Abstract
Oxidative modification of low-density lipoprotein (LDL) plays a causative role in the development of atherosclerosis. In this study, we demonstrate that minimally oxidized LDL (mmLDL) stimulates intracellular reactive oxygen species (ROS) generation in macrophages through NADPH oxidase 2 (gp91phox/Nox2), which in turn induces production of RANTES and migration of smooth muscle cells. Peritoneal macrophages from gp91phox/Nox2−/− mice or J774 macrophages in which Nox2 was knocked down by siRNA failed to generate ROS in response to mmLDL. Because mmLDL-induced cytoskeletal changes were dependent on TLR4, we analyzed ROS generation in peritoneal macrophages from wild type, TLR4−/−, or MyD88−/− mice and found that mmLDL-mediated ROS was generated in a TLR4-dependent, but MyD88-independent manner. Furthermore, we found that ROS generation required the recruitment and activation of spleen tyrosine kinase (Syk) and that mmLDL also induced PLCγ1 phosphorylation and PKC membrane translocation. Importantly, the PLCγ1 phosphorylation was reduced in J774 cells expressing Syk-specific shRNA. Nox2 modulated mmLDL activation of macrophages by regulating the expression of proinflammatory cytokines IL-1β, IL-6 and RANTES. We showed that purified RANTES was able to stimulate migration of mouse aortic smooth muscle cells (MASMC) and addition of neutralizing antibody against RANTES abolished the migration of MASMC stimulated by mmLDL-stimulated macrophages. These results suggest that mmLDL induces generation of ROS through sequential activation of TLR4, Syk, PLCγ1, PKC, and gp91phox/Nox2 and thereby stimulates expression of proinflammatory cytokines. These data help explain mechanisms by which endogenous ligands, such as mmLDL, can induce TLR4-dependent, proatherogenic activation of macrophages.
Keywords: minimally oxidized of LDL, reactive oxygen species, NADPH oxidase 2, RANTES, atherosclerosis
Introduction
Reactive oxygen species (ROS), including hydrogen peroxide and superoxide anion, are generally considered cytotoxic 1. However in recent years, many reports have demonstrated that intracellular ROS, produced in mammalian cells in response to the activation of various receptors, also serve as important second messengers in cell signaling1. The major source of ROS in phagocytes is an NADPH oxidase complex2. It is composed of six protein components, two transmembrane flavocytochrome b proteins (gp91phox and p22phox) and four cytosolic proteins (p47phox, p67phox, p40phox, and Rac). The catalytic protein in this complex is gp91phox, recently renamed as Nox2. The NH3-terminal region of gp91phox/Nox2 consists of transmembrane domains that bind two heme groups, and the COOH-terminal region contains NADPH/FAD binding sites. The cytosolic component of the complex, p47phox, plays a critical role in an assembly of the whole NADPH oxidase complex upon activation of resting phagocytic cells. p47phox is extensively phosphorylated by PKC and then helps recruit p67phox, p40phox and Rac. The translocation of the cytosolic components to the transmembrane component gp91phox results in the formation of a stable molecular structure of NADPH oxidase, which extracts an electron from NADPH that passes through FAD and heme groups and finally reduces an oxygen molecule to make a superoxide anion.
We and others demonstrated that Nox isozymes can be activated by toll-like receptors (TLRs), which are key regulators of innate immunity 3, 4. TLRs recognize pathogen-associated molecular patterns (PAMPs) on the surface of pathogens as well as altered host proteins and lipoproteins and stimulate inflammatory signaling pathways 5, 6. We reported that the stimulation of TLR4 with LPS induces ROS generation and NF-κB activation and that this process is mediated by the interaction of TLR4 with Nox4 4. Moreover, we have demonstrated that Nox4-dependent ROS generation plays an important role in LPS-induced proinflammatory cytokine production by endothelial cells (EC) and activation, leading to adhesion molecule expression 7. These results suggest that EC-generated ROS may play an important role in the process of atherogenesis because infiltration of blood vessel intima by leukocytes, aided by the expression of chemokines and adhesion molecules, is an initial and rate-limiting step in the development of atherosclerotic lesions 8. Indeed, increased levels of superoxide have been found in atherosclerotic lesions in human coronary arteries of explanted hearts, which were accompanied by increased expression of gp91phox and p22phox in phagocytic cells and of Nox4 in non-phagocytic cells in the lesions9.
