Abstract
Background
Patients with acute stroke are at risk for pneumonia and urinary tract infection (UTI). Identifying stroke patients at high risk for common infections could enhance timely treatment and improve clinical outcomes. We aimed to identify risk factors associated with the occurrence of pneumonia and UTI during stroke hospitalization.
Methods
We analyzed the frequency of pneumonia and UTI and their influence on outcomes during hospitalization in patients diagnosed with ischemic stroke in the California Acute Stroke Prototype Registry (CASPR). Generalized estimating equations were used to identify factors and outcomes independently associated with pneumonia and UTI.
Results
Overall, 663 patients were admitted with acute ischemic stroke at 11 hospitals. Pneumonia occurred in 66 (10%), and UTI in 84 (13%). Older age, atrial fibrillation and congestive heart failure were independently associated with greater risk for developing pneumonia, while a history of dementia was associated with lesser risk. Women and patients with a prior history of cerebrovascular events were significantly more likely to experience a UTI. Both pneumonia and UTI were associated with significantly greater length of stay, but only pneumonia was independently associated with higher inpatient mortality and poorer discharge ambulatory status.
Conclusions
Several factors are associated with an increased risk of developing pneumonia and UTI during ischemic stroke hospitalization. Early identification and treatment of these patients may improve clinical outcomes.
Keywords: Infection, hospitalization, stroke, pneumonia, UTI
INTRODUCTION
Medical complications may account for 30% or more of the deaths resulting from acute ischemic stroke. 1 In-hospital infection is one of the leading post-stroke complications, 2,3 and its occurrence predicts prolonged hospital stay and significant disability after stroke. 4,5 Furthermore, several studies have shown that elevated body temperature (which often results from infection) early after ischemic stroke is associated with severe neurological deficit and a poor outcome. 6–9 Indeed, pneumonia and urinary tract infection (UTI) are among the most frequent individual infective complications encountered following acute stroke. 1,10
Given the aforementioned, heightened awareness of infective complications during stroke hospitalization is underscored in national guidelines, and antibiotics to treat infection after stroke are strongly recommended. 11 However, beyond knowledge that infection is more likely to occur in stroke patients unable to cough or who are immobile, 11 the identification of risk factors associated with common post-stroke infections could permit clinicians to provide the close surveillance and timely management of stroke patients at highest risk for infective complications, thereby optimizing clinical outcomes. Few studies have explored risk factors for post-stroke infection outside of a clinical trial setting, 3 or included a broad spectrum of hospital types that cater to the acute stroke patient. 6, 10, 12–14
In this prospective study of representative California hospitals, we aimed to identify factors associated with an increased risk for experiencing pneumonia or UTI during acute stroke hospitalization.
METHODS
Data from the California Acute Stroke Prototype Registry (CASPR) were analyzed. CASPR’s study methods have been previously described.15 CASPR collected prospective data on acute stroke care in individuals with a diagnosis of suspected stroke or TIA, in 11 hospitals in five major population regions of California from November 1, 2002 through January 31, 2003, and from November 1, 2003 though January 31, 2004. CASPR hospitals comprised four university hospitals, four community hospitals, two county hospitals and one health maintenance organization hospital. Human subjects review boards at each participating center approved CASPR. For these analyses, all patients with a discharge diagnosis of ischemic stroke who were admitted during either time period were included. Using a case report form containing a dedicated list of pre-specified variables, we identified incidence rates for any pneumonia and UTI per patient during the stroke hospitalization. We then assessed risk factors independently and separately associated with pneumonia and UTI. Furthermore, we examined the relationship between the occurrences of pneumonia and UTI and hospital outcomes, including length of hospital stay in days, ambulation status at the time of discharge, and inpatient mortality.
Statistical analysis
The chi-squared test was used to evaluate the homogeneity of hospitals in the registry with respect to the incidence of pneumonia and UTI. Because a significant difference in infection incidence rates was observed among hospitals, generalized estimating equations (GEE) were used to account for both within-hospital and between-hospital variances. Potential predictors of each infection were separately examined in univariate models, and variables significant at the p=0.10 level were included in multivariable models.
