Abstract
Background
Donor human hearts cannot be preserved for >5 hours between explantation and recipient implantation. A better approach is needed to preserve transplantable hearts for longer periods, ideally at ambient conditions for transport. We tested whether Lifor solution could satisfactorily preserve guinea pig isolated hearts perfused at low flow with no added oxygen at room temperature for 20 hours.
Methods
Hearts were isolated from 18 guinea pigs and perfused initially with oxygenated Krebs–Ringer (KR) solution at 37°C. Hearts were then perfused with recirculated Lifor or cardioplegia (CP) solution (K+ 15 mmol/liter) equilibrated with room air at 20% of control flow at 26°C for 20 hours. Hearts were then perfused at 100% flow with KR for 2 hours at 37°C.
Results
Lifor and CP arrested all hearts. During the 20-hour low-flow perfusion with Lifor coronary pressure increased by 6 ± 2 mm Hg and percent oxygen extraction by 29 ± 2%, whereas oxygen consumption (MVo2) decreased by 74 ± 4%. Similar changes were noted for CP, except that MVo2 was decreased by 86 ± 7%. After 20-hour low-flow perfusion with Lifor and 2 hours of warm reperfusion with KR solution, diastolic left ventricular pressure (LVP), maximal dLVP/dt and percent oxygen extraction returned completely to baseline values, whereas heart rate returned to 80 ± 3%, developed LVP to 76 ± 3%, minimal dLVP/dt (relaxation) to 65 ± 4%, coronary flow to 80 ± 4%, oxygen consumption to 82 ± 4% and cardiac efficiency to 85 ± 4% of baseline values. Flow responses to adenosine and nitroprusside after Lifor treatment were 65 ± 3% and 64 ± 3% of their baseline values. After cardioplegia, treatment there was no cardiac activity, with a diastolic pressure of 35 ± 14 mm Hg and a return of coronary flow to only 45 ± 3% of baseline value.
Conclusions
Compared with a cardioplegia solution at ambient air and temperature conditions, Lifor solution is a much better medium for long-term cardiac preservation in this model.
In cardiac transplantation, the transport time between harvest and recipient implantation is limited by the viability of the donor heart. Cold storage of human hearts for transplantation currently limits functional viability to 4 to 5 hours despite development and clinical availability of approximately 10 different heart preservation solutions. There remains a lack of consensus on the ideal solution. Two major problems with current approaches are the need for severe hypothermia (3° to 6°C) and the lack of tissue perfusion during transport. Very low-flow perfusion of hearts at room temperature without supplemental oxygen would facilitate a lengthening of the period of viability and reduce the need for complicated support equipment during transport. To do so requires a suitable preservation solution. Lifor is a fully artificial preservation medium containing both a non-protein oxygen and nutrient carrier (nanoparticles) and cellular nutrients, including amino acids and sugars. Our aim was to compare a cardioplegia (CP) solution with Lifor solution when recirculated into hearts at room temperature and atmospheric conditions for 20 hours. Our aim was to determine whether Lifor was more effective than CP in preserving cardiac electrical, mechanical, vascular and metabolic function on restitution with a normal physiologic crystalloid solution.
METHODS
Langendorff Heart Preparation
The present investigation conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, revised 1996). Prior approval was obtained from the institutional biomedical resource committee. Our Langendorff heart method has been described previously in detail.1–5 Isolated hearts were perfused with in-line-filtered (20-µm pore) and oxygenated (pH 7.39 ± 0.1, Po2 562 ± 11 mm Hg) Krebs–Ringer (KR) solution containing (in millimoles per liter): 137 Na+, 5 K+, 1.2 Mg2+, 2.5 Ca2+, 134 Cl−, 15.5 HCO3− 1.2 H2PO4−, 11.5 glucose, 2 pyruvate, 16 mannitol and 0.05 ethylene-diamine tetraacetic acid (EDTA), with 5 units/liter insulin. Perfusate temperature was maintained at 37.2 ± 0.1°C before and after CP (n = 7 hearts) or Lifor treatment (n = 11 hearts).
Lifor (Lifeblood Medical, Inc., Freehold, NJ) is a proprietary (U.S. Patent #7,220,538) solution containing sugars, amino acids, salts, buffers, colloids and lipid nanoparticles, as defined in the patent. The CP and Lifor solutions had the following compositions, respectively, when equilibrated with room air at 26°C: 295 ± 4 and 297 ± 5 mOsm/liter; pH 7.07 ± 0.01 and 7.08 ± 0.01; Pco2 5.0 ± 0.2 and 6.0 ± 0.3 mm Hg; Po2 169 ± 2 and 167 ± 3 mm Hg; Na+ 136 ± 2 and 98 ± 1 mmol/liter; K+ 15.1 ± 0.4 and 15.8 ± 0.5 mmol/liter; and Ca2+ 0.25 ± 0.03 and 0.17 ± 0.02 mmol/liter. Lifeblood Medical had no role in the collection, analysis and interpretation of data or the right to approve or disapprove its publication. Additives to CP and Lifor were 10 µmol/liter adenosine and 1 µmol/liter blebbistatin, a myosin II inhibitor.6,7 Bartel’s antibotic solution (3%, containing gentamycin, streptomycin and amphotericin B) was also added to the preservation solutions because hearts were not harvested in a sterile manner and the solution was exposed to room air. During the 20-hour treatment period with 250 ml of recirculated CP or Lifor, coronary inflow was set at 15% to 20% of the baseline flow of approximately 17 ml/min, so that this volume was recirculated through the vasculature approximately 12 times. Recirculation was achieved using pump and tubing between the right ventricle (coronary sinus) and aortic inflow (coronary ostia) cannula.
