotoxin-induced IL-12 production in normal and IL-10 deficient mice. J Neuroimmunol. 1998b;88:57–61. doi: 10.1016/s0165-5728(98)00073-3. [DOI] [PubMed] [Google Scholar]
- Heinz S, Haehnel V, Karaghiosoff M, Schwarzfischer L, Muller M, Krause SW, Rehli M. Species-specific regulation of Toll-like receptor 3 genes in men and mice. J Biol Chem. 2003;278:21502–21509. doi: 10.1074/jbc.M301476200. [DOI] [PubMed] [Google Scholar]
- Ignatowski TA, Gallant S, Spengler RN. Temporal regulation by adrenergic receptor stimulation of macrophage (M phi)-derived tumor necrosis factor (TNF) production post-LPS challenge. J Neuroimmunol. 1996;65:107–117. doi: 10.1016/0165-5728(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Cortez V, Kennedy SL, Foley TE, Hanson H, 3rd, Fleshner M. Role of central beta-adrenergic receptors in regulating proinflammatory cytokine responses to a peripheral bacterial challenge. Brain Behav Immun. 2008;22:1078–1086. doi: 10.1016/j.bbi.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitis J, Murdoch E, Gomez C, Ramirez L, Kovacs E. Acute ethanol exposure attenuates pattern recognition receptor activated macrophage functions. Journal of Interferon and Cytokine Research. 2008;28:413–422. doi: 10.1089/jir.2007.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Moynihan JA, Stevens SY, Grota LJ, Felten DL. Chemical sympathectomy has no effect on the severity of murine AIDS: murine AIDS alone depletes norepinephrine levels in infected spleen. Brain Behav Immun. 2002;16:118–139. doi: 10.1006/brbi.2001.0627. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Weise VK, Thoa NB, Kopin IJ. Effects of chronic guanethidine treatment and adrenal medullectomy on plasma levels of catecholamines and corticosterone in forcibly immobilized rats. J Pharmacol Exp Ther. 1979;209:287–291. [PubMed] [Google Scholar]
- Lew KH, Ludwig EA, Milad MA, Donovan K, Middleton E, Jr., Ferry JJ, Jusko WJ. Gender-based effects on methylprednisolone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 1993;54:402–414. doi: 10.1038/clpt.1993.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg AM, Drexler SK, Monaco C, Williams LM, Sacre SM, Feldmann M, Foxwell BM. Key differences in TLR3/poly I:C signaling and cytokine induction by human primary cells: a phenomenon absent from murine cell systems. Blood. 2007;110:3245–3252. doi: 10.1182/blood-2007-02-072934. [DOI] [PubMed] [Google Scholar]
- Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden KS. Catecholamines, sympathetic innervation, and immunity. Brain Behav Immun. 2003;17(Suppl 1):S5–10. doi: 10.1016/s0889-1591(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- McCoy CE, Carpenter S, Palsson-McDermott EM, Gearing LJ, O’Neill LA. Glucocorticoids inhibit IRF3 phosphorylation in response to Toll-like receptor-3 and -4 by targeting TBK1 activation. J Biol Chem. 2008;283:14277–14285. doi: 10.1074/jbc.M709731200. [DOI] [PubMed] [Google Scholar]
- Meltzer JC, MacNeil BJ, Sanders V, Pylypas S, Jansen AH, Greenberg AH, Nance DM. Stress-induced suppression of in vivo splenic cytokine production in the rat by neural and hormonal mechanisms. Brain Behav Immun. 2004;18:262–273. doi: 10.1016/j.bbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Milton NG, Hillhouse EW, Milton AS. Activation of the hypothalamo-pituitary-adrenocortical axis in the conscious rabbit by the pyrogen polyinosinic:polycytidylic acid is dependent on corticotrophin-releasing factor-41. J Endocrinol. 1992;135:69–75. doi: 10.1677/joe.0.1350069. [DOI] [PubMed] [Google Scholar]
- Munck A, Naray-Fejes-Toth A. The ups and downs of glucocorticoid physiology. Permissive and suppressive effects revisited. Mol Cell Endocrinol. 1992;90:C1–4. doi: 10.1016/0303-7207(92)90091-j. [DOI] [PubMed] [Google Scholar]
- Naitoh Y, Fukata J, Tominaga T, Nakai Y, Tamai S, Mori K, Imura H. Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious, freely-moving rats. Biochem Biophys Res Commun. 1988;155:1459–1463. doi: 10.1016/s0006-291x(88)81305-6. [DOI] [PubMed] [Google Scholar]
- Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q. Characterization of glucocorticoid receptor translocation, cytoplasmic IkappaB, nuclear NFkappaB, and activation of NFkappaB in T lymphocytes exposed to stress-inducible concentrations of corticosterone in vivo. Int Immunopharmacol. 2003a;3:1–16. doi: 10.1016/s1567-5769(02)00081-4. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q. Acute ethanol administration profoundly alters poly I:C-induced cytokine expression in mice by a mechanism that is not dependent on corticosterone. Life Sci. 2003b;72:1825–1839. doi: 10.1016/s0024-3205(02)02507-9. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Myers LP, Hebert P. Modeling and predicting immunological effects of chemical stressors: characterization of a quantitative biomarker for immunological changes caused by atrazine and ethanol. Toxicol Sci. 2003c;75:343–354. doi: 10.1093/toxsci/kfg200. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Schwab C, Zheng Q, Fan R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J Immunol. 2004;173:2715–2724. doi: 10.4049/jimmunol.173.4.2715. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Ross MH, Kaye GI, Pawlina W. Histology: A Text and Atlas With Cell and Molecular Biology. Lippincott Williams and Wilkins; Baltimore, MD: 2003. [Google Scholar]
- Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol. 1997;158:4200–4210. [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol. 1990;145:1430–1434. [PubMed] [Google Scholar]
- Svensson A, Bellner L, Magnusson M, Eriksson K. Role of IFN-alpha/beta signaling in the prevention of genital herpes virus type 2 infection. J Reprod Immunol. 2007;74:114–123. doi: 10.1016/j.jri.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Dunn AJ. Mouse interleukin-6 stimulates the HPA axis and increases brain tryptophan and serotonin metabolism. Neurochem Int. 1998;33:143–154. doi: 10.1016/s0197-0186(98)00016-3. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Seibring M, Nelson TF, Lee H. Trends in college binge drinking during a period of increased prevention efforts. Findings from 4 Harvard School of Public Health College Alcohol Study surveys: 1993-2001. J Am Coll Health. 2002;50:203–217. doi: 10.1080/07448480209595713. [DOI] [PubMed] [Google Scholar]
- Weiss PA, Collier SD, Pruett SB. Role of glucocorticoids in ethanol-induced decreases in expression of MHC class II molecules on B cells and selective decreases in spleen cell number. Toxicol Appl Pharmacol. 1996;139:153–162. doi: 10.1006/taap.1996.0154. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Pruett SB. Suppression of splenic natural killer cell activity in a mouse model for binge drinking. II. Role of the neuroendocrine system. J Pharmacol Exp Ther. 1996;278:1331–1339. [PubMed] [Google Scholar]
- Wu WJ, Pruett SB. Involvement of catecholamines and glucocorticoids in ethanol-induced suppression of splenic natural killer cell activity in a mouse model for binge drinking. Alcohol Clin Exp Res. 1997;21:1030–1036. [PubMed] [Google Scholar]