To examine the association between infectious events and length of hospital stay, we utilized Cox proportional hazards to model time to discharge. Pneumonia and UTI as predictors of both ambulatory status at discharge (defined as a binary variable, walking vs. not walking) and in-hospital mortality were modeled using GEE. Analyses included both unadjusted models, and models adjusted for potential confounders, including age, gender, diabetes, prior vascular event, smoking, dementia and atrial fibrillation.. Variables significant at the 0.10 level in univariate analysis were included in multivariable models. SAS (version 8e, SAS Institute, Cary, NC) was used for all statistical analysis.
RESULTS
Overall, 663 patients were diagnosed with ischemic stroke at the 11 CASPR hospitals. Complete data regarding presence or absence of UTI were available for all 663 CASPR patients, and complete pneumonia data for 660 patients. Males constituted 45.0% of the cohorts and 56.1% were white. During stroke hospitalization, pneumonia occurred in 66 (10%) patients, while UTI occurred in 84 (13%) patients. Seventeen patients (2.6%) had both pneumonia and UTI. Rates of infection varied significantly among hospitals for both pneumonia, which ranged from 0 to 27% of patients, and UTI, which varied between 5 and 22% of patients.
Risk Factors Associated with Infection
In univariate analyses, patients were more likely to experience pneumonia if they were older, had atrial fibrillation, were treated at an academic facility, or had a history of coronary artery disease, congestive heart failure, and valve prosthesis (Table 1). Patients with dementia appeared less likely to contract pneumonia. In multivariable analysis, older age, current atrial fibrillation, and a history of congestive heart failure remained significantly associated with an increased risk of pneumonia, and dementia with a lessened risk. In univariate analyses, patients who were older, female, had atrial fibrillation, and a history of vascular events or hypertension were more likely to be diagnosed with a UTI (Table 2). In multivariable analysis, only female gender and a prior history of cerebrovascular events were significantly associated with increased risk of infection at the p = 0.05 level.
Table 1.
Risk Factors for Pneumonia (N=660)
| Characteristic | Pneumonia (N=66) | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | OR | 95% CI | P value | OR | 95% CI | P value | ||
| Decade | 1.35 | (1.20, 1.52) | <.0001 | 1.20 | (1.08, 1.34) | 0.001 | |||
| Gender | |||||||||
| Female (n=363) | 33 | (9.1) | Reference | ||||||
| Male (n=297) | 33 | (11.1) | 1.11 | (0.66, 1.87) | 0.70 | ||||
| Race/Ethnicity | |||||||||
| White (n=370) | 40 | (10.8) | Reference | ||||||
| Others (n=290) | 26 | (9.0) | 0.90 | (0.52, 1.55) | 0.69 | ||||
| History of | |||||||||
| Stroke/TIA (n=233) | 24 | (10.3) | 1.35 | (0.81, 2.23) | 0.24 | ||||
| MI (n=53) | 6 | (11.5) | 1.14 | (0.44, 2.96) | 0.79 | ||||
| CAD (n=108) | 16 | (15.0) | 1.74 | (0.96, 3.13) | 0.07 | 1.22 | (0.65, 2.27) | 0.54 | |
| CHF (n=81) | 16 | (19.8) | 3.02 | (1.71, 5.31) | .0001 | 2.04 | (1.06, 3.93) | 0.03 | |
| Valve Prosthesis (n=15) | 3 | (20.0) | 2.16 | (0.97, 4.81) | 0.06 | 1.25 | (0.43, 3.61) | 0.68 | |
| Hypertension (n=461) | 52 | (11.3) | 1.62 | (0.79, 3.29) | 0.19 | ||||
| Dyslipidemia (n=210) | 19 | (9.1) | 0.76 | (0.29, 2.02) | 0.59 | ||||
| Diabetes Mellitus (n=166) | 22 | (13.3) | 1.56 | (0.83, 2.95) | 0.17 | ||||
| Dementia (n=42) | 1 | (2.4) | 0.23 | (0.08, 0.68) | 0.008 | 0.18 | (0.05, 0.69) | 0.01 | |
| Atrial Fibrillation (n=129) | 24 | (18.6) | 2.57 | (1.55, 4.26) | .0003 | ||||
| Current Atrial Fibrillation (n=160) | 30 | (18.8) | 2.79 | (1.86, 4.19) | <.0001 | 2.01 | (1.39, 2.89) | 0.0002 | |
| Academic Ctr. (n=225) | 53 | (23.6) | 2.62 | (1.05, 6.49) | 0.04 | 2.45 | (0.86, 7.00) | 0.10 | |
TIA=transient ischemic attack; MI=myocardial infarction; CAD=coronary artery disease; CHF=congestive heart failure; Ctr=center
Table 2.