Left ventricular pressure (LVP) and its first derivative (dLVP/dt) were measured isovolumetrically with a transducer and a saline-filled balloon inserted into the LV through the mitral valve. Balloon volume was initially adjusted to a diastolic LVP of 0 mm Hg so that any subsequent increase in diastolic LVP reflected diastolic contracture. Heart rate was monitored from bipolar electrodes in the right atrial appendage and right ventricular free wall. Coronary flow was measured by an ultrasonic flowmeter placed into the aortic in-flow line. Coronary sinus effluent was collected from a catheter placed into the right ventricle through the pulmonary artery after ligating both venae cavae. Coronary effluent Na+, K+, Ca2+, Po2, Pco2 and pH were measured intermittently. Coronary outflow (sinus) oxygen tension was also measured in-line with a Clark-type oxygen electrode. Percent oxygen extraction was calculated as 100 · (Pao2 − PVo2)/Pao2 (where Pao2 and PVo2 are arterial and venous Po2, respectively). Myocardial oxygen consumption (MVo2) was calculated as (coronary flow/g) · (arterial Pao2 − PVo2) · 24 µl O2/ml (37°C) or 26.5 µl O2/ml (26°C) at 760 mm Hg; and cardiac work efficiency was calculated as: systolic– diastolic LVP · heart rate/MVo2.
All data were collected from hearts in sinus rhythm at baseline. After the 20-hour treatment period and a 2-hour reperfusion period with KR, hearts were removed and the ventricles were cut into 4 or 5 horizontal sections and stored overnight in 10% formaldehyde. Wet heart weight was 1.46 ± 0.07 g averaged for both groups. Ventricular infarct size (percent of total ventricular weight) was determined by one of the investigators (J.S.H.) without knowledge of the treatment, using the 2,3,5-triphenyltetrazolium chloride (TTC) staining method.3,5
Protocol
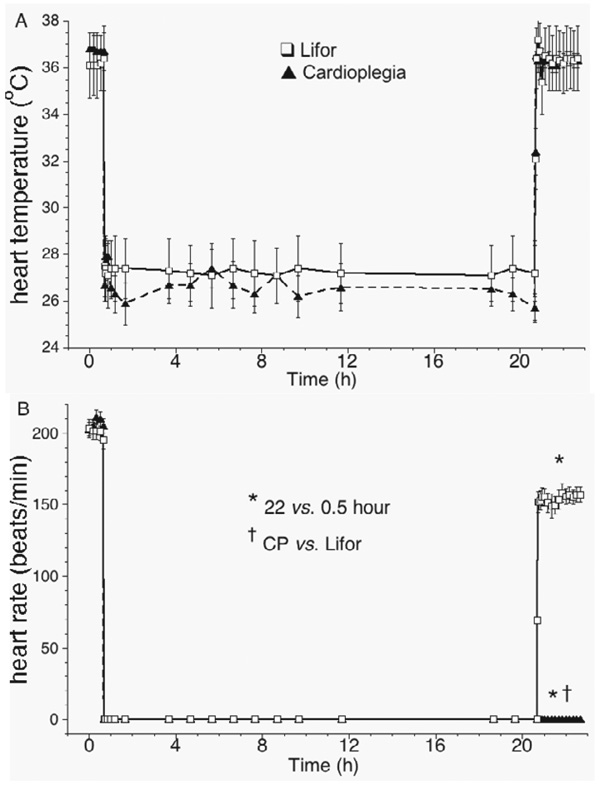
Each experiment lasted 23 hours, beginning after 30 minutes of equilibration. Ten-second recordings of atrial and ventricular electrograms, LVP, coronary flow, venous Po2, perfusion pressure and temperature were recorded automatically every 30 minutes (PowerLab, ADInstruments, info@adinstruments.com). There was no time-control group. We previously reported a comparison of Lifor and ViaSpan treatments for 10 hours to a time-control group.5 Because the ViaSpan-treated groups were non-viable, in the present study we compared the Lifor treatment with a cardioplegia treatment. Hearts were allowed to cool from 37°C to 26.2 ± 0.05°C over 10 minutes at the initiation of treatment and were rewarmed from 26.2°C to 37°C over 10 minutes on reperfusion with KR as during baseline conditions (Figure 1A). LVP, coronary flow and coronary venous Po2 were measured continuously before, during and after treatment. Hearts arrested immediately with CP or Lifor treatments.
Figure 1.
(A) Cardiac temperature before, during and after treatment with air-equilibrated, recirculated CP or Lifor solution for 20 hours at 20% baseline flow. (B) Both treatment-arrested hearts; after treatment, CP treated hearts did not beat. Heart rate in the Lifor-treated group was lower than before treatment.
Statistical Analysis
All data are presented as mean ± SEM. Data were analyzed by 1-way (within-group) analysis of variance (SuperANOVA 1.11; Abacus Concepts, Berkeley, CA) for comparison of data collected at the selected time-points of 10 and 20 hours (during treatment) and 22 hours (2 hours after treatment) vs that at 0 hours (37°C pretreatment baseline). Two-way analysis of variance (between groups) was determined at these same time-points. If F-values from the analyses of variance were significant, Duncan’s and least-significant-difference (LSD) post hoc tests were used to clarify differences over time and between the two groups (p < 0.05, 2-tailed).
