Abstract
Mitochondria play critical roles in neuronal function and almost all aspects of mitochondrial function are altered in Alzheimer neurons. Emerging evidence shows that mitochondria are dynamic organelles that undergo continuous fission and fusion, the balance of which not only controls mitochondrial morphology and number, but also regulates mitochondrial function and distribution. In this review, after a brief overview of the basic mechanisms involved in the regulation of mitochondrial fission and fusion and how mitochondrial dynamics affects mitochondrial function, we will discuss in detail our and others’ recent work demonstrating abnormal mitochondrial morphology and distribution in AD models and how these abnormalities may contribute to mitochondrial and synaptic dysfunction in AD. We propose that abnormal mitochondrial dynamics plays a key role in causing the dysfunction of mitochondria that ultimately damage AD neurons.
Keywords: Alzheimer Disease, Mitochondrial Dynamics, Mitochondrial Dysfunction, Mitochondrial Distribution, Synaptic Dysfunction, DLP1
Introduction
Mitochondria are involved in a number of cellular function and essential to both life and death. While it has long been appreciated that mitochondria are very dynamic organelles in the sense that they are actively transported in cells and they can have defined subcellular distributions which can rapidly change according to physiological needs (Chan 2006a), emerging evidence has added several additional dimensions to mitochondrial dynamics. Specifically, it is now apparent that mitochondria constantly divide and fuse with each other, thus leading to changes in mitochondrial morphology, length, size and number. Also, the internal structure of mitochondria can change in response to their physiological state. It becomes apparent that mitochondrial dynamics affects mitochondrial function and vice versa.
Neurons are particularly sensitive to changes in mitochondrial function (Kann & Kovacs 2007). Not surprisingly, mitochondrial injury can have severe consequences for neuronal function and survival and the prevalence of neuronal diseases associated with mutations in mitochondrial genes underscores the important functional relationship between neurons and mitochondria (Chan 2006a). Along this line of reasoning, perhaps it is not coincidental that mitochondrial dysfunction is one of the most prominent and early features in Alzheimer disease (AD). While the mechanisms underlying mitochondrial dysfunction in AD and how mitochondrial dysfunction leads to Alzheimer pathogenesis remains unclear, recent studies in the field show that abnormal mitochondrial dynamics likely play a key role in AD pathogenesis, which is the topic of this review.
Mitochondrial Dynamics: A Delicate Balance of Fusion and Fission
The number and morphology of mitochondria are controlled by a delicate balance of mitochondrial fusion and fission (Chan 2006b) such that genetic inactivation of the fusion leads to fragmentation while genetic inactivation of fission results in elongation (Bleazard et al. 1999, Sesaki & Jensen 1999). Mitochondrial fission and fusion are regulated by a machinery involving large dynamin-related GTPases that exert opposing effects (McBride et al. 2006). Mitochondrial fission in mammals involves at least two proteins: a large GTPase, dynamin-like protein 1 (DLP1, or DRP1) and a small molecule, Fis1. The majority of DLP1 resides in the cytoplasm. During fission, DLP1 is recruited to punctate spots on the mitochondrial surface, a fraction of which progress to the actual fission site and it is thought that DLP1 uses GTP hydrolysis to constrict microtubules during fission (Smirnova et al. 2001). As a mitochondrial outer membrane protein, Fis1 evenly resides on the surface of mitochondria (James et al. 2003) and is believed to recruit DLP1 to mitochondria during fission through its N-terminus (Wells et al. 2007). On the other hand, mitochondrial fusion is regulated by three large GTPases: Mitofusin 1 (Mfn1), Mitofusin 2 (Mfn2) and optic atrophy protein 1(OPA1) (Chan 2006a, Knott et al. 2008). Both Mfn1 and Mfn2 are mitochondrial transmembrane proteins localized to the outer membrane. During mitochondrial fusion, through interactions of their coiled-coil domains, Mfn 1 and Mfn 2 could form homo-oligomeric and hetero-oligomeric complexes, and tether neighboring mitochondria together (Ishihara et al. 2004, Zuchner et al. 2004). OPA1 is localized to the inner membrane and is primarily involved in inner membrane fusion (Chen et al. 2005, Cipolat et al. 2004).