Risk Factors for Urinary Tract Infection (UTI) (N=663)
| Characteristic | UTI (N=84) | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | OR | 95% CI | P value | OR | 95% CI | P value | ||
| Decade | 1.20 | (1.01, 1.43) | 0.04 | 1.06 | (0.86, 1.32) | 0.58 | |||
| Gender | |||||||||
| Female (n=365) | 57 | (15.6) | Reference | Reference | |||||
| Male (n=298) | 27 | (9.1) | 0.50 | (0.33, 0.74) | .0006 | 0.54 | (0.34, 0.86) | 0.009 | |
| Race/Ethnicity | |||||||||
| White (n=372) | 45 | (12.1) | Reference | ||||||
| Others (n=291) | 39 | (13.4) | 1.20 | (0.63, 2.29) | 0.59 | ||||
| History of | |||||||||
| Stroke/TIA (n=233) | 37 | (15.9) | 1.69 | (1.18, 2.41) | 0.004 | 1.51 | (1.10, 2.06) | 0.01 | |
| MI (n=53) | 6 | (11.3) | 0.86 | (0.36, 2.05) | 0.73 | ||||
| CAD (n=108) | 13 | (12.0) | 0.91 | (0.47, 1.74) | 0.77 | ||||
| CHF (n=81) | 10 | (12.4) | 0.99 | (0.52, 1.87) | 0.98 | ||||
| Valve Prosthesis (n=15) | 1 | (6.7) | 0.47 | (0.09, 2.35) | 0.36 | ||||
| Hypertension (n=461) | 64 | (13.9) | 1.49 | (1.05, 2.12) | 0.02 | 1.27 | (0.87, 1.86) | 0.21 | |
| Dyslipidemia (n=210) | 30 | (14.3) | 1.19 | (0.76, 1.84) | 0.45 | ||||
| Diabetes Mellitus (n=166) | 23 | (13.9) | 1.14 | (0.65, 1.97) | 0.65 | ||||
| Dementia (n=42) | 3 | (7.1) | 0.54 | (0.14, 2.02) | 0.36 | ||||
| Atrial Fibrillation (n=129) | 25 | (19.4) | 1.94 | (1.28, 2.95) | 0.002 | ||||
| Current Atrial Fibrillation (n=160) | 31 | (19.4) | 2.01 | (1.19, 3.40) | 0.009 | 1.74 | (0.92, 3.27) | 0.09 | |
| Academic Ctr. (n=225) | 37 | (16.4) | 1.59 | (0.89, 2.85) | 0.12 | ||||
TIA=transient ischemic attack; MI=myocardial infarction; CAD=coronary artery disease; CHF=congestive heart failure; Ctr=center
Infection as a Risk Factor for Hospital Outcomes
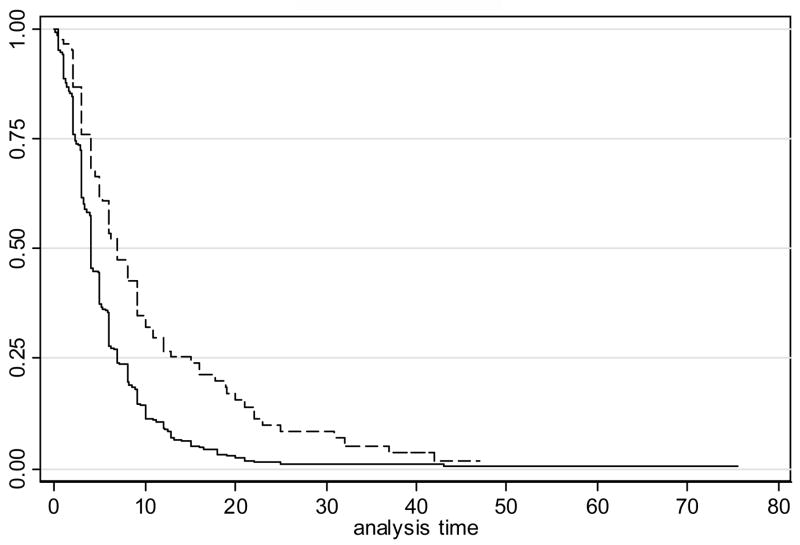
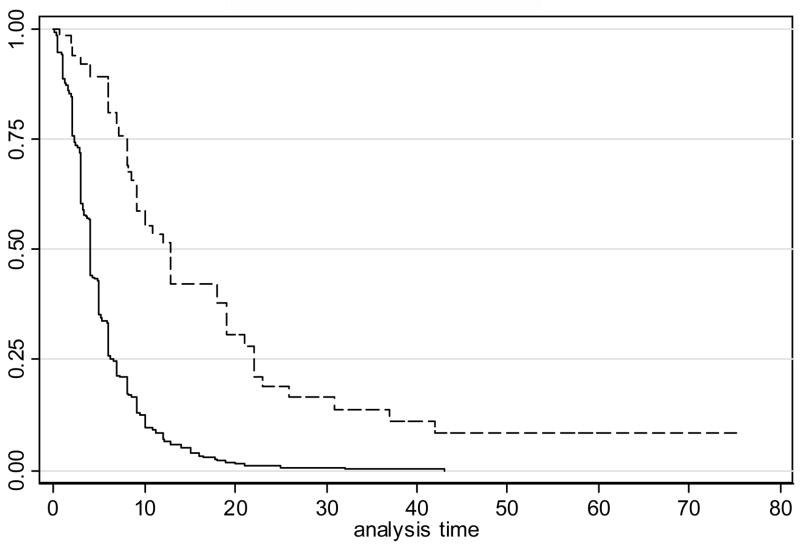