RESULTS
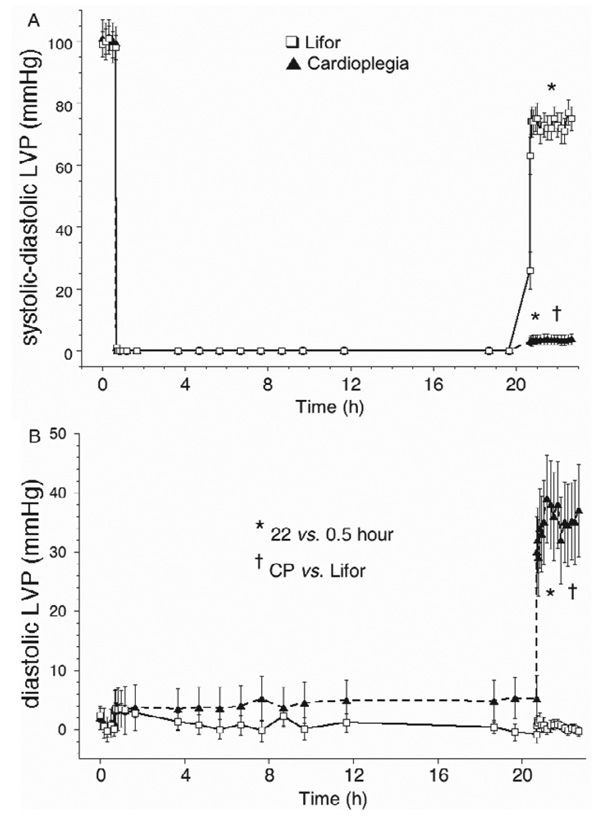
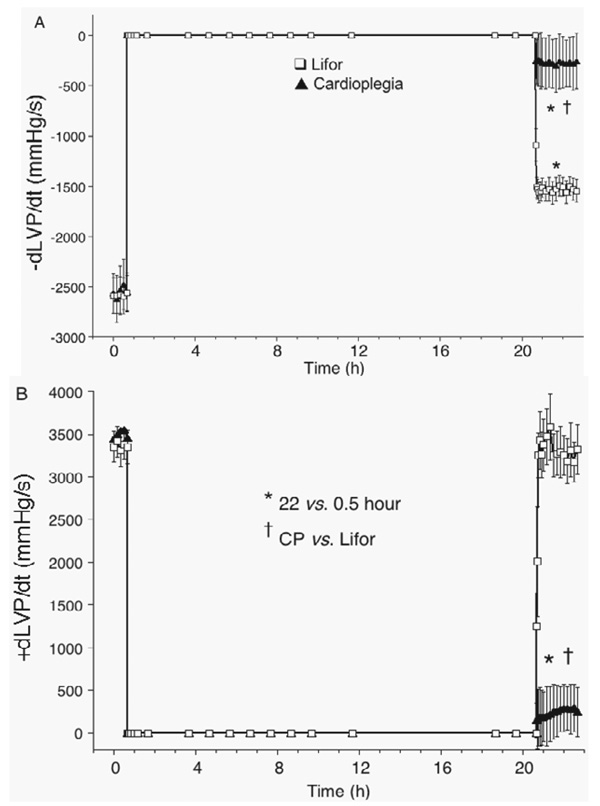
CP and Lifor solutions arrested all hearts (Figure 1B). Each heart in the CP-treated group exhibited no electrical or contractile activity on reperfusion 22 hours later with KR solution, whereas each Lifor-treated heart had rhythmic contractions on reperfusion. None of the Lifor-treated hearts exhibited ventricular dysrhythmias throughout the post-treatment period. Two hours after terminating Lifor treatment, heart rate was 20 ± 3% lower than baseline and developed (systolic– diastolic) LVP (Figure 2A) returned to 76 ± 3% of baseline values after 2-hour reperfusion. Diastolic LVP (Figure 2B) remained very low on reperfusion with KR in the Lifor group, but diastolic contracture (35 ± 14 mm Hg) occurred in the CP group. With 2-hour reperfusion, minimal dLVP/dt, an index of relaxation (Figure 3A), returned to 65 ± 4%, whereas maximal dLVP/dt, an index of contractility (Figure 3B), returned to 100 ± 3% of baseline values in the Lifor group.
Figure 2.
(A) Developed (systolic–diastolic) left ventricular pressure (LVP) was near zero after CP treatment and reduced after Lifor treatment. (B) Diastolic LVP was not altered during or after Lifor treatment but was markedly increased after CP treatment.
Figure 3.
(A) Minimal dLVP/dt was lower after treatment with air-equilibrated, re-circulated Lifor for 20 h at 26°C at 20% baseline coronary flow. (B) Maximal dLVP/dt was fully restored after Lifor treatment. CP-treated hearts exhibited no contractility or relaxation.
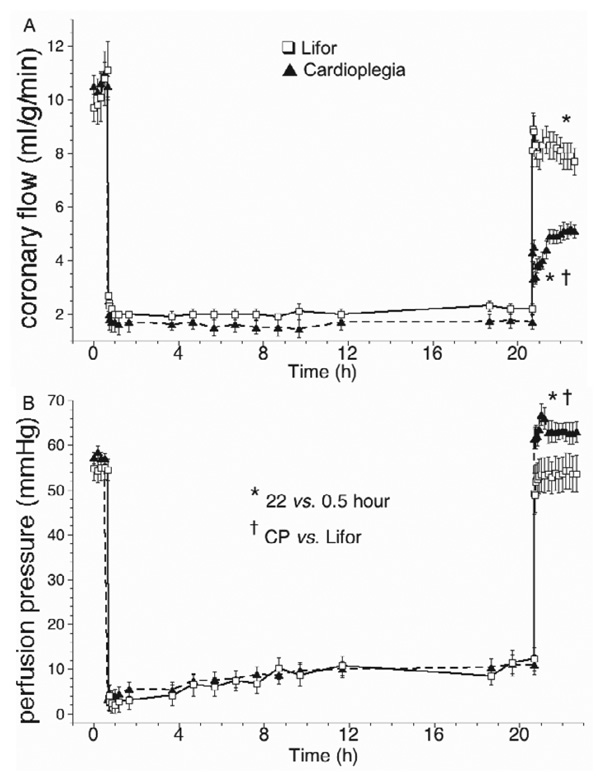
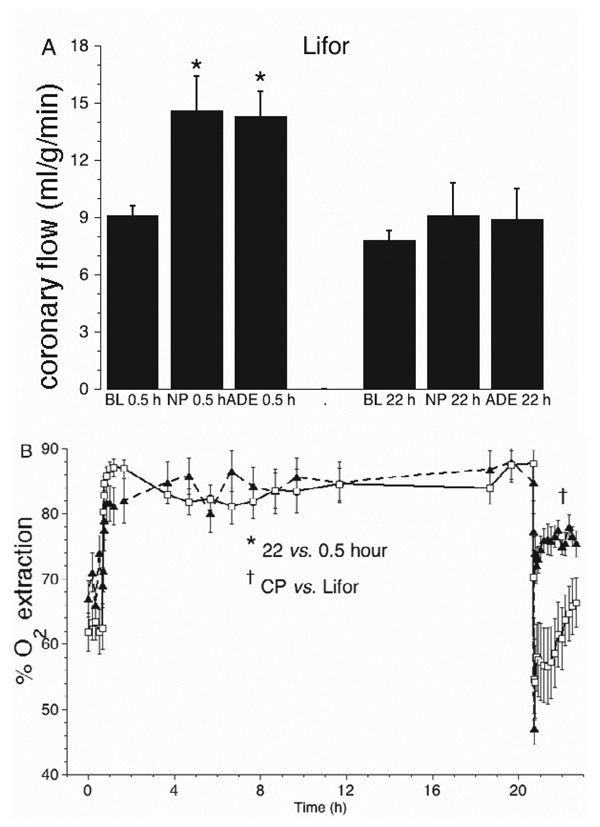
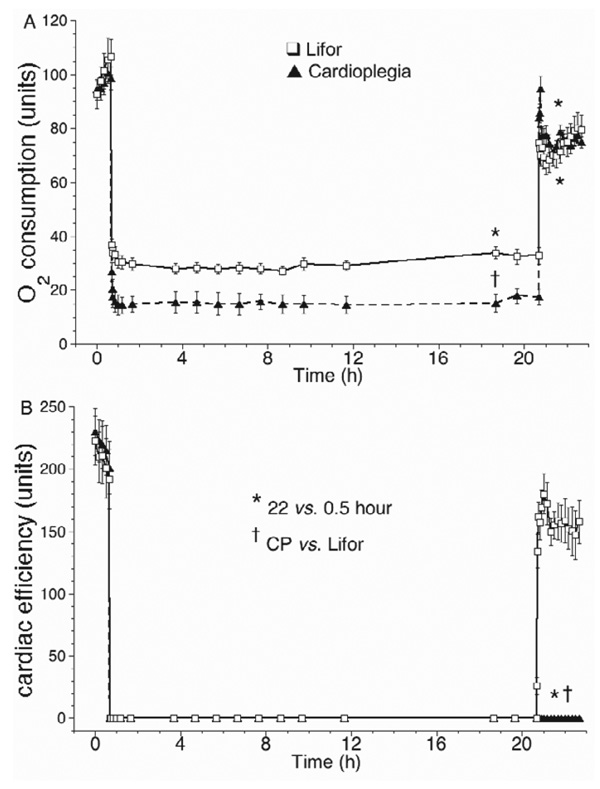
Coronary flow (Figure 4A) was set constant (2.0 ± 0.4 ml/g/min) at 20% of the baseline flow (10 ± 2 ml/g/min) during CP and Lifor treatments. On reperfusion after Lifor, coronary flow returned to 80 ± 4% of the baseline value, but to only 45 ± 3% of baseline after CP. Coronary perfusion pressure was set to 55 ± 2 mm Hg before and after CP or Lifor treatments. Constant flow perfusion pressure (Figure 4B) increased slightly from 4 ± 1 to 10 ± 2 mm Hg between 1 and 20 hours in both groups and was higher on reperfusion after CP than after Lifor. Responses to adenosine and nitroprusside (Figure 5A) were lower by 35 ± 3% and 36 ± 3% compared with their baseline values in the Lifor group; there were no responses in the CP group (data not shown). Percent oxygen extraction (Figure 5B) increased by approximately 29% during CP or Lifor treatment and returned to the baseline level on reperfusion after Lifor treatment but remained higher after CP treatment. Oxygen consumption (Figure 6A) fell to 26 ± 6% and 15 ± 8% of baseline during the Lifor and CP treatments and returned to 82 ± 4% and 83 ± 6% of control after 2-hour reperfusion, respectively. Cardiac efficiency (Figure 6B) was zero during treatments as hearts did not beat or generate pressure and returned to 85 ± 4% of baseline during 2-hour reperfusion with Lifor compared with its baseline. Cardiac efficiency was zero on reperfusion after CP. Apparent percent infarct size after Lifor treatment and 2-hour warm reperfusion was 18.6 ± 0.1%; for the CP group infarct size was 59.5 ± 0.2%.
Figure 4.
(A) Coronary flow was natural at constant pressure (55 mm Hg) before and after treatment, and pump perfused at 20% of the natural flow during treatment. Natural coronary flow was lower than baseline after Lifor treatment but even lower after CP treatment. (B) During either treatment, perfusion pressure gradually increased at the constant coronary flow of 20% baseline, indicating increased flow resistance.