Both mitochondrial fission and fusion are critical in maintaining a healthy population of mitochondria: mitochondrial fusion allows the exchange of lipid membrane and intramitochondrial contents including mtDNA between different mitochondria which will effectively lower the percentage of defective mitochondria while mitochondrial fission, coupled with fusion and followed by autophagy, allows sequestration and elimination of irreversibly damaged mitochondria and mitochondrial content, thus maintaining the integrity and homogeneity mitochondria population throughout the cell (Chen et al. 2007, Cheng et al. 2005, Twig et al. 2008). Growing evidence suggests that the delicate equilibrium between mitochondria fission and fusion is vital for mitochondrial functions including metabolism, energy production, Ca2+ signaling, ROS production, apoptosis and senescence (Frank et al. 2001, Lee et al. 2004, Parone et al. 2006, Chen et al. 2005, McBride et al. 2006, Yu et al. 2006, Lee et al. 2007). For example, unbalanced mitochondrial dynamics, which causes either mitochondrial elongation or excessive mitochondrial fragmentation, would result in reduced metabolism and loss of mtDNA (Chen et al. 2005, Chen et al. 2003, Benard et al. 2007, Parone et al. 2008). Mitochondrial dynamics are also involved in apoptosis with mitochondrial fragmentation being an early event during apoptosis that precedes cytochrome c release and caspase activation (Frank et al. 2001).
Mitochondria are not free floating in the cytoplasm and the cytoskeleton controls their position and movement within cells. It is generally believed that mitochondrial distribution is adapted to cellular physiology such that mitochondria concentrate in subcellular regions with high metabolic requirement (Frazier et al. 2006). In this regard, it is worth noting that mitochondrial dynamics can also impact cellular function by influencing mitochondrial distribution since both fission mutants (i.e., DLP1) with elongated mitochondria (Smirnova et al. 1998) and fusion mutants (i.e., OPA1) with short, rounded mitochondria (Griparic et al. 2004, Spinazzi et al. 2008) caused mitochondrial distribution changes.
Mitochondrial Dysfunction in AD
A large number of studies implicate metabolic defects in AD, such that a reduced rate of brain metabolism is one of the best documented abnormalities in AD (Blass 2000). Most importantly, such cerebral metabolic rate abnormalities precede rather than follow any evidence for functional impairment by neuropsychological testing or of brain atrophy by neuroimaging (Blass 2000). Consistently, deficiency in several key enzymes of oxidative metabolism is well documented in AD brain (Reddy & Beal 2008). Damage to both the components and the structure of mitochondria as well as increased oxidative stress are also extensively reported in AD (Zhu et al. 2006). Altered calcium homeostasis is also reported in AD and studies of animal models of AD suggest that mitochondrial impairment in these models enhances a dysregulation of neuronal calcium homeostasis (Keller et al. 1998). Additionally, mtDNA genetic markers have been linked to an increased incidence of AD (Wallace et al. 1997, Coskun et al. 2004). More recently, It was determined that there were many more sporadic mutations in the mtDNA control region in AD patients compared with control cases and several mutations in the mtDNA control region (e.g., T414G, T414C, and T477C) that were unique to AD (Coskun et al. 2004).
Abnormal Mitochondrial Dynamics in AD
In an effort to characterize specific cytological abnormalities in mitochondria in vulnerable neurons, we performed quantitative ultrastructural morphometric measurements of the percentage of different types of mitochondria (intact and broken cristae) in AD neurons (Hirai et al. 2001). These initial studies revealed that AD neurons contained a significantly lower percentage of normal mitochondria and a significantly higher percentage of the mitochondria with broken cristae compared to aged-matched control group. Most interestingly, we also found significant changes in mitochondrial size and number in vulnerable neurons in AD (Hirai et al. 2001). Given that mitochondrial number and morphology are tightly regulated by mitochondrial fission and fusion, these findings implicate that an abnormal mitochondrial dynamics may be involved.
