Abstract
The epoxyeicosatrienoic acids (EETs) are products of cytochrome P450 epoxygenases that have vasodilatory properties similar to that of endothelium-derived hyperpolarizing factor. The cytochrome P450 isoform CYP2J2 was cloned and identified as a potential source of EETs in human endothelial cells. Physiological concentrations of EETs or overexpression of CYP2J2 decreased cytokine-induced endothelial cell adhesion molecule expression, and EETs prevented leukocyte adhesion to the vascular wall by a mechanism involving inhibition of transcription factor NF-κB and IκB kinase. The inhibitory effects of EETs were independent of their membrane-hyperpolarizing effects, suggesting that these molecules play an important nonvasodilatory role in vascular inflammation.
The metabolism of arachidonic acid by cytochrome P450 epoxygenases leads to the formation of various biologically active eicosanoids such as epoxyeicosatrienoic acids (EETs), dihydroxyeicosatrienoic acids (DHETs), and hydroxyeicosatetraenoic acids (HETEs) (1, 2). The EETs are potential candidates for endothelium-derived hyperpolarizing factor (EDHF) because they hyperpolarize and relax vascular smooth muscle cells by activating calcium-sensitive potassium (KCa) channels (3, 4). Indeed, the predominant mediator of endothelium-dependent relaxation is in the coronary microcirculation EDHF, and not in nitric oxide (NO) (5–7). EETs increase coronary blood flow (5) and protect the myocardium from ischemia-reperfusion injury (8), but the specific cytochrome P450 enzymes that synthesize EETs in human vascular endothelial cells remain unidentified.
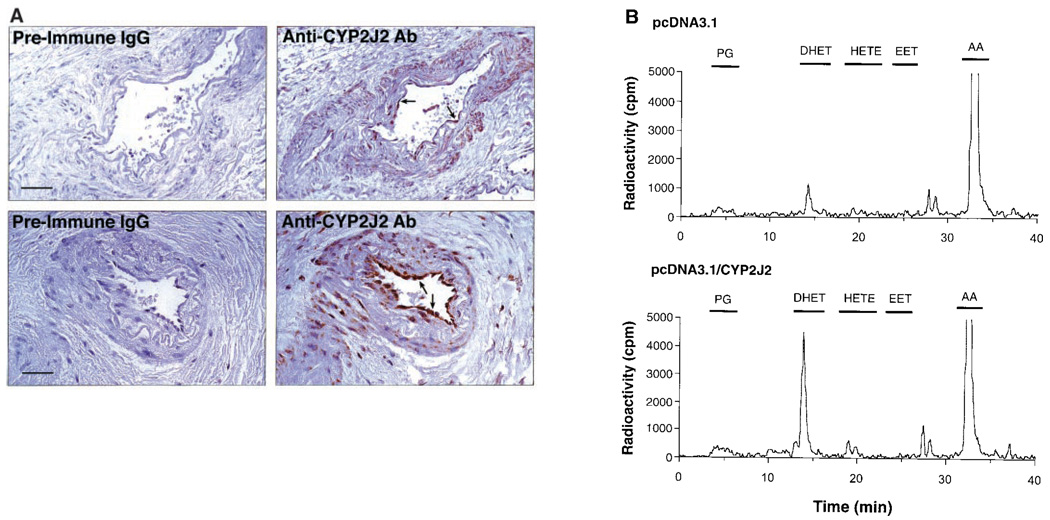
Using reverse transcription–polymerase chain reaction (RT-PCR) on human endothelial cell mRNA, we cloned a cytochrome P450 epoxygenase that was identical in sequence to the gene encoding CYP2J2, previously cloned from human liver and subsequently found to be highly expressed in human heart (GenBank/European Molecular Biology Laboratory accession numbers AF144566 and U37143) (9, 10). To determine whether CYP2J2 is localized to the human coronary artery, we stained formalin-fixed, paraffin-embedded human coronary artery tissue sections with an antibody specific for CYP2J2 (11). CYP2J2 immunoreactivity was abundant in the endothelium of both large and small coronary arteries (Fig. 1A). Staining was also present, although much less intense, in vascular smooth muscle cells, whereas surrounding connective tissue stained relatively poorly. Although other cytochrome P450 epoxygenases have been identified in endothelial cells such as CYP1A subfamily members (12), CYP2C8 (13), and CYP2B1 (14), their presence and functional role in the human coronary artery have not been demonstrated.
Fig. 1.