Figure 5.
(A) Before Lifor treatment (0.5 hours), coronary flow increased with nitroprusside (NP) and adenosine (ADE) compared with baseline (BL). After treatment (22 hours), vasodilator responses were attenuated. After CP treatment, there were no flow responses to ADE or NP (data not shown). (B) Percent oxygen extraction increased during Lifor treatment when Po2 was reduced from 97% to 20% (room air), but returned to baseline levels after treatment. After CP treatment, percent oxygen extraction remained high.
Figure 6.
(A) Oxygen consumption decreased markedly during Lifor or CP treatment, while hearts were arrested and Po2 was lower, but was less decreased during Lifor than during CP treatment. After treatment, oxygen consumption was lower than baseline in both groups. (B) Cardiac efficiency (heart rate · systolic — diastolic LVP/O2 consumption) was zero during CP and Lifor treatments and was zero after CP treatment and lower than baseline after Lifor treatment.
DISCUSSION
This is a second report on Lifor, a nanoparticle salt-based solution containing amino acids and other additives as a heart preservation solution. In our first report5 we found that Lifor was a much better preservation solution for isolated hearts than was ViaSpan, a commonly used preservation solution, when each were low-flow perfused at ambient air and temperature conditions for 10 h. We found that re-circulated Lifor solution, supplemented with adenosine and butanedione monoxime (BDM) and given both as a cardioplegic agent and as a preservation medium, protected hearts against damage for 10 hours at 26°C.5 Lifor-treated hearts exhibited a full return of developed LVP and dLVP/dt minimum (relaxation), and initially, of dLVP/dt maximum (contractility).5 Under the same experimental conditions as for Lifor, hearts treated with ViaSpan were completely non-functional (no heart beat or contractile effort), with diastolic contracture and high coronary vascular resistance during the 2-hour reperfusion period with KR solution.
In the present study we extended the treatment time from 10 to 20 hours. CP-treated hearts were nonfunctional on reperfusion after 20 hours. In contrast, we found that low-flow perfusion of Lifor for 20 hours at ambient atmospheric and temperature conditions was followed on reperfusion with KR solution by complete cardiac protection for a number of variables, such as diastolic LVP, contractility and percent oxygen extraction, and small functional declines in other variables, such as heart rate, developed LVP, relaxation, coronary flow, oxygen consumption and cardiac efficiency. The gradual but small decline in function over time in constant-pressure-perfused, isolated hearts at 37°C is well documented. We have typically noted a fall in developed LVP of approximately 3% to 4% per hour or 9% to 12% over 3 hours.8–10 In the present study, developed LVP decreased by 24% over the 3-hour perfusion with KR solution and 20-hour perfusion with Lifor. In our prior study,5 15-hour perfusion with KR at 37°C resulted in a 35% decline in developed LVP. Thus, there was effectively little fall in LVP during this 23-hour study compared with a shorter time-control study. Decreases in heart rate and functional indices can be largely attributed to catecholamine depletion over time.