To test this hypothesis, we first used fibroblasts from AD patients and found profound alterations in mitochondria dynamics in these cells (Wang et al. 2008a). Specifically, as evidenced by both confocal and electron microscopy, mitochondria were significantly longer, and two or more mitochondria often joined together to form a highly connected networks in sAD fibroblasts which is distinctly different from those of age-matched normal human fibroblasts (NHFs) where mitochondria were much shorter and appeared sausage- or round-shaped (Wang et al. 2008a). These findings suggest that the balance of mitochondrial fission and fusion is likely tipped toward less fission in AD fibroblasts. Indeed, DLP1 levels were significantly decreased while OPA1 levels were not changed. Moreover, DLP1 knockdown and expression of dominant-negative DLP1 in NHFs caused changes in mitochondrial morphology comparable to those seen in sAD fibroblasts and DLP1 overexpression in sAD fibroblasts rescued the abnormal mitochondrial morphology in sAD fibroblasts. Therefore, reductions of DLP1 likely cause the abnormal mitochondrial morphology in sAD fibroblasts (Wang et al. 2008a).
Interestingly, while mitochondria were evenly distributed throughout the cytoplasm in most NHFs, we observed a dramatic change in mitochondrial distribution in many sAD fibroblasts such that the majority of mitochondria clustered around the perinuclear area and mitochondria at more remote regions became sparse. We demonstrated that this mitochondrial redistribution is also due to DLP1 reduction in sAD fibroblasts (Wang et al. 2008a). While there was no apparent functional consequence of such mitochondrial re-distribution in sAD fibroblasts, this is of great interest to us because we reasoned that if a similar phenomenon occurs in more polarized cells such as neurons, it could have profound effects. To begin to explore this possibility, we investigated the expression pattern of mitochondrial fission/fusion proteins in hippocampal tissues from AD patients (Wang et al. 2008c). Immunoblot analysis revealed significantly reduced levels of DLP1, OPA1, Mfn1/2 and increased levels of Fis1. Interestingly, although all these proteins demonstrate even distribution in the cytoplasm and processes of pyramidal neurons in age-matched control hippocampus, they appeared to accumulate in the soma but not in the processes of pyramidal neurons in AD hippocampus (Wang et al. 2008c). Since OPA1, Fis1, Mfn1 and Mfn2 are all mitochondrial membrane proteins, their re-distribution to the soma in AD neurons suggest that mitochondrial re-distribution occurs in AD neurons.
To pursue the potential cause and consequence of abnormal mitochondrial dynamics in AD neurons, we investigated the effect of APP and Aβ on mitochondrial dynamics in cultured neurons. Interestingly, confocal and electron microscopic analysis demonstrated that most control M17 cells demonstrate normal short tubular forms of mitochondria while M17 cells overexpressing wild type APP contain mitochondria populations that are more heterogeneous in morphology with approximately 50% of cells containing a fragmented, punctiform structure of mitochondria and a small population (10-15%) showing an elongated net-like structure of mitochondria (Wang et al. 2008b). The mitochondrial fragmentation phenotype became even more severe in M17 cells overexpressing mutant APP where the percentage of cells with fragmented mitochondria exceeded 80%. Not surprisingly, total number of mitochondria was reduced and the number of damaged mitochondria was greatly increased in cells overexpressing APP compared to control M17 cells. Through time lapse study, we demonstrated that mitochondria were able to fuse with each other but at a much slower rate in APP overexpressing cells. More importantly, we again observed a striking difference in mitochondrial distribution: in control M17 cells, mitochondria were evenly distributed throughout the cytoplasm while in M17 cells overexpressing APP they accumulated around the perinuclear area such that more remote areas were devoid of mitochondria. Overexpression of APP in differentiated primary hippocampal neurons also led to mitochondrial fragmentation and reduced mitochondrial coverage in neuronal processes (Wang et al. 2008b). Functional analysis revealed that overexpression of APP or mutant APP in M17 cells caused an increase in ROS production, reduced ATP generation and lowered mitochondrial membrane potential, indicative of impaired mitochondrial function. Moreover, M17 cells overexpressing APP lost the capability to differentiate upon retinoic acid treatment up to one month. Our further studies demonstrated that APP overexpression led to a significant decrease in DLP1 and OPA1 levels and significant increase in Fis1 levels. Very intriguingly, overexpression of DLP1 in APP-overexpressing M17 cells rescued the abnormal mitochondrial distribution and differentiation deficiency, but failed to rescue mitochondrial fragmentation and functional parameters, while overexpression of OPA1 rescued mitochondrial fragmentation and functional parameters, but failed to restore normal mitochondrial distribution. The effect of APP overexpression is likely through the overproduction of Aβ since these mitochondrial changes and changes in mitochondrial fission/fusion proteins were abolished by treatment with BACE inhibitor IV. This is consistent with prior studies demonstrating that extracellular Aβ treatment could induce mitochondrial fragmentation in neurons (Barsoum et al. 2006a).
