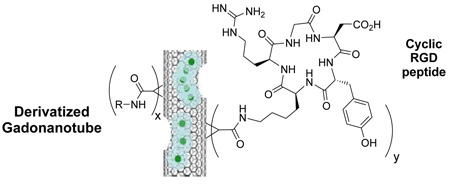
Abstract
A new Rh6(CO)16-catalyzed functionalization of gadonanotube MRI probes offers the opportunity to prepare a number of amino acid- and peptide-derivatized gadonanotubes under RT conditions, containing, for example, the cyclic RGD peptide for the biological targeting of cancer.
Gadonanotubes (Gd@US-tubes) are recently reported high-performance magnetic resonance imaging (MRI) contrast agents derived from ultra-short single-walled carbon nanotubes (US-tubes) internally loaded with clusters of Gd3+ ions.1,2 In fact, the gadonanotubes are the highest performing T1-weighted MRI contrast agent known with a relaxivity (per Gd3+ ion) of up to 40 times greater than that for Magnevist®, a typical Gd3+-ion clinical agent. Here we report the first functionalization of gadonanotubes with various amino acids and peptides using a strategy designed to produce both biocompatibility and biological targeting. The formation of SWNT η2 complexes with rhodium compounds have been described and the catalytic properties of these complexes explored,3 however, to our knowledge, the new functionalization procedure reported here is the first transition-metal-catalyzed derivatization of a carbon nanotube material of any nature. Other US-tube materials such as I2@US-tubes4 and 211AtCl@US-tubes5 could also benefit from the derivatization procedure described in this work.
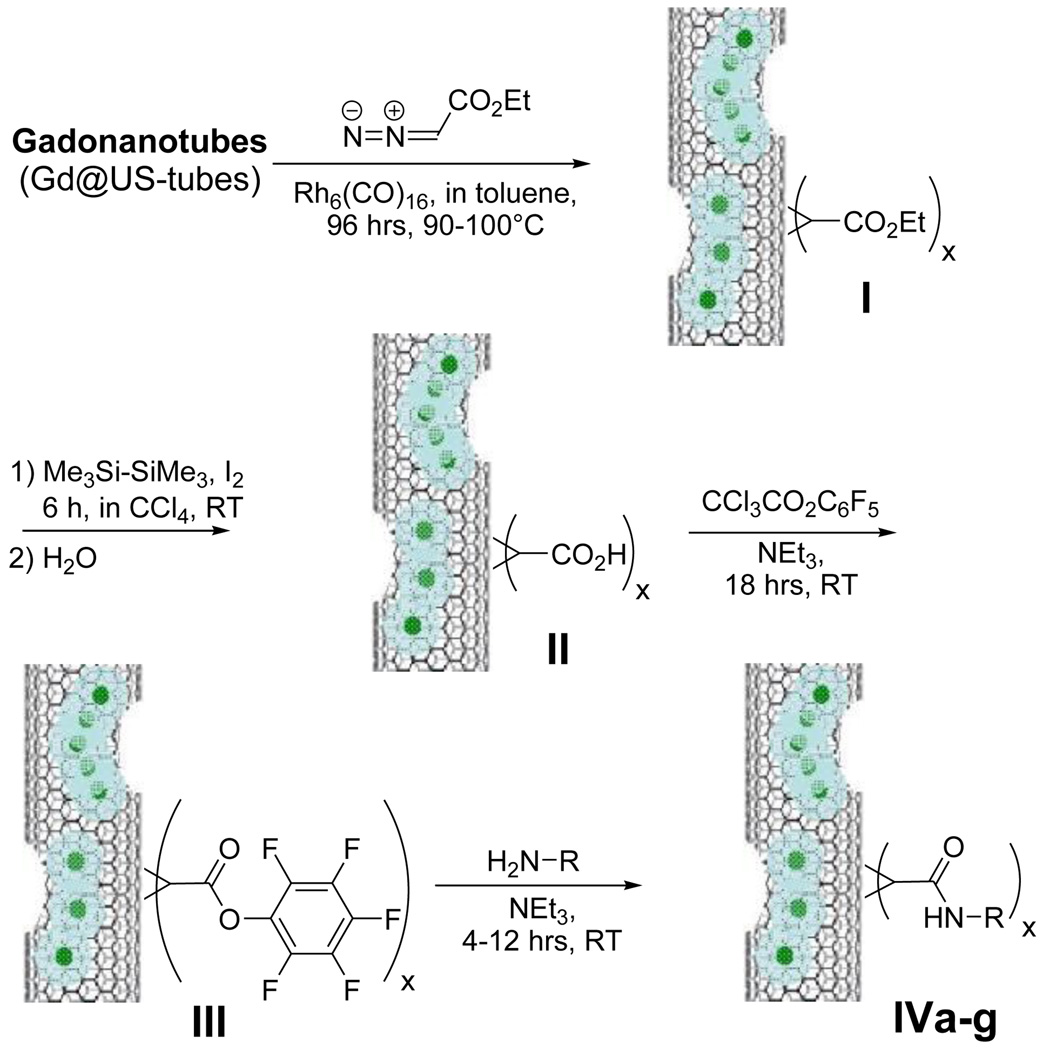
Gadonanotubes were prepared from HiPCO-produced single-walled carbon nanotubes (SWNTs) and characterized as previously described.2 In general, we selected HiPCO gadonanotubes for this study because they are among of the smallest diameter carbon nanotubes, and thus, they exhibit greater sidewall chemical reactivity than most other SWNT materials. Derivatization of the gadonanotubes was accomplished by the cyclopropanation reaction of Scheme 1 using diazoacetic ester in the presence of the catalyst hexarhodium(0) hexadecacarbonyl, Rh6(CO)16. Although the non-catalyzed reaction of arenediazonium reagents with SWNTs has been described,6 functionalization of SWNTs by diazoesters has not been previously reported. As