The decline in oxygen consumption was primarily due to the small decrease in coronary flow because percent oxygen extraction was unchanged after compared with before treatment. Cardiac efficiency by definition, however, was more decreased by the decline in heart rate and developed LVP than by the fall in oxygen consumption. Vascular resistance to flow increased progressively but mildly during Lifor treatment. Moreover, the maximal vasodilatory responses to nitroprusside and adenosine were depressed after Lifor treatment compared with before Lifor treatment. These findings likely reflect mild vascular edema because both direct vascular smooth muscle relaxation (adenosine) and endothelium-mediated (nitroprusside) muscle relaxation were blunted.
In our prior companion study,5 BDM and adenosine were added because we reported improved function with these additives in a severe-cold-storage heart model.1,11 BDM is a non-specific reversible inhibitor of the actinomyosin complex.12,13 In the present study we used blebbistatin, a recently discovered inhibitor of the ATPases associated with Class II myosin isoforms in an actin-detached state.6,7 Blebbistatin is highly specific for myosin II inhibition in cardiac and skeletal muscle, but not in smooth muscle. Our rationale for its use was that it might reduce or prevent diastolic contracture because the myosin II head regions are responsible for attachment and movement along the actin-based thin filaments that generate muscle contraction.
The apparent small infarct size in the Lifor group could represent true infarction, but it is rather likely a result of the inaccuracy in identifying and cutting out suspected small infarcted areas for weighing. The values obtained are within the detection error of the method,14 because the TTC staining-by-weight method to determine the percentage of infarcted tissue is not reliable at lower degrees of infarction, as indicated by the apparent 11 ± 3% infarct size we measured after 3-hour KR perfusion without ischemia in another study.15
Quest for Better Techniques and Solutions
During the past decade, over 600 articles have been published on cardiac preservation techniques and solutions, but there is no consensus on the best approaches to long-term preservation of hearts for transplant. Although there are suitable long-term preservation techniques for the liver and kidney, hearts cannot be well protected for periods of more than 4 or 5 hours.16–20 More popular experimentally tested and clinically used solutions include ViaSpan21–24 (also called UW solution), HTK (histidine–tryptophan–ketoglutarate-based solution, or Bretschneider’s),25,26 Celsior (anti-oxidant–based solution with mannitol, reduced glutathione, plus high Mg2+, lactobionate and glutamate)27–31 and STH (St Thomas’ Hospital, a high K+, high Mg+, low Ca2+, lidocaine-containing solution)32–35; others include Euro-Collins and Stanford solutions. Most of these solutions have a “cardioplegic” (high [K+]) base to arrest the heart.
All these solutions have limitations regarding adequacy and length of protection,36 and their protective effects are dependent on study conditions.32,37,38 Many of the heart studies have compared one preservation solution with another,26,29–33,35,39–44 and with or without additives, such as channel activators or blockers,1,11,45–49 exchange inhibitors,50–52 anesthetics,2,9,49 nitric oxide (NO) donors,53 perfluorocarbons54 or BDM.1,30,55 For example, Celsior solution preserved function better than ViaSpan in several studies,44,56 whereas others found that ViaSpan was better than Celsior.29,30 HTK was found to be more protective than ViaSpan in one study,43 whereas another found the reverse to be true.26 A newer solution, LYPS (extracellular type with low Ca2+ and Mg2+, added pyruvate, polyethylene glycol and chlorpromazine), was tested using a biopsy technique for tissue viability to evaluate the independent effects of 19 compounds found in other preservation solutions.57 This solution was better for preserving pig hearts stored for 8 hours at 4°C than was STH solution.57 In a rat model (8 hours, 4°C), LYPS was better than ViaSpan (intracellular-type UW, or extracellular-type UW-1), but was equivalent to Celsior.41
Perfusion storage of hearts is rarely used clinically compared with simple immersion into an ice-jacketed container, because it is more complicated and costly to undertake. Perfusion storage could require a mechanical pump, a cooling system, an oxygen supply tank and a very large volume of non-recirculated solution to perfuse the coronary vasculature. Moreover, to be warranted as the best technique, perfusion preservation must lead to superior return of function after a long period compared with simple storage, particularly if severe cooling is to be avoided. A recent review suggested that a perfusion system is required to effectively preserve hearts for increasingly longer periods between explant and implant.17 Animal studies have shown the superiority of low-flow perfusion techniques.1,2,58,59
Another concern for long-term protection of hearts is the need for severe hypothermia. The colder the hearts the longer they can be protected against no-flow ischemia.8,50,52 Severe hypothermia reduces energy demand and therefore it is useful to protect hearts metabolically against ischemic injury during cardiac storage prior to transplantation. Hypothermia preserves essential mechanisms during heart transport to rapidly regenerate ATP on reperfusion by decreasing energy utilization.
Although hypothermia is the most effective method to preserve hearts during ischemic storage, hypothermia itself has deleterious effects on the contractile element and endothelial cell function as cooling is more severe. Two of these effects include cytosolic60 and mitochondrial3 Ca2+ loading, another is excess release of reactive oxygen species (ROS).61 Either of these can result in mitochondrial and cellular damage proportional to the degree and duration of hypothermia. For example, we reported that cardiac perfusion at 17°C before ischemia itself caused a moderate and steady-state increase in mitochondrial Ca2+, a more reduced redox state (increased NADH), and moderate production of ROS.3 Under different mitochondrial conditions, either low or high tissue oxygen levels can lead to ROS generation.4,62
Less cooling and oxygenation in cell-free preservation solutions should be offset by methods to increase tissue oxygen and nutrient delivery, particularly if a solution is to be recirculated to reduce the volume of coronary perfusate required. Our goal in this experimental model was to apply this approach, but to preserve hearts at room temperature rather than expose them to severe hypothermia and to do so with no added oxygen.