(A) Immunohistochemical localization of CYP2J2 in human coronary artery (arrows). Shown are photomicrographs of adjacent sections of one large (top panels; bar, 250 µm) and one small (bottom panels; bar, 100 µm) human coronary artery stained with anti-human CYP2J2 or preimmune IgG (11). (B) Reversed-phase HPLC chromatogram of metabolites generated during incubation of transfected endothelial cells with radiolabeled arachidonic acid (15). Endothelial cells were transfected with either empty vector (pcDNA3.1) or CYP2J2-containing expression vector (pcDNA3.1/CYP2J2). The retention times of authentic standards are indicated by bars above the respective peaks. (Top) Cells transfected with pcDNA3.1; (bottom) cells transfected with pcDNA3.1/CYP2J2.
To study the formation of EETs by CYP2J2, we measured EET synthesis in bovine aortic endothelial cells that are transfected with CYP2J2 cDNA (15). Bovine endothelial cells were used because of their higher level of transfection efficiency compared with human endothelial cells (16). In endothelial cells that are transfected with pcDNA3.1 (control), the epoxygenase metabolites (the EETs and their stable hydration products, the DHETs) accounted for about half of the total reaction products and were formed at a rate of 7.7 ± 2.1 pmol/min per 107 cells (n = 3) (Fig. 1B, top). This metabolic profile is comparable to that reported for bovine coronary artery endothelial cells (4) and is consistent with the ability of endothelial cells to rapidly hydrate EETs to DHETs (17). The CYP2J2-transfected cells produced epoxygenase metabolites at a rate of 17.8 ± 2.3 pmol/min per 107 cells (n = 5, P < 0.05 versus control cells) (Fig. 1B, bottom). The cytochrome P450 epoxygenase inhibitor SKF-525A (100 µM) inhibited the formation of these epoxygenase metabolites in CYP2J2-transfected cells by >80%.
To elucidate the biological effects of EETs in vascular inflammation, we treated cytokine-activated human endothelial cells with physiologically relevant concentrations (1 to 100 nM) of [5,6]-, [8,9]-, [11,12]-, [14,15]-EET, or [11,12]-DHET. Maximal 72% inhibition of tumor necrosis factor–α (TNF-α)–induced vascular cell adhesion molecule–1 (VCAM-1) expression was achieved with [11,12]-EET, followed by [8,9]-EET, [5,6]-EET, and [11,12]-DHET (Fig. 2A). The calculated median inhibitory concentration (IC50) of [11,12]-EET was 20 nM. [14,15]-EET did not inhibit TNF-α–induced VCAM-1 expression, indicating differential bioactivity of specific EET regioisomers. [11,12]-EET also inhibited VCAM-1 expression in response to other inflammatory mediators such as interleukin-1α (IL-1α) and bacterial lipopolysaccharide (LPS) (Fig. 2A), and it inhibited the expression of other adhesion molecules such as ICAM-1 and E-selectin, although to a lesser extent compared with VCAM-1 (Fig. 2B). These findings suggest that the mechanism by which EETs inhibit adhesion molecule expression may depend on the proximal signaling pathway elicited by particular cytokines. In addition, two selective KCa channel blockers, iberiotoxin (100 nM) and charybdotoxin (100 nM), did not block the inhibitory effect of [11,12]-EET on TNF-α–induced VCAM-1 expression, indicating that the mechanism is independent of EET’s hyperpolarizing effects.
Fig. 2.
Cell-surface enzyme immunoassay. (A) The effects of cytochrome P450 epoxygenase-derived eicosanoids (100 nM) on endothelial cell VCAM-1 expression [measured in optical density (OD) units] in response to TNF-α (10 ng/ml), IL-1α (10 ng/ml), or LPS (10 ng/ml). (B) The effects of [11,12]-EET (100 nM) on unstimulated (control) or TNF-α (10 ng/ml)–stimulated VCAM-1, E-selectin, and ICAM-1 expression. The differences between treatment with TNF-α alone and in the presence of EETs were statistically significant (*P < 0.05, **P < 0.01). (C) Effect of [11,12]-EET (100 nM) or [14,15]-EET (100 nM) on bovine endothelial cells transiently transfected with a κB heterologous promoter construct (pκB.Luc). The κB promoter activity was determined relative to basal activity (*P < 0.01 versus TNF-α). (D) Effect of transfection with empty vector (pcDNA3.1) or CYP2J2 cDNA subcloned into pcDNA3.1 on TNF-α–induced VCAM-1 or heterologous κB promoter activity in the presence or absence of SKF-525A (100 µM) (*P < 0.01 versus TNF-α alone; **P < 0.05 versus TNF-α + CYP2J2).