Abnormal Mitochondrial Dynamics and Mitochondrial and Synaptic Dysfunction in AD Neurons
Emerging evidence suggests that mitochondrial fragmentation, not necessarily associated with eventual cell death, is a mechanism that leads to mitochondrial structural damage and dysfunction in neurons. For example, there is a burst in ROS production along with mitochondrial fragmentation when neuronal cells were exposed to high glucose concentrations (Yu et al. 2006). Mitochondrial fragmentation induced by nitric oxide is accompanied by ultrastructural damage of the mitochondria, autophagy, ATP decline and ROS production that occurs long before neurite injury and neuronal cell death (Barsoum et al. 2006b). It is now becoming clear that the balance of mitochondrial fission and fusion is altered in AD cells. At least in neuronal cells, Aβ overproduction induced mitochondrial fragmentation and dysfunction including heightened ROS production, reduced ATP generation and lower MMP as well as increased number of mitochondria with damaged structure while OPA1 overexpression restored the mitochondrial morphology and these mitochondrial functional parameters (Wang et al. 2008b). These findings confirm the involvement of mitochondrial fragmentation in mediating mitochondrial dysfunction and structural damage induced by AD-related insults in neuronal cells. Since the expression of mitochondrial fission/fusion proteins and the mitochondrial morphology, size and number in AD hippocampus were also changed in a way similar to that in APP overexpressing M17 cells, it is likely mitochondrial fragmentation is also involved in mitochondrial dysfunction in AD neurons. Interestingly, unlike that in AD neurons, mitochondria become elongated in sAD fibroblasts. However, it must be noted that, despite the fact that both AD fibroblasts and neurons are under a chronic oxidative stress, unlike massive neuronal degeneration, no apparent fibroblasts death is ever observed in AD patients. In fact, although sAD fibroblasts contain 3 times higher basal level cellular ROS than NHFs (Wang et al. 2008a), they are more resistant to ROS-induced apoptosis (Naderi et al. 2006). These data suggest that AD fibroblasts achieve an altered redox homeostasis with a heightened basal intracellular level of ROS presumably eliciting some adaptive responses. We suspect that DLP1 reduction and mitochondrial elongation in sAD fibroblasts may serve as a protective adaptation to chronic oxidative stress because decreased mitochondrial fission and enhanced fusion could effectively decrease mitochondrial respiration rate and diminish ROS production. It is likely a lack of, or failure to elicit, such an adaptive response that leads AD neurons eventually to succumb to the chronic oxidative stress. In this regard, it is known that AD neurons are subject to various insults which may deplete their adaption potential and leave them vulnerable when in face with additional insults that require further adaptation (Zhu et al. 2007, Zhu et al. 2004).
Our finding that mitochondria fuse at a much slower rate in APP overexpressing neurons may cause mitochondrial dysfunction in AD neurons. The mtDNA genome encodes proteins essential for oxidative phosphorylation and thus mtDNA defects may severely affect mitochondrial function (Melov 2004). Mitochondrial fusion and fission result in the mixing and exchange of mitochondrial contents including mtDNA and its encoded proteins, and enable individual mitochondrion that have suffered stochastic loss of essential components to rapidly replenish their stores, thus effectively lowering the effect of defect mtDNA. In the extreme situation where mitochondrial fusion is entirely abolished, mitochondrial fragmentation results in a majority of mitochondria lacking mtDNA (Chen et al., 2007). These nucleotide-deficient mitochondria lack essential respiratory subunits encoded by mtDNA and have no mechanisms for regaining these proteins or mtDNA and thus lack electron transport activity. Metabolic defects caused by these nucleotide-deficient mitochondria likely cannot be compensated by those mitochondria with mtDNA since they represent too small a mitochondrial mass to support the respiratory requirement of the whole cell. M17 cells overexpressing mutant APP demonstrated more than 4 fold decrease in the rate of mitochondrial fusion (Wang et al. 2008b). It is likely in these cells, due to unbalanced ongoing fission, that each mtDNA nucleotide will be encased by smaller mitochondrial mass, and therefore the functional mitochondrial mass is greatly reduced. Additionally, the coupled fission and fusion, followed by autophagy, plays an important role in removing dysfunctional mitochondrial components (Twig et al. 2008) making it possible that the altered balance of mitochondrial fission and fusion also affects the removal of mutant mtDNA and thus contributes to the accumulation of mutant mtDNA in AD neurons which obviously will exacerbate the above situation. Indeed, some dominant missense mutations in OPA1 cause the accumulation of multiple mtDNA deletions in skeletal muscle (Amati-Bonneau et al. 2008, Hudson et al. 2008).