Oxidation of LDL is a major pathogenic factor in the development of atherosclerosis 10. Oxidized LDL (OxLDL) accumulates in atherosclerotic lesions and activates macrophages and other vascular cells. The resulting chronic inflammation in the vascular wall makes atherosclerotic plaques vulnerable to rupture, leading to acute cardiovascular events. Because TLR4 and TLR2 are key regulators of inflammation and MyD88 is an adaptor molecule essential for the TLR4- and TLR2-mediated signaling, several laboratories studied atherogenesis in TLR4-, TLR2- and MyD88-deficient mice. In general, these knockout mice have no obvious phenotype, but they are unresponsive to specific microbial TLR ligands. When crossed to apoE−/− or LDLR−/− mice and fed a high fat diet, TLR4-, TLR2- and MyD88-deficient mice developed less atherosclerosis than apoE−/− or LDLR−/− controls 11-13. We have developed and characterized a model of minimally oxidized LDL (mmLDL), which interacts with CD14 and activates cytoskeletal rearrangements in macrophages and induces secretion of certain cytokines via TLR4 14, 15. Because mmLDL activates TLR4 in macrophages and TLR4 mediates Nox4 activation in EC, we asked whether mmLDL induces TLR4-mediated activation of Nox2, the predominant NADPH oxidase in macrophages. In this paper, we demonstrate that mmLDL stimulates ROS generation in macrophages via activation of TLR4, and a subsequent signaling cascade involving Syk, PLCγ, PKC and Nox2. Moreover, we report that the production and functional role of specific proinflammatory cytokines in response to mmLDL requires the presence of Nox2.
Materials and Methods
Detailed information on materials, animals and primary macrophages, cell culture and immunoblot analysis, LDL isolation and modification, generation of a retrovirus containing siRNA against Nox2, Syk shRNA transient transfection, measurements of ROS, yeast two-hybrid, subcellular fractionation, immunoblotting, and immunostaining are described in the expanded Material and Methods section in the online data supplement, available at http://circres.ahajournals.org.
Results
MmLDL stimulates ROS generation in J774 macrophages
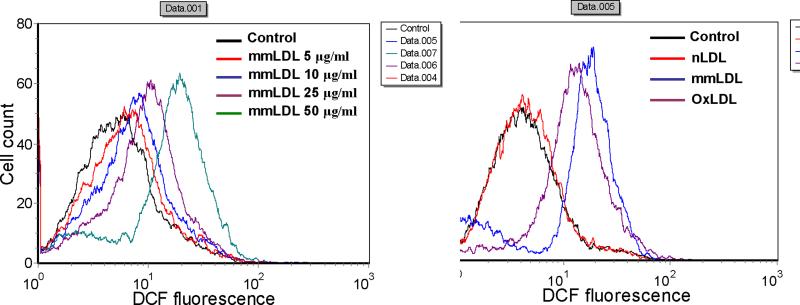
Our earlier results indicate that LPS activation of TLR4 stimulates ROS generation in EC 4 and that mmLDL activates macrophages via TLR4 14. Thus, we reasoned that mmLDL may stimulate TLR4-dependent ROS generation in macrophages. The intracellular levels of ROS in J774 cells, a murine macrophage-like cell line, were determined by measuring the oxidation of DCF-DA to DCF using FACS analysis. The exposure of J774 cells to the indicated concentrations of mmLDL resulted in increased ROS generation in a dose-dependent manner (Fig. 1A). Equal concentrations of extensively oxidized LDL (OxLDL) induced ROS generation as well, though less than mmLDL, whereas native LDL was inactive (Fig. 1B).
Figure 1.