To localize cis-acting elements within the VCAM-1 5′-flanking region that might mediate the inhibitory effects of [11,12]-EET, we transiently transfected bovine aortic endothelial cells with a −755-bp VCAM-1 promoter construct (16). Stimulation with TNF-α increased the relative VCAM-1 promoter activity by 13-fold, which was abolished by cotreatment with [11,12]-EET (data not shown). A similar inhibitory effect was observed with [11,12]-EET when a −98-bp VCAM-1 promoter construct was used that contained the tandem κB cis-acting elements. To confirm that the tandem κB sites in the VCAM-1 promoter are repressed by [11,12]-EET, we subcloned these tandem κB sites into a heterologous promoter reporter gene construct containing the SV40 enhancer (pκB.Luc). Stimulation of transfected cells with TNF-α increased pκB.Luc promoter activity by sixfold, which was abolished by cotreatment with [11,12]-EET, but not [14,15]-EET (Fig. 2C). To determine whether CYP2J2-derived eicosanoids can functionally inhibit TNF-α–induced VCAM-1 gene transcription, we overexpressed CYP2J2 in bovine aortic endothelial cells cotransfected with the −755 bp VCAM-1 promoter construct or pκB.Luc. Compared to transfection with the empty pcDNA3.1 vector, overexpression of CYP2J2 decreased TNF-α–induced VCAM-1 and pκB.Luc promoter activity by 60 and 70%—effects that were reversed in the presence of SKF-525A (Fig. 2D). These findings suggest that CYP2J2-derived eicosanoids repress VCAM-1 gene transcription, in part by inhibiting κB cis-acting elements.
Because NF-κB activation involves the nuclear translocation of NF-κB subunit Rel A, we assessed the effect of [11,12]-EET on Rel A subcellular localization. In unstimulated endothelial cells, Rel A is predominantly localized to the cytoplasm, whereas cells stimulated with TNF-α show an intense nuclear accumulation of Rel A (Fig. 3A). Cotreatment with [11,12]-EET prevented the nuclear accumulation of Rel A. Because the nuclear translocation of Rel A requires the degradation of its endogenous cytoplasmic inhibitor, inhibitor kappa B–α (IκB-α), we monitored the fate of IκB-α after TNF-α stimulation with and without [11,12]-EET. Stimulation of endothelial cells with TNF-α caused a rapid and almost complete disappearance of IκB-α that was prevented by cotreatment with [11,12]-EET, but not [14,15]-EET (Fig. 3B). The IκB-α phosphorylation is regulated by IκB kinase (IKK), which specifically phosphorylates Ser32 and Ser36 and targets IκB-α for subsequent degradation by 26S proteasomes (18). Indeed, the 26S proteasome inhibitor MG132 prevented IκB-α degradation. Using purified glutathione-S-transferase (GST)–IκB-α fusion protein as substrate for IKK, we found minimal IKK activity in unstimulated endothelial cells (Fig. 3C). Stimulation with TNF-α markedly increased IKK activity, which was inhibited by >90% with [11,12]-EET, and to a lesser extent with [5,6]-EET or [8,9]-EET. Specificity of IKK was confirmed by the absence of phosphorylation when the mutated IκB-α substrate GST–IκB-α (Ser32, Ser36→T), which cannot be phosphorylated, was substituted for wild-type GST–IκB-α.
Fig. 3.
(A) Immunofluorescence studies showing the subcellular localization of NF-κB subunit Rel A in unstimulated endothelial cells (control) and endothelial cells stimulated with TNF-α (10 ng/ml, 15 min) in the presence or absence of [11,12]-EET (100 nM). Experiments were performed three times with similar results. Bar, 10 µm. (B) The fate of IκB-α after treatment with TNF-α (10 ng/ml, 15 min) with or without [11,12]-EET (100 nM), [14,15]-EET (100 nM), or 26S proteasome inhibitor, MG132 (10 µM). NS, non-specific bands. (C) IKK activity in unstimulated endothelial cells and endothelial cells stimulated with TNF-α (10 ng/ml, 15 min) with and without [11,12]-EET (100 nM), [5,6]-EET (100 nM), or [8,9]-EET (100 nM). In some experiments, [11,12]-EET (100 nM) was added directly to the IKK assay (in vitro). As a control for nonspecific phosphorylation, the IκB-α mutant (Ser32, Ser36→T ) was used as substrate. The assay was performed three times with similar results.