Early changes in the AD brain include loss of synapses and, among all the early changes, synaptic loss is the most robust correlate of AD-associated cognitive deficits, leading to the notion that synaptic dysfunction plays a critical role in the pathogenesis of AD (Selkoe 2002). Under normal conditions, it is known that mitochondria are abundantly localized at synaptic terminals (Sheehan et al. 1997), presumably reflecting the intense ATP demands of an active neuron engaged in synaptic transmission and/or need of calcium buffering at these sites. Our studies repeatedly show that mitochondrial re-distribution from an evenly distributed pattern to perinuclear accumulation occurs in AD neurons and peripheral cells. Although it may not cause any apparent functional significance in peripheral cells, it may be of critical importance to neurons since the great morphological complexity and dependency on mitochondria for energy at multiple selective sites make neurons particularly sensitive to perturbations in mitochondrial distribution. In this regard, not surprisingly, in the Drosophila with mutant Milton, a mitochondrial protein involved in mitochondrial transport via binding to kinesin heavy chain, the loss of mitochondria from axon terminals leads to defective synaptic transmission in photoreceptor cells (Stowers et al. 2002); Loss of Miro, another mitochondrial protein that may be involved in mitochondrial morphology regulation, results in mitochondrial accumulation in neuronal cell bodies, absence of mitochondrial from the neuromuscular junction and impaired larva movement as well as synaptic dysfunction in flies (Guo et al. 2005, Melov 2004). Perturbation of DLP1 or OPA1 affected the number of dendritic mitochondria which in turn affected the number and plasticity of spines and synapses and, indeed, there is a correlation between dendritic spine morphogenesis and recruitment of nearby mitochondria (Li et al. 2004). Similarly, DLP1 mutations in Drosophila led to elongated mitochondria and failure to populate the distal axon with mitochondria, which resulted in elevated resting calcium levels and modestly impaired calcium buffering as well as dysfunction in the mobilization of reserve pool vesicles (Verstreken et al. 2005). Therefore, we suggest that defects in mitochondrial distribution likely cause localized energy and calcium defects in AD neurons, resulting in synaptic dysfunction. Along this same reasoning, it is likely that Aβ-induced abnormal mitochondrial distribution underlies its toxic effect on dendritic spine and synapses (Lacor et al. 2007).