shown in Scheme 1 and Table 1, the resulting ester-functionalized gadonanotubes (I) are especially useful as intermediates for the covalent attachment of an assortment of amino acid and peptide moieties to the external sidewalls. After trying a variety of potential metal catalysts and solvent combinations that have been used for olefin cyclopropanation with diazoester,7 Rh6(CO)16 and toluene, were found to be the most effective catalyst/solvent combination for the initial cyclopropanation reaction in Scheme 1 (see also Supporting Information). Without the Rh6(CO)16 catalyst, the reaction does not proceed to I. Substitution of full-length, purified HiPCO SWNTs8 for gadonanotubes in Scheme 1 results in derivatization but with less than one-fourth the coverage per nanometer (nm−1) (data not shown), suggesting that much of the cyclopropanation reaction for gadonanotubes occurs at the sidewall defect sites through which Gd3+-ion loading occurs.2,9 Using empty US-tubes in Scheme 1 instead of gadonanotubes produces derivatived US-tubes with approximately the same coverage nm−1 as the gadonanotubes (Table 1).
Scheme 1.
Catalytic functionalization of the gadonanotubes.
Table 1.
Characterization of the gadonanotubes and their derivatives.
| Compound | Number1 of groups nm−1 |
Solubility2 in water at RT, mg/ml |
Relaxivity3, r1/Gd3+, mM−1·s−1 |
|||
|---|---|---|---|---|---|---|
| x | y | |||||
| Gd@US-tube | n/a | 0 | 0 | 0 | 64 | |
| I | - OEt | 14–28 | - | 0 | 28 | |
| II | - OH | 14–19 | - | 0 | ||
| III | - OC6F5 | 14–19 | - | reacts | n/a | |
| IVa | 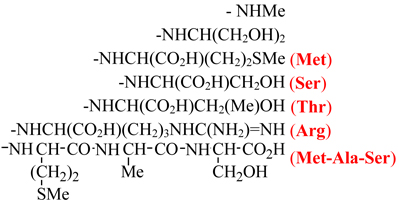 |
12–19 | - | 0 | 60 | |
| IVb | 8–13 | - | 1–2 | >30 | ||
| IVc | 8–12 | - | 0 | 21 | ||
| IVd | 12–15 | - | 1–2 | 47 | ||
| IVe | 8–10 | - | 1–3 | 49 | ||
| IVf | 10–13 | - | 1–2 | 33 | ||
| IVg | 3–11 | - | 0.05–0.2 | -4 | ||
| Va | RGD | - | 0.65–1 | 0 | -4 | |
| Vb | RGD+ Met | 8–12 | 0.65–1 | 0 | -4 | |
| Vc | RGD + Ser | 8–12 | 0.65–1 | 1–2 | 33 | |
From combined XPS (for C, N and S) and TGA data. The range of values shown is for three different sample preparations. Ref.13 describes the method used to determine the number of groups nm−1.