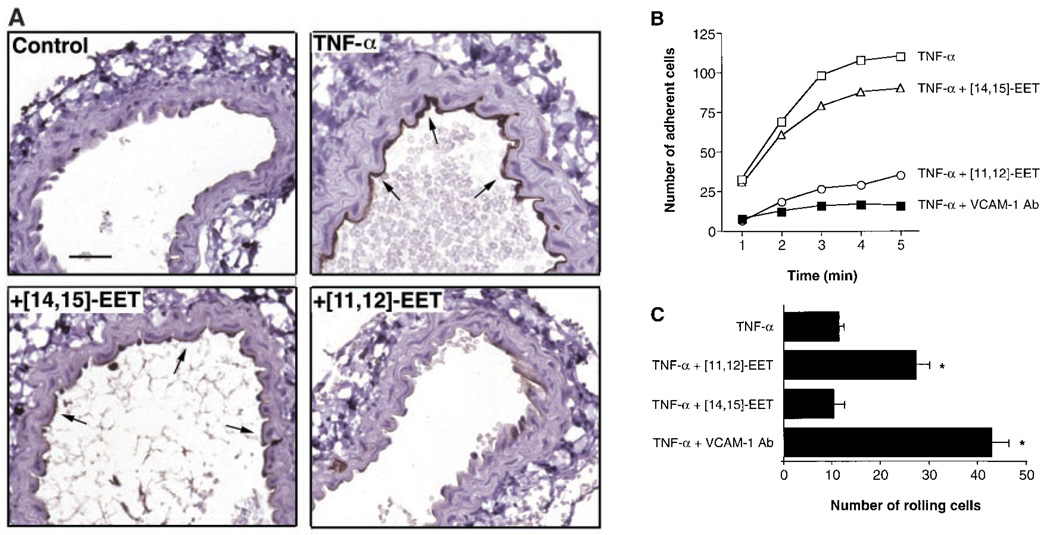
To determine the functional relevance of EETs in vascular inflammation, we tested [11,12]-EET and its regioisomer, [14,15]-EET, in an in vivo model of TNF-α–induced, VCAM-1–mediated mononuclear cell adhesion (19, 20). In this murine carotid artery model, vascular cell adhesion is primarily dependent on U937 mononuclear cell α4β1 integrin interacting with endothelial VCAM-1. Under baseline conditions, VCAM-1 is not expressed on the endothelial cell surface, and mononuclear cells neither roll along nor adhere to the arterial endothelium. However, 5 hours after an intraperitoneal injection of TNF-α, VCAM-1 is expressed on the endothelial surface and mononuclear cells adhere to the vessel wall (Fig. 4, A and B). Intra-arterial infusion of [11,12]-EET, but not [14,15]-EET or vehicle (saline), decreased endothelial VCAM-1 expression and inhibited mononuclear cell adhesion. The mononuclear cell adhesion was also blocked by an antibody to VCAM-1 (MK2) (21), confirming the importance of VCAM-1– mediated mononuclear cell adhesion in this model of vascular inflammation. Because of reduced recruitment into the adherent pool, there was a shift in the number of adherent U937 cells into the nonadherent population, leading to increased mononuclear cell rolling in carotid arteries harvested from mice treated with [11,12]-EET or VCAM-1–blocking antibody (Fig. 4C). Our findings are also consistent with studies in mice showing augmentation of fever by inhibitors of cytochrome P450 epoxygenases (22, 23).
Fig. 4.
(A) Expression of endothelial VCAM-1 (arrows) in the murine carotid artery 5 hours after an intraperitoneal injection of saline (control) or TNF-α (10 µg/kg) in the presence or absence of continuous intra-arterial infusion of [11,12]-EET or [14,15]-EET (both at 100 ng/kg per minute for 5 hours) (25). Bar, 250 µm. Effect of EETs on TNF-α–induced U937 mononuclear cell (B) adhesion and (C) rolling in the murine carotid artery. The number of adherent and rolling cells per mm2 of endothelium was measured after infusion of U937-labeled cells (20). Mononuclear cell adhesion or rolling was not detected in control mice. Mice were treated with TNF-α alone (□) and in the presence of [11,12]-EET (○), [14,15]-EET (∆), or a mAb (MK2) directed against VCAM-1 (■) (23). For mononuclear cell adhesion, threeseparate experiments yielded similar results with less than 10% variation (*P < 0.05 versus TNF-α alone).