Conclusion
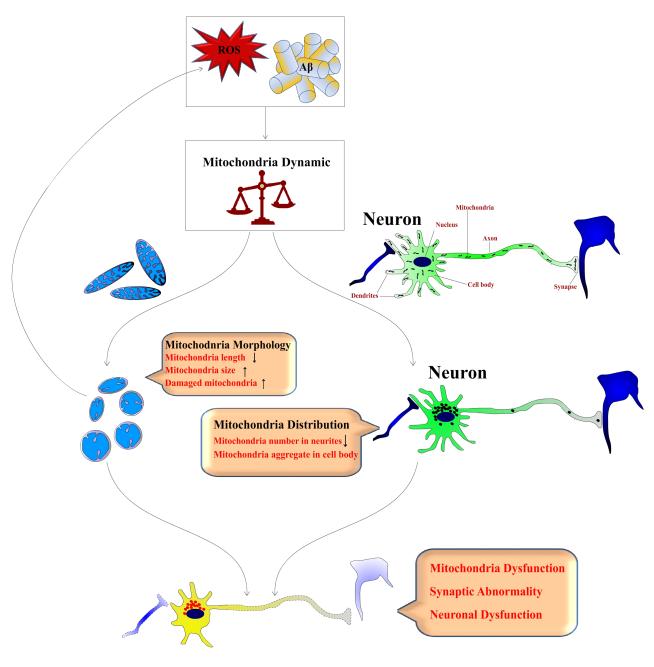
The brain is a highly metabolic tissue, and neurons in the central nervous system have an intense demand for mitochondria (Isaacs et al. 2006). The delicate balance of mitochondrial fission and fusion controls not only mitochondrial morphology and number but also mitochondrial function and distribution, which are of paramount importance in neuronal homeostasis including synaptic function. Not surprisingly, an impaired balance of mitochondrial fission and fusion is increasingly implicated in neurodegenerative diseases. For example, Charcot-Marie-Tooth neuropathy type 2A (CMT-2A) is caused by mutations in Mfn2 (Zuchner et al. 2004, Kijima et al. 2005), and the most commonly inherited optic neuropathy is caused by mutations in OPA1 (Delettre et al. 2000, Alexander et al. 2000). More recently, it was reported that PINK1, mutations of which lead to Parkinson disease (PD), appears to play a role in mitochondrial fission through regulation of DLP1 (Poole et al. 2008) and fibroblasts from PD patients bearing PINK1 mutations also demonstrate abnormal mitochondrial morphology (Exner et al. 2007). Given the critical role of mitochondria dynamics in neurons and the fact that mitochondria morphological abnormalities seem to be a feature of all AD cases, sporadic or familial, it is very likely that abnormal mitochondrial dynamics may be a common pathway leading to mitochondrial and neuronal dysfunction critical to the pathogenesis of AD (Fig.1).
Figure 1. Schematic Illustration of the Involvement of Abnormal Mitochondrial Dynamics in the Pathogenesis of Alzheimer Disease.
Pathogenic factors such as oxidative stress and Aβ impair the delicate balance of mitochondria fission and fusion dynamics in the vulnerable neurons, which not only results in mitochondrial morphological change and structural damage that in turn further enhance ROS production and thus a vicious cycle ensues, but also leads to mitochondrial re-distribution from an evenly distribution pattern to accumulation in the cell body and depletion in the remote area such as axon or dendrite. Together, the abnormal mitochondrial dynamics causes mitochondrial dysfunction and neuronal dysfunction including synaptic dysfunction, and eventually neurodegeneration.
Acknowledgement
This study is supported by the NIH (AG031852) and Alzheimer’s Association (IIRG-07-60196).
REFERENCES
- Alexander C, Votruba M, Pesch UE, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- Amati-Bonneau P, Valentino ML, Reynier P, et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain. 2008;131:338–351. doi: 10.1093/brain/awm298. [DOI] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. The EMBO journal. 2006a;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. Embo Journal. 2006b;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R. Mitochondrial bioenergetics and structural network organization. Journal of cell science. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- Blass JP. The mitochondrial spiral. An adequate cause of dementia in the Alzheimer’s syndrome. Ann N Y Acad Sci. 2000;924:170–183. doi: 10.1111/j.1749-6632.2000.tb05576.x. [DOI] [PubMed] [Google Scholar]
- Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, Nunnari J, Shaw JM. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nature cell biology. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006a;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annual review of cell and developmental biology. 2006b;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. The Journal of biological chemistry. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. The Journal of cell biology. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Cheng X, Kanki T, Fukuoh A, Ohgaki K, Takeya R, Aoki Y, Hamasaki N, Kang D. PDIP38 Associates with Proteins Constituting the Mitochondrial DNA Nucleoid. Journal of biochemistry. 2005;138:673–678. doi: 10.1093/jb/mvi169. [DOI] [PubMed] [Google Scholar]
- Cipolat S, de Brito O. Martins, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- Exner N, Treske B, Paquet D, et al. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Developmental cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Frazier AE, Kiu C, Stojanovski D, Hoogenraad NJ, Ryan MT. Mitochondrial morphology and distribution in mammalian cells. Biological chemistry. 2006;387:1551–1558. doi: 10.1515/BC.2006.193. [DOI] [PubMed] [Google Scholar]
- Griparic L, van der Wel NN, Orozco IJ, Peters PJ, van der Bliek AM. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. The Journal of biological chemistry. 2004;279:18792–18798. doi: 10.1074/jbc.M400920200. [DOI] [PubMed] [Google Scholar]
- Guo XF, Macleod GT, Wellington A, et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G, Amati-Bonneau P, Blakely EL, et al. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain. 2008;131:329–337. doi: 10.1093/brain/awm272. [DOI] [PubMed] [Google Scholar]