Determined by UV–vis spectroscopy, using the method described in Ref. 13. The range of values shown is for three different sample preparations.
T1-weighted relaxivity per Gd3+ ion at 1.5T and 40° C. All compounds, except IVd and IVf, were suspended in 1% Pluronic® F 108 solution (aq.); IVd and IVf were suspended in DI water.
The sample was prepared using US-tubes (no Gd3+).
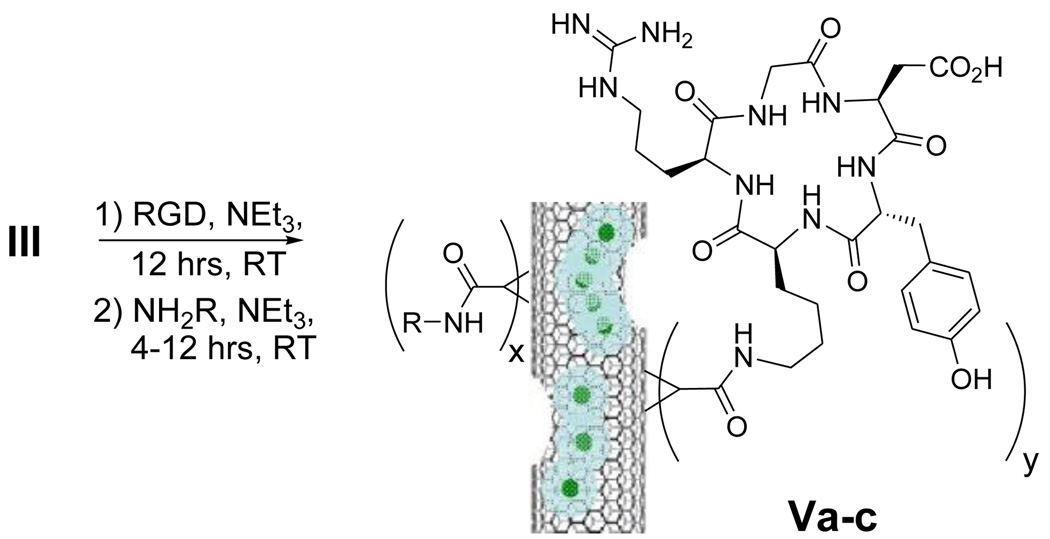
Next, I was hydrolyzed using hexamethyldisilane to produce 1H-cyclopropan-Gd@US-tube-carboxylic acid (II).10 Compound II was then converted into the reactive intermediate, pentafluorophenyl 1H-cyclopropan-Gd@US-tube-1-carboxylate (III), by means of perfluorophenyl 2,2,2-trichloroacetate.11 Compound III, which is sensitive to moisture but stable under anhydrous conditions for months, readily reacts under mild conditions with sterically-unhindered primary amines in solvents like dioxane, methylene chloride, DMF, or their mixtures, giving various amides (compounds IV, Scheme 1). Compound III was used as a reactant to produce a number of amino acid derivatives (IVc-f), a serinol derivative (IVb), and a methylamine derivative (IVa) (Scheme 1, Table 1). Compound IVa was then used to estimate the number of R groups nm−1 of gadonanotube sidewall using XPS to determine the atomic percentage of N. Thus, Scheme 1 is a convenient method to make gadonanotubes water-soluble by attaching hydroxyl group-containing compounds such as serinol or amino acids like threonine and serine, without the need to protect the carboxylic acid group of the amino acid. The reaction with higher molecular weight compounds like peptides takes longer and never proceeds quantitatively. For example, the reaction with the cyclic RGD peptide, which is known to bind selectively to receptors expressed on metastatic cancer cells,12 gave Va with a coverage of up to 1 peptide nm−1 after 12 hours of reaction time, although only a few percent of the pentafluorophenyl groups reacted. Under the same conditions, Va can be easily converted into Vb and water-soluble Vc by reacting it with the free amino acids added to the same reaction mixture as shown in Scheme 2.
Scheme 2.
Synthesis of the water-soluble RGD-peptide derivatives of the gadonanotubes.
Compounds I, II, IVa-IVg and Va-Vc have all been characterized by X-ray photoelectron spectroscopy (XPS) for F, C, N, O, S (as appropriate), by TGA, and by Raman and FTIR spectroscopy (Supplementary Information).
