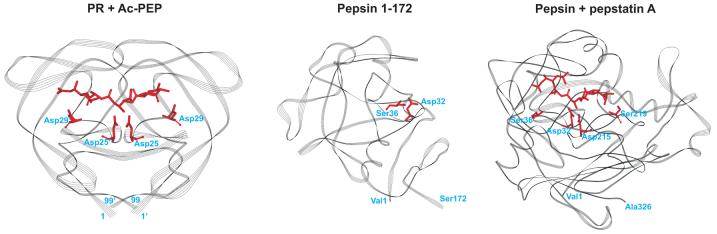
Fig. 6. Line ribbon representations of the crystal structures of PR co-crystallized with the generic aspartic protease inhibitor, acetyl-pepstatin (5HVP, left) and human pepsin co-crystallized with pepstatin A (1PSO, right).
The two pepstatins differ only in the presence of an acetyl or an isovalyl group at the N-terminus. Only the residues common to both pepstatins are shown (as red sticks). The highly conserved Asp25 (which form part of the active site AspThrGly triad, unique to all aspartic acid proteases) and Asp29 residues in HIV-1 protease that invariably interact with substrates and clinical inhibitors are indicated in red as stick representations. Catalytic Asp32 and Asp215 residues of pepsin, and Ser219 and Ser36 residues that are in equivalent positions to that of Asp29 and Asp29′ of HIV-1 are also indicated in red. The structure of the N-terminal half of pepsin (residues 1-172, middle) mimics that of the single subunit of the HIV-1 protease dimer in its overall structure, substrate binding site and flaps3, 6.