The median time to discharge among patients without UTI was 4 days {Interquartile range [IQR] (2, 7)}, compared to a median of 7 days {IQR (4, 15)} for those with a UTI (RH=0.54, p<0.001). The relative hazard remained highly significant even when adjusted for age, gender, atrial fibrillation, and a history of prior vascular events, diabetes, smoking and dementia (p<0.001). The median time to discharge for patients without pneumonia was 4 days {IQR (2, 6)}, compared to a median time of 13 days {IQR (8, 22)} among those with pneumonia (RH=0.28, p<0.0001 without adjustment, 0.26, p<0.0001 with adjustment). Kaplan-Meier curves depicting time to discharge for those with and without UTI, and those with and without pneumonia, are displayed in Figures 1 and 2, respectively.
Figure 1.
Kaplan-Meier curves depicting time to discharge for those with and without urinary tract infection (UTI)
Figure 2.
Kaplan-Meier curves depicting time to discharge for those with and without pneumonia
At hospital discharge, 26.2% of CASPR subjects who had a UTI were independently ambulatory compared to 42.5% of those without a UTI. This difference was not significant in either unadjusted (OR = 0.52 {95% CI (0.23, 1.17)} p=0.12) or adjusted (OR = 0.65 {95% CI (0.32, 1.58)} p=0.34) analyses. Among those with pneumonia, 9.1% were able to walk without assistance at discharge, compared to 42.5% of those who had not contracted pneumonia. This difference was highly significant (p<0.0001) in both unadjusted (OR = 0.14 {95% CI (0.08, 0.25)} and adjusted (OR = 0.16 {95% CI (0.09, 0.29) analyses. Death occurred during hospitalization in 8.3% of patients with a UTI compared to 7.4% of patients without a UTI (1.05 (0.56, 1.96) p=0.88, unadjusted; 0.83 (0.43, 1.57) p=0.56, adjusted). On the other hand, patients with pneumonia were about 6 times as likely (27.3% vs. 5.3%) to die in hospital as those without pneumonia (6.38 (3.83, 10.6) p=<0.0001, unadjusted; 5.96 (3.02, 11.7) p<0.0001, adjusted). This risk remained significantly elevated even when adjusted for age, gender, atrial fibrillation, and a history of prior vascular event, diabetes, smoking or dementia.