Transplant programs could benefit from a preservation technique with a single solution that does not require severe cooling of the heart or supplemental oxygen, and requires only a small volume of coronary perfusate for transport between centers. Lifor solution has potential in reaching this goal. A prolonged preservation time, particularly at room temperature, would increase the available donor pool of viable hearts and improve post-transplant outcomes. An increase in preservation times and improvements in banking and transport of hearts over longer distances should greatly increase the availability of viable hearts with good tissue matches to recipients in need.
Conclusions and Limitations
In this experimental model, which consisted of a low-flow, coronary recirculation system at room temperature and room air, Lifor solution maintained hearts for up to 20 hours. Simple CP solutions rendered hearts non-viable. The experimental conditions of this study were set up to mimic the potential for preserving human hearts over a long period for eventual transplant. An obvious limitation is that the use of a small animal heart model to examine preservation solutions may not reflect clinical conditions or use of these solutions in the large mammalian or human heart. Future studies should determine the optimal conditions and maximal length of protection afforded by Lifor solution and compare Lifor with other available preservation solutions in a large animal heart transplant model. The mechanism of protection by nanoparticle based amino acid solutions will be a focus of future studies.
Acknowledgments
Supported in part by a contract from Lifeblood Medical, Inc., Freehold, NJ, and by a grant from Eckhart Grohmann to the Cardiovascular Research Center.
This work was published previously in part in abstract form (Stowe DF, Camara AKS, Heisner JS, Aldakkak M, Harder DR: 20 h preservation of isolated hearts perfused at low flow with air-saturated Lifor solution at room temperature. Proc Int Acad Cardiol 13:38, 2007)
REFERENCES
- 1.Stowe DF, Graf BM, Fujita S, Gross GJ. One-day cold perfusion of bimakalim and butanedione monoxime restores ex situ cardiac function. Am J Physiol Heart Circ Physiol. 1996;271:H1884–H1892. doi: 10.1152/ajpheart.1996.271.5.H1884. [DOI] [PubMed] [Google Scholar]
- 2.Stowe DF, Habazettl H, Graf BM, Kampine JP, Bosnjak ZJ. One-day hypothermic preservation of isolated hearts with halothane improves cardiac function better than low calcium. Anesthesiology. 1995;83:1065–1077. doi: 10.1097/00000542-199511000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Riess ML, Camara AK, Kevin LG, An J, Stowe DF. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17°C ischemia in intact hearts. Cardiovasc Res. 2004;61:580–590. doi: 10.1016/j.cardiores.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 5.Stowe DF, Camara AK, Heisner JS, Aldakkak M, Harder DR. Ten-hour preservation of guinea pig isolated hearts perfused at low flow with air-saturated Lifor solution at 26°C: comparison to ViaSpan solution. Am J Physiol Heart Circ Physiol. 2007;293:H895–H901. doi: 10.1152/ajpheart.00149.2007. [DOI] [PubMed] [Google Scholar]
- 6.Fedorov VV, Lozinsky IT, Sosunov EA, et al. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007;4:619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 7.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 8.An J, Camara AK, Rhodes SS, Riess ML, Stowe DF. Warm ischemic preconditioning improves mitochondrial redox balance during and after mild hypothermic ischemia in guinea pig isolated hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2620–H2627. doi: 10.1152/ajpheart.01124.2004. [DOI] [PubMed] [Google Scholar]
- 9.An J, Camara AK, Riess ML, Rhodes SS, Varadarajan SG, Stowe DF. Improved mitochondrial bioenergetics by anesthetic preconditioning during and after 2 hours of 27°C ischemia in isolated hearts. J Cardiovasc Pharmacol. 2005;46:280–287. doi: 10.1097/01.fjc.0000175238.18702.40. [DOI] [PubMed] [Google Scholar]
- 10.Riess ML, Kevin LG, McCormick J, et al. Anesthetic preconditioning: the role of free radicals in sevoflurane-induced attenuation of mitochondrial electron transport in guinea pig isolated hearts. Anesth Analg. 2005;100:46–53. doi: 10.1213/01.ANE.0000139346.76784.72. [DOI] [PubMed] [Google Scholar]
- 11.Stowe DF, Boban M, Kampine JP, Bosnjak ZJ. Reperfusion with adenosine and nitroprusside improves preservation of isolated guinea pig hearts after 22 hours of cold perfusion with 2,3 butanedione monoxime. J Cardiovasc Pharmacol. 1993;21:578–586. doi: 10.1097/00005344-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Scaduto RC, Jr, Grotyohann LW. 2,3-butanedione monoxime unmasks Ca2+-induced NADH formation and inhibits electron transport in rat hearts. Am J Physiol Heart Circ Physiol. 2000;279:H1839–H1848. doi: 10.1152/ajpheart.2000.279.4.H1839. [DOI] [PubMed] [Google Scholar]
- 13.Forer A, Fabian L. Does 2,3-butanedione monoxime inhibit nonmuscle myosin? Protoplasma. 2005;225:1–4. doi: 10.1007/s00709-004-0077-z. [DOI] [PubMed] [Google Scholar]
- 14.Riess M, Camara AKS, Chen Q, et al. Comparison of two methods to determine infarct size after global ischemia in isolated guinea pig hearts. FASEB J. 2002;16:A492. [Google Scholar]
- 15.Riess ML, Novalija E, Camara AK, et al. Preconditioning with sevoflurane reduces changes in nicotinamide adenine dinucleotide during ischemia-reperfusion in isolated hearts: reversal by 5-hydroxydecanoic acid. Anesthesiology. 2003;98:387–395. doi: 10.1097/00000542-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Nelson SK, Bose S, Rizeq M, McCord JM. Oxidative stress in organ preservation: a multifaceted approach to cardioplegia. Biomed Pharmacother. 2005;59:149–157. doi: 10.1016/j.biopha.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Rivard AL, Gallegos RP, Bianco RW, Liao K. The basic science aspect of donor heart preservation: a review. J Extra Corpor Technol. 2004;36:269–274. [PubMed] [Google Scholar]
