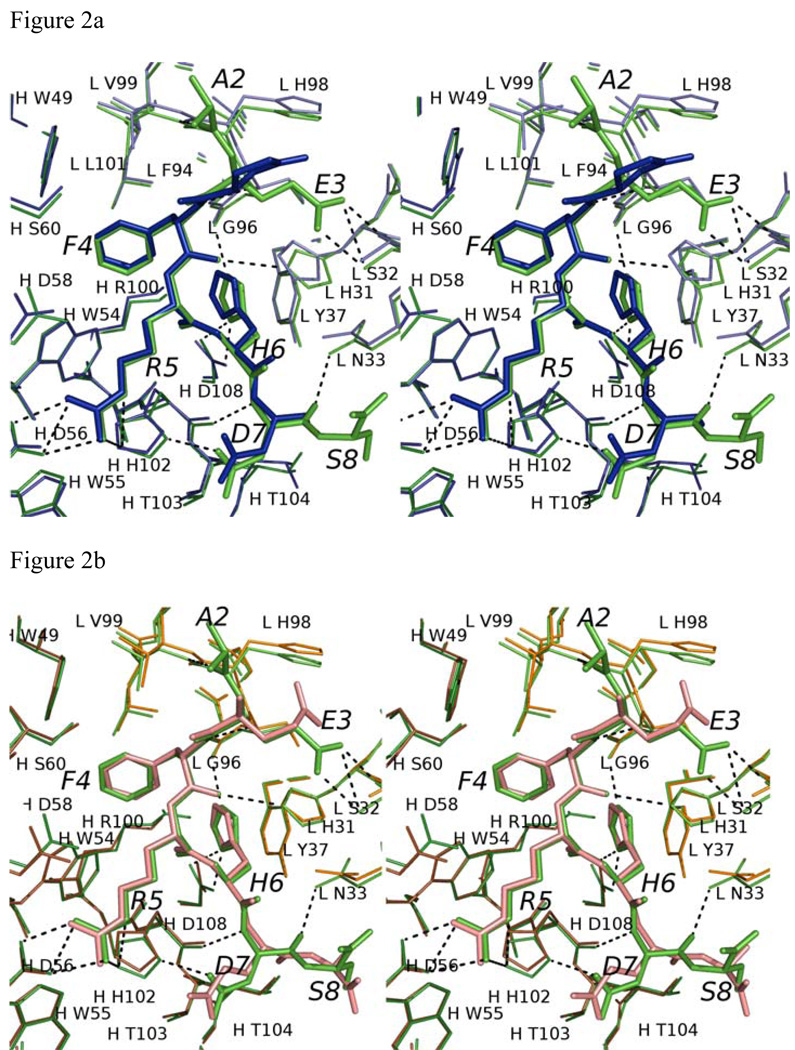
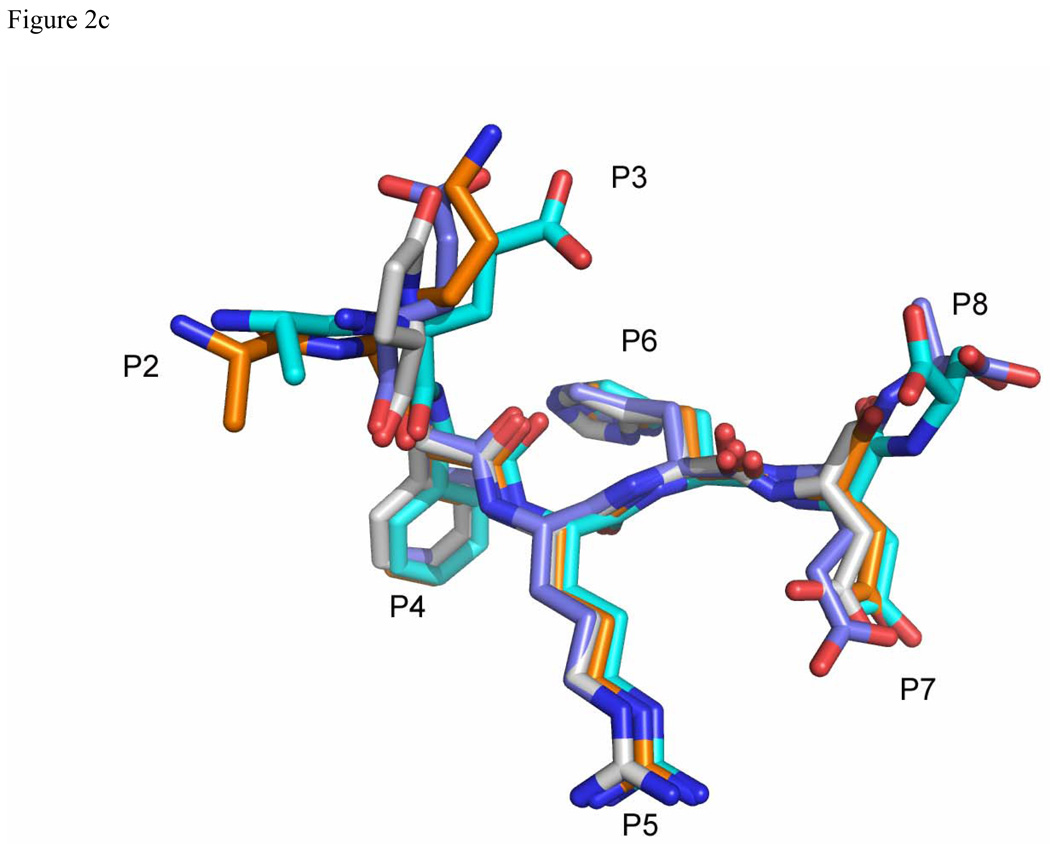
Figure 2.
Comparison of Aβ and related peptide structures. Stereo view of the overlay of Aβ(1–8) WT peptide (green) with (a) pyro-peptide (blue), (b) Ror2(518–525) (pink). The PFA1 residues are drawn with thinner bonds and similarly color-coded, save that the light chain is shown in a paler shade and the heavy chain is shown in a darker shade. The numbering scheme is that of Aβ(1–8) WT. The WWDDD motif appears in the lower left corner of (a) and (b); D57 points away from the bound peptide and does not bind to it. (c) Superposition of Aβ-related peptide structures demonstrating coupled position changes (“cross-talk”) of residues 3 and 8: Aβ(1–8) (cyan), Grip1(110–115) (orange), pyro-Glu3 Aβ (white) and Ror2(518–525) (purple) bound to PFA1. The side-chains are marked P2–P8, where P stands for the peptide position.