DISCUSSION
This study shows that at representative hospitals in California, almost one of every eight individuals discharged from the hospital following an ischemic stroke or TIA develops either a UTI or pneumonia, and it confirms that the incidence of UTI tends to be more common than pneumonia. 3, 16 A major advantage of our study over previously conducted analyses of hospital infection during stroke is the evaluation of routine stroke practice at multiple hospitals operating within different healthcare settings.
Our study confirmed the association of pneumonia with older age and atrial fibrillation noted in prior studies of hospitalized stroke patients, 3, 12, 17 but also identified an independent relationship between pneumonia and having a history of congestive heart failure. Congestive heart failure has not been previously evaluated as a potential predictor of pneumonia. Another unique finding of our study was that CASPR patients with a history of dementia were less likely to contract pneumonia. The reason for this association is not entirely clear, but we speculate that perhaps increased surveillance and prophylactic measures (such as airway protection) against pneumonia were undertaken early during the stroke hospitalization, because of the known baseline cognitive impairment in these patients. We found that women and having a history of prior stroke or TIA predicted in-hospital UTI. Female gender and prior stroke have both previously been shown to predict UTI following acute ischemic stroke. 3, 17
There was a wide variation in incidence rates among participating hospitals with regard to the occurrence of pneumonia. Univariately, stroke patients who were treated at academic institutions were more likely to have pneumonia. Although this difference between pneumonia rates in academic institutions vs. others did not reach statistical significance in multivariable analysis, the tertiary referral role which academic stroke centers often play would likely have led to sicker patients, with more severe strokes, being treated at these academic hospitals. On the other hand, the disparity in pneumonia rates may also signify the need for a more uniform approach across hospital types, towards the detection and prevention of infections during stroke hospitalization.
We also evaluated the influence of pneumonia and UTI on several outcomes. The occurrence of both infections was significantly associated with greater length of stay, as has been previously reported, 4, 18 although it is not clear whether the presence of the infections delayed the time of hospital discharge, or if longer hospital stay (for other reasons) created more of an opportunity for exposure to the risk of nosocomial contamination, thereby increasing the incidence of these infections. 12 We noted a marked difference in the influence of UTI vs. pneumonia on discharge ambulatory status and inpatient mortality rates. Patients with pneumonia were much less likely to be independently ambulatory and alive at the time of hospital discharge. The higher incidence of poor outcomes, including mortality in stroke patients with pneumonia vs. UTI, has been shown in a previous study. 3
This study has limitations. First, it was a secondary analysis of a completed study, and was not specifically designed to address the issues being evaluated. Thus, although we controlled for clinical and biological factors that are known to influence outcomes in ischemic stroke, we cannot exclude the possibility that unmeasured confounding may explain some of our findings. Furthermore, the CASPR questionnaire did not include any index of stroke severity. Assessment of stroke severity may have confounded the apparent independent contribution of atrial fibrillation to the incidence of pneumonia, 19 although it has been shown that atrial fibrillation may confer increased risk for pneumonia beyond just severity of clinical presentation.17 Lastly, stroke location, which may also influence the extent of medical complications following stroke, 10, 12 was not evaluated in this study.