Our findings suggest an important nonvasodilatory property of EETs that is distinct from their membrane-hyperpolarizing effect and that involves the inhibition of NF-κB–mediated VCAM-1 expression. Indeed, the concentration of [11,12]-EET used in our study was subthreshhold for augmenting cerebral blood flow at sites distal to the carotid artery infusion (24). We propose that cytochrome P450 epoxygenase-derived eicosanoids may be useful therapeutic agents for treating vascular and nonvascular inflammatory disorders. The physiological role and the relative contributions of specific cytochrome P450 isoforms in mediating these effects remain to be determined.
References and Notes
- 1.Capdevila JH, Falck JR, Estabrook RW. FASEB J. 1992;6:731. doi: 10.1096/fasebj.6.2.1537463. [DOI] [PubMed] [Google Scholar]
- 2.Campbell WB, Harder DR. Circ. Res. 1999;84:484. doi: 10.1161/01.res.84.4.484. [DOI] [PubMed] [Google Scholar]
- 3.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. ibid. 1996;78:415. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 4.Rosolowsky M, Campbell WB. Biochim. Biophys. Acta. 1996;1299:267. doi: 10.1016/0005-2760(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 5.Oltman CL, Weintraub NL, VanRollins M, Dellsperger KC. Circ. Res. 1998;83:932. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 6.Pfister SL, Falck JR, Campbell WB. Am. J. Physiol. 1991;261:H843. doi: 10.1152/ajpheart.1991.261.3.H843. [DOI] [PubMed] [Google Scholar]
- 7.Pritchard KA, Jr, Wong PY, Stemerman MB. Am. J. Pathol. 1990;136:1383. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, et al. J. Biol. Chem. 1997;272:12551. doi: 10.1074/jbc.272.19.12551. [DOI] [PubMed] [Google Scholar]
- 9.Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. ibid. 1996;271:3460. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- 10.Using 15 different pairs of degenerate oligonucleotides corresponding to conserved regions of cytochrome P450 epoxygenases, we performed RT-PCR on mRNA obtained from cultured human endothelial cells. Only one pair of primers (sense: 5′-GCTGACTTTCTCAAAAGACG-3′ antisense: 5′-CTCTGCACCTCATGGATGAC-3′ annealing/elongation temperature: 51°/72°C, 35 cycles) yielded a single 1-kb band that is identical to the gene encoding CYP2J2 (9).
- 11.Tissues were fixed in 10% neutral buffered formalin, processed, and embedded in paraffin. An afFInity-purified rabbit polyclonal antiserum to CYP2J2 (anti-CYP2J2, 1:200 dilution) was used to detect CYP2J2 on serial sections (5 to 6 mm) of human heart (9). The specificity of anti-CYP2J2 was demonstrated by immunoblots of microsomal and S9 fractions prepared from various human tissues, in which anti-CYP2J2 produced a single band at 56 kD corresponding to endogenous CYP2J2 protein, and the FInding that anti-CYP2J2 strongly bound recombinant CYP2J2 but did not recognize other cytochrome P450 isoforms including members of the CYP1A, CYP2A, CYP2B, CYP2C, CYP2D, and CYP2E subfamilies (8, 9) and CYP4A1, CYP4A2, CYP4A3, CYP4A8, and CYP4A11.
- 12.Farin FM, Pohlman TH, Omiecinski CJ. Toxicol. Appl. Pharmacol. 1994;124:1. doi: 10.1006/taap.1994.1001. [DOI] [PubMed] [Google Scholar]
- 13.Lin JH-C, et al. Endothelium. 1996;4:219. [Google Scholar]
- 14.Hoebel BG, Steyrer E, Graier WF. Clin. Exp. Pharmacol. Physiol. 1998;25:826. doi: 10.1111/j.1440-1681.1998.tb02162.x. [DOI] [PubMed] [Google Scholar]
- 15.Subconfluent bovine aortic endothelial cells were transfected at 10 to 20% effciency with either pcDNA3.1 or pcDNA3.1/CYP2J2 by using Fugene 6 reagent (Boehringer Mannheim). Forty-eight hours after transfection, cells were incubated with freshly purified [5,6,8,9,11,12,14,15-3H]arachidonic acid (185 Ci/mmol, 4 to 5 µCi/175-mm2 flask) and unlabeled arachidonic acid (10 µM) in serum-free medium at 37°C. In some experiments, SKF-525A (100 µM) was added before the addition of arachidonic acid. At various time points, the media and cells were removed and extracted with diethyl ether. The combined organic phases were dried under a nitrogen stream, resolved by reversed-phase high-performance liquid chromatography (HPLC), and quantified by liquid scintillation as described (9). Products were identified by comparing their HPLC properties with those of authentic EET, DHET, HETE, and prostaglandin (PG) standards.