- Isaacs AM, Senn DB, Yuan M, Shine JP, Yankner BA. Acceleration of amyloid beta-peptide aggregation by physiological concentrations of calcium. The Journal of biological chemistry. 2006;281:27916–27923. doi: 10.1074/jbc.M602061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. Journal of cell science. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. The Journal of biological chemistry. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- Kann O, Kovacs R. Mitochondria and neuronal activity. American journal of physiology. 2007;292:C641–657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- Keller JN, Guo Q, Holtsberg FW, Bruce-Keller AJ, Mattson MP. Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J Neurosci. 1998;18:4439–4450. doi: 10.1523/JNEUROSCI.18-12-04439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijima K, Numakura C, Izumino H, et al. Mitochondrial GTPase mitofusin 2 mutation in Charcot-Marie-Tooth neuropathy type 2A. Hum Genet. 2005;116:23–27. doi: 10.1007/s00439-004-1199-2. [DOI] [PubMed] [Google Scholar]
- Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nature reviews. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. The Journal of biological chemistry. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Molecular biology of the cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Melov S. Modeling mitochondrial function in aging neurons. Trends in Neurosciences. 2004;27:601–606. doi: 10.1016/j.tins.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Naderi J, Lopez C, Pandey S. Chronically increased oxidative stress in fibroblasts from Alzheimer’s disease patients causes early senescence and renders resistance to apoptosis by oxidative stress. Mechanisms of ageing and development. 2006;127:25–35. doi: 10.1016/j.mad.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou JC. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE. 2008;3:e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F, Martinou JC. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Molecular and Cellular Biology. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends in molecular medicine. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science (New York, N.Y. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. The Journal of cell biology. 1999;147:699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Miller SW, Davis RE, Parks JK, Parker WD, Tuttle JB. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer’s disease. J Neurosci. 1997;17:4612–4622. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Molecular biology of the cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. The Journal of cell biology. 1998;143:351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinazzi M, Cazzola S, Bortolozzi M, et al. A NOVEL DELETION IN THE GTPase DOMAIN OF OPA1 CAUSES DEFECTS IN MITOCHONDRIAL MORPHOLOGY AND DISTRIBUTION, BUT NOT IN FUNCTION. Human molecular genetics. 2008 doi: 10.1093/hmg/ddn225. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO journal. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Stugard C, Murdock D, Schurr T, Brown MD. Ancient mtDNA sequences in the human nuclear genome: a potential source of errors in identifying pathogenic mutations. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14900–14905. doi: 10.1073/pnas.94.26.14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. The American journal of pathology. 2008a;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak S, Moreira P, Fujioka H, Wang Y, Casadesus G, Zhu X. Aβ Overproduction Causes Abnormal Mitochondrial Dynamics via Modulation of Mitochondrial Fission/Fusion Proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008b doi: 10.1073/pnas.0804871105. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Smith MA, Perry GZ, X. Impaired balance of mitochondrial fission and fusion in susceptible neurons of Alzheimer disease. ICAD 2008. 2008c doi: 10.1523/JNEUROSCI.1357-09.2009. Program No. P3-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RC, Picton LK, Williams SC, Tan FJ, Hill RB. Direct binding of the dynamin-like GTPase, Dnm1, to mitochondrial dynamics protein Fis1 is negatively regulated by the Fis1 N-terminal arm. The Journal of biological chemistry. 2007;282:33769–33775. doi: 10.1074/jbc.M700807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Perry G, Moreira PI, Aliev G, Cash AD, Hirai K, Smith MA. Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J Alzheimers Dis. 2006;9:147–153. doi: 10.3233/jad-2006-9207. [DOI] [PubMed] [Google Scholar]
- Zhu XW, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: An update. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2007;1772:494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Zhu XW, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet neurology. 2004;3:219–226. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- Zuchner S, Mersiyanova IV, Muglia M, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]