In conclusion, a highly proactive approach is warranted in all hospitalized stroke patients, in order to identify and treat any infections early, thereby improving outcome. Potentially modifiable factors are possible targets for interventions to reduce the burden of illness and healthcare costs of stroke. Otherwise, a general intervention, such as the implementation of a stroke clinical care pathway, may be a suitable option for promoting favorable stroke outcomes. Indeed, it has been shown that stroke patients managed with care pathways are less likely to suffer inpatient infections and more likely to have a reduced length of hospital stay. 18, 20, 21
Acknowledgments
Grant Support: This study was supported by the Center for Disease Control (Johnston, U50 CCU920271), and in part by NIH-NINDS (Saver, P50 NS044378).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brott T. Prevention and management of medical complications of the hospitalized elderly stroke patient. Clin Geriatr Med. 1991;7:475–482. [PubMed] [Google Scholar]
- 2.Hilker R, Poetter C, Findeisen N, et al. Nosocomial pneumonia after acute stroke: Implications for neurological intensive care medicine. Stroke. 2003;34:975–981. doi: 10.1161/01.STR.0000063373.70993.CD. [DOI] [PubMed] [Google Scholar]
- 3.Aslanyan S, Weir CJ, Diener HC, et al. Pneumonia and urinary tract infection after acute ischaemic stroke: A tertiary analysis of the gain international trial. Eur J Neurol. 2004;11:49–53. doi: 10.1046/j.1468-1331.2003.00749.x. [DOI] [PubMed] [Google Scholar]
- 4.Spratt N, Wang Y, Levi C, et al. A prospective study of predictors of prolonged hospital stay and disability after stroke. J Clin Neurosci. 2003;10:665–669. doi: 10.1016/j.jocn.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima M, Watanabe-Hara R, Inatomi Y, et al. [Respiratory infectious complications after acute ischemic stroke] Rinsho Shinkeigaku. 2002;42:917–921. [PubMed] [Google Scholar]
- 6.Grau A, Buggle F, Schnitzler P, et al. Fever and infection early after ischemic stroke. J Neurol Sci. 1999;171:115–120. doi: 10.1016/s0022-510x(99)00261-0. [DOI] [PubMed] [Google Scholar]
- 7.Azzimondi G, Bassein L, Nonino F, et al. Fever in acute stroke worsens prognosis. A prospective study. Stroke. 1995;26:2040–2043. doi: 10.1161/01.str.26.11.2040. [DOI] [PubMed] [Google Scholar]
- 8.Hajat C, Hajat S, Sharma P. Effects of poststroke pyrexia on stroke outcome : A meta-analysis of studies in patients. Stroke. 2000;31:410–414. doi: 10.1161/01.str.31.2.410. [DOI] [PubMed] [Google Scholar]
- 9.Reith J, Jorgensen HS, Pedersen PM, et al. Body temperature in acute stroke: Relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347:422–425. doi: 10.1016/s0140-6736(96)90008-2. [DOI] [PubMed] [Google Scholar]
- 10.Hamidon B, Raymond AA, Norlinah MI, et al. The predictors of early infection after an acute ischaemic stroke. Singapore Med J. 2003;44:344–346. [PubMed] [Google Scholar]
- 11.Adams H, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the stroke council of the american stroke association. Stroke. 2003;34:1056–1083. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 12.Upadya A, Thorevska N, Sena KN, et al. Predictors and consequences of pneumonia in critically ill patients with stroke. J Crit Care. 2004;19:16–22. doi: 10.1016/j.jcrc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa T, Sekizawa K, Arai H, et al. High incidence of pneumonia in elderly patients with basal ganglia infarction. Arch Intern Med. 1997;157:321–324. [PubMed] [Google Scholar]
- 14.Ween J, Alexander MP, D'Esposito M, et al. Incontinence after stroke in a rehabilitation setting: Outcome associations and predictive factors. Neurology. 1996;47:659–663. doi: 10.1212/wnl.47.3.659. [DOI] [PubMed] [Google Scholar]
- 15.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64:654–659. doi: 10.1212/01.WNL.0000151850.39648.51. [DOI] [PubMed] [Google Scholar]
- 16.Hung J, Tsay TH, Chang HW, et al. Incidence and risk factors of medical complications during inpatient stroke rehabilitation. Chang Gung Med J. 2005;28:31–38. [PubMed] [Google Scholar]
- 17.Hanchaiphiboolkul S. Risk factors for early infection after an acute cerebral infarction. J Med Assoc Thai. 2005;88:150–155. [PubMed] [Google Scholar]
- 18.Werner G, Gadomski M, Scheinert B. [The significance of urinary tract infections in patients with cerebrovascular diseases during clinical rehabilitation] Rehabilitation (Stuttg) 1998;37:64–67. [PubMed] [Google Scholar]
- 19.Kimura K, Minematsu K, Yamaguchi T. Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76:679–683. doi: 10.1136/jnnp.2004.048827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwan J, Sandercock P. In-hospital care pathways for stroke. Cochrane Database Syst Rev. 2004:CD002924. doi: 10.1002/14651858.CD002924.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esteve M, Serra-Prat M, Zaldivar C, et al. [impact of a clinical pathway for stroke patients] Gac Sanit. 2004;18:197–204. doi: 10.1016/s0213-9111(04)71833-6. [DOI] [PubMed] [Google Scholar]