The procedures described above and documented in more detail in the Supplementary Information offer the opportunity to prepare a number of amino acid- and peptide-derivatized gadonanotubes under RT conditions, containing, for example, the covalently-attached cyclic RGD peptide for the biological targeting of cancer. The r1 relaxivities for the underivatized Gd@US-tubes (64 mM−1·s−1) and several of the amino-acid and peptide-derivatized Gd@US-tubes are also shown in Table 1. The data in the table demonstrate that the gadonanotubes remain high-performance T1-weighted MRI contrast agents even when derivatized, although the r1 relaxivities vary depending upon the size and the hydrophilicity of the R group. We are currently exploring the biological and MR imaging properties of these new water-soluble gadonanotube MRI probes.
Supplementary Material
Acknowledgment
At Rice University this research was supported by the Robert A. Welch Foundation (grant C-0624) and the Nanoscale Science and Engineering Initiative of the National Science Foundation under NSF Award number EEC-0647452. At Baylor College of Medicine research was supported by the Cancer Center (grant number 1 P30 CA 125123), the Helis Medical Research Foundation and the NCI/NIH SPORE grant.
Footnotes
Supporting information available Syntheses and spectral and TGA data for the Gd@US-tube derivatives, are available free of charge via the internet at http://pubs.acs.org.
References
- 1.Gu Z, Peng H, Hauge RH, Smalley RE, Margrave JL. Nano Lett. 2002;2(9):1009–1013. [Google Scholar]
- 2.Sitharaman B, Kissell KR, Hartman KB, Tran LA, Baikalov A, Rusakova I, Sun Y, Khant HA, Ludtke SJ, Chiu W, Laus S, Toth E, Helm L, Merbach AE, Wilson LJ. Chem. Commun. 2005;(31):3915–3917. doi: 10.1039/b504435a. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S, Hemraj-Benny T, Wong SS. Adv. Mater. 2005;17:17. [Google Scholar]
- 4.Ashcroft JM, Hartman KB, Kissell KR, Mackeyev Y, Pheasant S, Young S, Van der Heide PAW, Mikos AG, Wilson LJ. Adv. Mater. 2007;19:573–576. [Google Scholar]
- 5.Hartman KB, Hamlin DK, Wilbur DS, Wilson LJ. Small. 2007;3(9):1496–1499. doi: 10.1002/smll.200700153. [DOI] [PubMed] [Google Scholar]
- 6.Dyke CA, Tour JM. Chem. Eur. J. 2004;10:813–817. [Google Scholar]
- 7.(a) Doyle MP, Tamblyn WH, Buhro WE, Dorow RL. Tetrahedron Letters. 1981;22:1783–1786. [Google Scholar]; (b) Urbano J, Izarra R, Gomez-Ariza JL, Trofimenko S, Diaz-Requejo MM, Perez PJ. Chem. Commun. 2006:1000–1002. doi: 10.1039/b517337j. [DOI] [PubMed] [Google Scholar]
- 8.Mackeyev Y, Bachilo S, Hartman KB, Wilson LJ. Carbon. 2007;45:1013–1017. [Google Scholar]
- 9.Hartman KB, Laus S, Bolskar RD, Muthupillai R, Helm L, Toth E, Merbach AE, Wilson LJ. Nano Lett. 2008;8(2):415–419. doi: 10.1021/nl0720408. [DOI] [PubMed] [Google Scholar]
- 10.Olah GA, Narang SC, Gupta BGB, Malhorta R. Angew. Chem. 1979;91:648–649. [Google Scholar]
- 11.Gudkov AT, Shekhvatova GV. J. Gen. Chem. USSR (Engl.Transl.) 1978;48:1955. [Google Scholar]; Zh. Obshch. Khim. 1978;48:2146. [Google Scholar]
- 12.Langer M, Kratz F, Rothen-Rutishauser B, Wunderli-Allenspach H, Beck-Sickinger AG. J. Med. Chem. 2001;44:1341–1348. doi: 10.1021/jm001065f. [DOI] [PubMed] [Google Scholar]
- 13.Ashcroft JM, Hartman KB, Mackeyev Y, Hofmann C, Pheasant S, Alemany LB, Wilson LJ. Nanotechnology. 2006;17:5033–5037. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.