- 18.Dobson GP. Organ arrest, protection and preservation: natural hibernation to cardiac surgery. Comp Biochem Physiol B Biochem Mol Biol. 2004;139:469–485. doi: 10.1016/j.cbpc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Stringham JC, Southard JH, Hegge J, et al. Limitations of heart preservation by cold storage. Transplantation. 1992;53:287–294. doi: 10.1097/00007890-199202010-00007. [DOI] [PubMed] [Google Scholar]
- 20.D’Alessandro AM, Southard JH, Love RB, Belzer FO. Organ preservation. Surg Clin N Am. 1994;74:1083–1095. [PubMed] [Google Scholar]
- 21.Swanson DK, Pasaoglu I, Berkoff HA, Southard JA, Hegge JO. Improved heart preservation with UW preservation solution. J Heart Transplant. 1988;7:456–467. [PubMed] [Google Scholar]
- 22.Kawai A, Morita S, Kormos RL, et al. A clinical trial comparing University of Wisconsin solution and cold cardioplegic solution with load-independent mechanical parameters. J Heart Lung Transplant. 1994;13:150–155. [PubMed] [Google Scholar]
- 23.Mankad PS, Severs NJ, Lachno DR, Rothery S, Yacoub MH. Superior qualities of University of Wisconsin solution for ex vivo preservation of the pig heart. J Thorac Cardiovasc Surg. 1992;104:229–240. [PubMed] [Google Scholar]
- 24.Oshima K, Takeyoshi I, Mohara J, et al. Long-term preservation using a new apparatus combined with suppression of proinflammatory cytokines improves donor heart function after transplantation in a canine model. J Heart Lung Transplant. 2005;24:602–608. doi: 10.1016/j.healun.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Saitoh Y, Hashimoto M, Ku K, et al. Heart preservation in HTK solution: role of coronary vasculature in recovery of cardiac function. Ann Thorac Surg. 2000;69:107–112. doi: 10.1016/s0003-4975(99)01190-x. [DOI] [PubMed] [Google Scholar]
- 26.Galinanes M, Murashita T, Hearse DJ. Long-term hypothermic storage of the mammalian heart for transplantation: a comparison of three cardioplegic solutions. J Heart Lung Transplant. 1992;11:624–635. [PubMed] [Google Scholar]
- 27.Oshima Y, Mohri S, Shimizu J, et al. Celsior preserved cardiac mechanoenergetics better than popular solutions in canine hearts. Ann Thorac Surg. 2006;81:658–664. doi: 10.1016/j.athoracsur.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez A, Borrego JM, Gomez S, et al. Myocardial preservation using Celsior: clinical results in high-risk cardiac transplantation. Transplant Proc. 2005;37:1543–1545. doi: 10.1016/j.transproceed.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 29.Boku N, Tanoue Y, Kajihara N, et al. A comparative study of cardiac preservation with Celsior or University of Wisconsin solution with or without prior administration of cardioplegia. J Heart Lung Transplant. 2006;25:219–225. doi: 10.1016/j.healun.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Kajihara N, Morita S, Tanoue Y, et al. The UW solution has greater potential for longer preservation periods than the Celsior solution: comparative study for ventricular and coronary endothelial function after 24-h heart preservation. Eur J Cardiothorac Surg. 2006;29:784–789. doi: 10.1016/j.ejcts.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Ohwada S, Sunose Y, Tsutsumi H, et al. Celsior is superior to UW for graft preservation from non-heart-beating donors in a canine liver transplantation model. Transplant Proc. 2001;33:922–923. doi: 10.1016/s0041-1345(00)02270-3. [DOI] [PubMed] [Google Scholar]
- 32.Karck M, Frantzen V, Haverich A, Uretzky G. Prolonged myocardial protection with St. Thomas’ Hospital solution and University of Wisconsin solution. The importance of preservation techniques. Eur J Cardiothorac Surg. 1992;6:261–266. doi: 10.1016/1010-7940(92)90109-b. [DOI] [PubMed] [Google Scholar]
- 33.Ledingham SJ, Katayama O, Lachno DR, Yacoub M. Prolonged cardiac preservation. Evaluation of the University of Wisconsin preservation solution by comparison with the St. Thomas’ Hospital cardioplegic solutions in the rat. Circulation. 1990;82 suppl IV:351–358. [PubMed] [Google Scholar]
- 34.Besirli K, Burhani SM, Arslan C, Suzer O, Sayin AG. Effect of combining phosphodiesterase III inhibitors with St Thomas Hospital’s solution used as transplantation preservative solution in isolated rat hearts. Transplant Proc. 2006;38:1253–1258. doi: 10.1016/j.transproceed.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 35.Choong YS, Gavin JB. Functional recovery of hearts after cardioplegia and storage in University of Wisconsin and in St. Thomas’ Hospital solutions. J Heart Lung Transplant. 1991;10:537–546. [PubMed] [Google Scholar]
- 36.Smolens IA, Follette DM, Berkoff HA, Castellanos LM, Segel LD. Incomplete recovery of working heart function after twenty-four-hour preservation with a modified University of Wisconsin solution. J Heart Lung Transplant. 1995;14:906–915. [PubMed] [Google Scholar]
- 37.Humphrey SM, Choong YS, Buckman JE, Gavin JB. Influence of storage volume on functional recovery and metabolism of explanted hearts following cold cardioplegia: studies in the rat. Cardiovasc Res. 1991;25:719–726. doi: 10.1093/cvr/25.9.719. [DOI] [PubMed] [Google Scholar]
- 38.Mohara J, Tsutsumi H, Takeyoshi I, et al. The optimal pressure for initial flush with UW solution in heart procurement. J Heart Lung Transplant. 2002;21:383–390. doi: 10.1016/s1053-2498(01)00388-6. [DOI] [PubMed] [Google Scholar]
- 39.Perrault LP, El-Hamamsy I, Dumont E, Malo O, Carrier M. Effects of crystalloid, blood and Celsior solutions on porcine coronary endothelial function after heart transplantation. J Heart Lung Transplant. 2005;24:912–920. doi: 10.1016/j.healun.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Wilson CH, Stansby G, Haswell M, Cunningham AC, Talbot D. Evaluation of eight preservation solutions for endothelial in situ preservation. Transplantation. 2004;78:1008–1013. doi: 10.1097/01.tp.0000135465.00738.ed. [DOI] [PubMed] [Google Scholar]