- 16.De Caterina R, et al. J. Clin. Invest. 1995;96:60. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanRollins M, Kaduce TL, Knapp HR, Spector AA. J. Lipid Res. 1993;34:1931. [PubMed] [Google Scholar]
- 18.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. Cell. 1994;78:773. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 19.Ramos CL, et al. Circ. Res. 1999;84:1237. doi: 10.1161/01.res.84.11.1237. [DOI] [PubMed] [Google Scholar]
- 20.Mononuclear cell adhesion to isolated perfused mouse carotid arteries was investigated as described (19). C57BL/6 mice (Hilltop, Scottsdale, PA) were anesthetized with ketamine and xylazine. The right common carotid artery was cannulated with a PE10 perfusion catheter and the tip was advanced past the right subclavian artery into the brachiocephalic trunk to ensure direct delivery to the left carotid artery. Vehicle (saline), [11,12]-EET, or [14,15]-EET was infused at a flow rate of 5 µl/min for a calculated final dose of 100 ng/kg per minute, which is comparable to the in vitro dose of 10 to 100 nM. After 30 min, the mice were treated with a single intraperitoneal injection of recombinant murine TNF-α (10 µg/kg; R&D Systems, Minneapolis, MN). Five hours later, the left common carotid artery was ligated and cannulated with a PE10 catheter in the cranial direction and perfused with heparinized MOPS-buffered physiological salt solution. The external and internal carotid branches were ligated and perforated at the ends to produce similar outflow. The vessel was then perfused with a suspension of mononuclear cells [U937 labeled with calcein AM (Molecular Probes, Eugene, OR)] at a wall shear stress of about 3 dyn/cm2. Cell rolling and adhesion were recorded on videotape by means of stroboscopic epifluoresence illumination with an intravital microscope ( Zeiss ×20 objective, 0.5 numerical aperture). In mice receiving TNF-α only, the isolated vessels were also perfused with monoclonal antibody (mAb) MK-2 (40 µg/ml) for 20 min to block VCAM-1 function (21)
- 21.Miyake K, et al. J. Cell Biol. 1991;114:557. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakashima T, Harada Y, Miyata S, Kiyohara T. Am. J. Physiol. 1996;271:R1274. doi: 10.1152/ajpregu.1996.271.5.R1274. [DOI] [PubMed] [Google Scholar]
- 23.Kozak W, et al. ibid. 1998;275:R1031. doi: 10.1152/ajpregu.1998.275.4.R1031. [DOI] [PubMed] [Google Scholar]
- 24.Liao JK. unpublished observation. [Google Scholar]
- 25.Immunostaining for VCAM-1 expression was performed on paraffin sections (5 µm thick) of murine carotid arteries. Slides were incubated with avidin-biotin blocking reagent containing 10% horse serum ( Vector Laboratories, Burlingame, CA) to reduce background staining, then incubated with primary antibody (polyclonal goat anti-mouse VCAM-1, 5 µg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C followed by biotin-conjugated horse anti-goat immunoglobulin G (IgG), ABC complex, and 3,3′-diaminobenzidine as substrate (Vector Laboratories). All antibody incubations were performed in the presence of 10% horse serum. Slides were counterstained with hematoxylin and dehydrated in ethanol followed by xylene. Slides were examined under a light microscope ( Zeiss ×100/1.4 oil immersion objective)
- 26.We thank J. R. Falck for EET and DHET standards and advice on EET stability in vivo; J. DiDonato and M. Karin for GST-IκB-α proteins; J. M. Sanders for immunostaining; J. Ma and J. Foley for technical assistance; and J. Bonner and J. Cidlowski for helpful suggestions. Supported by NIH Grant HL-52233 (J.K.L.), HL-58108 (K.L.), and NIEHS Division of Intramural Research (B.Y. and D.C.Z.). M.S. is a recipient of the Feodor Lynen Fellowship (Alexander von Humboldt-Stiftung). J.K.L. is an Established Investigator, and K.N. is a Circulation Council/Otsuka Research Fellow of the American Heart Association.