- 41.Michel P, Vial R, Rodriguez C, Ferrera R. A comparative study of the most widely used solutions for cardiac graft preservation during hypothermia. J Heart Lung Transplant. 2002;21:1030–1039. doi: 10.1016/s1053-2498(02)00414-x. [DOI] [PubMed] [Google Scholar]
- 42.Wildhirt SM, Weis M, Schulze C, et al. Effects of Celsior and University of Wisconsin preservation solutions on hemodynamics and endothelial function after cardiac transplantation in humans: a single-center, prospective, randomized trial. Transplant Int. 2000;13 suppl 1:S203–S211. doi: 10.1007/s001470050326. [DOI] [PubMed] [Google Scholar]
- 43.Ku K, Oku H, Alam MS, Saitoh Y, Nosaka S, Nakayama K. Prolonged hypothermic cardiac storage with histidine-tryptophan-ketoglutarate solution: comparison with glucose-insulin-potassium and University of Wisconsin solutions. Transplantation. 1997;64:971–975. doi: 10.1097/00007890-199710150-00006. [DOI] [PubMed] [Google Scholar]
- 44.Warnecke G, Schulze B, Haverich A, Klima U. Celsior solution provides superior post-ischemic right ventricular function as compared with UW solution in a porcine heart transplantation model. J Heart Lung Transplant. 2002;21:586–589. doi: 10.1016/s1053-2498(01)00406-5. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Long C, Ji B, Zhang H, Wen F. Myocardial protective effects of nicorandil, an opener of potassium channels on senile rat heart. Perfusion. 2006;21:179–183. doi: 10.1191/0269216306pf858oa. [DOI] [PubMed] [Google Scholar]
- 46.Feng J, Li H, Rosenkranz ER. KATP channel opener protects neonatal rabbit heart better than St. Thomas’ solution. J Surg Res. 2003;109:69–73. doi: 10.1016/s0022-4804(02)00064-1. [DOI] [PubMed] [Google Scholar]
- 47.Hoenicke EM, Damiano RJ., Jr Superior 12-hour heart preservation with pinacidil hyperpolarizing solution compared to University of Wisconsin solution. J Heart Lung Transplant. 2001;20:1106–1114. doi: 10.1016/s1053-2498(01)00307-2. [DOI] [PubMed] [Google Scholar]
- 48.Dobson GP, Jones MW. Adenosine and lidocaine: a new concept in nondepolarizing surgical myocardial arrest, protection, and preservation. J Thorac Cardiovasc Surg. 2004;127:79–805. doi: 10.1016/s0022-5223(03)01192-9. [DOI] [PubMed] [Google Scholar]
- 49.Ross JD, Ripper R, Law WR, et al. Adding bupivacaine to high-potassium cardioplegia improves function and reduces cellular damage of rat isolated hearts after prolonged, cold storage. Anesthesiology. 2006;105:746–752. doi: 10.1097/00000542-200610000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Camara AK, An J, Chen Q, et al. Na+/H+ exchange inhibition with cardioplegia reduces cytosolic [Ca2+] and myocardial damage after cold ischemia. J Cardiovasc Pharmacol. 2003;41:686–698. doi: 10.1097/00005344-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Scheule AM, Jost D, Beierlein W, et al. Sodium-hydrogen inhibitor cariporide (HOE 642) improves in situ protection of hearts from non-heart-beating donors. J Heart Lung Transplant. 2003;22:1335–1342. doi: 10.1016/s1053-2498(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 52.Stowe DF, Heisner JS, An JZ, Camara A, et al. Inhibition of Na+/H+ exchange-1 isoform protects hearts perfused after six hour cardioplegic cold storage. J Heart Lung Transplant. 2002;21:374–382. doi: 10.1016/s1053-2498(01)00383-7. [DOI] [PubMed] [Google Scholar]
- 53.Ramzy D, Rao V, Mallidi H, et al. Cardiac allograft preservation using donor-shed blood supplemented with L-arginine. J Heart Lung Transplant. 2005;24:1665–1672. doi: 10.1016/j.healun.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Johnson DL, Greene PS, Gott VL, Gardner TJ. New pluronic-free perfluorocarbon cardioplegia improves myocardial oxygenation. Circulation. 1988;78:III-153–III-157. [PubMed] [Google Scholar]
- 55.Stringham JC, Paulsen KL, Southard JH, Fields BL, Belzer FO. Improved myocardial ischemic tolerance by contractile inhibition with 2,3-butanedione monoxime. Ann Thorac Surg. 1992;54:852–859. doi: 10.1016/0003-4975(92)90636-i. [DOI] [PubMed] [Google Scholar]
- 56.Michel P, Hadour G, Rodriguez C, Chiari P, Ferrera R. Evaluation of a new preservative solution for cardiac graft during hypothermia. J Heart Lung Transplant. 2000;19:1089–1097. doi: 10.1016/s1053-2498(00)00194-7. [DOI] [PubMed] [Google Scholar]
- 57.Ferrera R, Michel P, Hadour G, et al. An optimal experimental design for the development of preservative heart solutions. J Heart Lung Transplant. 2002;21:260–270. doi: 10.1016/s1053-2498(01)00361-8. [DOI] [PubMed] [Google Scholar]
- 58.Ferrera R, Michel P, Hadour G, et al. Microperfusion techniques for long-term hypothermic preservation. J Heart Lung Transplant. 2000;19:792–800. doi: 10.1016/s1053-2498(00)00146-7. [DOI] [PubMed] [Google Scholar]
- 59.Fitton TP, Wei C, Lin R, et al. Impact of 24 h continuous hypothermic perfusion on heart preservation by assessment of oxidative stress. Clin Transplant. 2004;18 suppl 12:22–27. doi: 10.1111/j.1399-0012.2004.00213. [DOI] [PubMed] [Google Scholar]
- 60.Stowe DF, Fujita S, An J, et al. Modulation of myocardial function and [Ca2+] sensitivity by moderate hypothermia in guinea pig isolated hearts. Am J Physiol Heart Circ Physiol. 1999;277:H2321–H2332. doi: 10.1152/ajpheart.1999.277.6.H2321. [DOI] [PubMed] [Google Scholar]
- 61.Camara AK, Riess ML, Kevin LG, Novalija E, Stowe DF. Hypothermia augments reactive oxygen species detected in the guinea pig isolated perfused heart. Am J Physiol Heart Circ Physiol. 2004;286:H1289–H1299. doi: 10.1152/ajpheart.00811.2003. [DOI] [PubMed] [Google Scholar]
- 62.Becker LB, Vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol Heart Circ Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]