MmLDL stimulates ROS generation in J774 macrophages. (A) J774 cells were incubated for 10 min with indicated concentrations of mmLDL, followed by a 10 min incubation with DCF-DA. The generation of ROS (H2O2) was then monitored by FACS analysis as an increase in DCF fluorescence. Fluorescence was analyzed in 10,000 cells with excitation at 488nm and emission at 530nm. (B) J774 cells were incubated with equal amounts (50μg/ml) of native LDL (nLDL), minimally oxidized LDL (mmLDL), or extensively oxidized LDL (OxLDL) for 10 min and ROS generation was measured as in A.
To identify the specific species of ROS generated, we used an adenovirus expressing mutant catalase with the COOH-terminal KANL peroxisomal targeting sequence deleted (pAd5-CMV-CatΔP, deleted K524ANL527). This mutation results in the CatΔP localization in the cytosol instead of its targeting to the peroxisomes compartment, and the enzyme retains its catalytic activity 16,17. Expression of mutant catalase (CatΔP) in J774 cells resulted in inhibited DCFH oxidation indicating that the generated ROS species are effectively reduced. Therefore, it appears that hydrogen peroxide is a dominant component of the ROS generated (Online Figure I).
Gp91phox/Nox2 is responsible for mmLDL-induced ROS generation
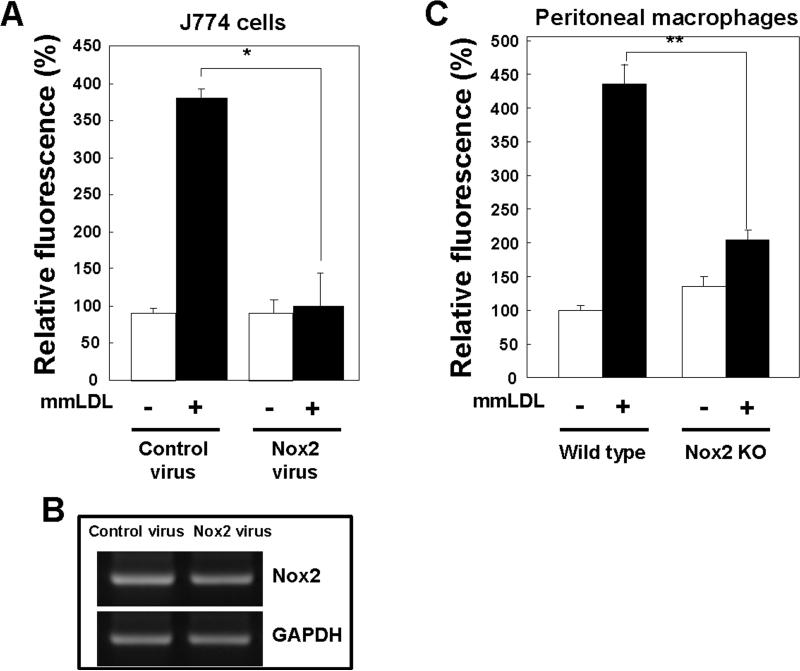
Several lines of evidence indicate that ROS generation is mediated by an NADPH oxidase complex in phagocytic cells, in which gp91phox/Nox2 is the major catalytic component 18. First, we explored whether gp91phox/Nox2 was involved in the mmLDL-dependent ROS generation in J774 cells. We generated a retrovirus encoding a siRNA specific to gp91phox/Nox2. J774 cells infected with the retrovirus-gp91phox/Nox2 siRNA exhibited a marked reduction in the expression of endogenous gp91phox/Nox2 (Fig. 2B) and failed to generate ROS in response to mmLDL, whereas cells infected with the control virus generated a robust ROS response (Fig. 2A). Next, we explored whether peritoneal resident macrophages from gp91phox/Nox2 knockout (gp91phox/Nox2−/−) mice would have a blunted ROS response to mmLDL. Indeed, gp91phox/Nox2 deficient macrophages, identified as CD11b positive cells in the peritoneal cell lavage, exhibited significantly reduced ROS levels in response to mmLDL compared to wild type macrophages (Fig. 2C). These results indicate that gp91phox/Nox2 is responsible for mmLDL-induced ROS generation in macrophages.
Figure 2.
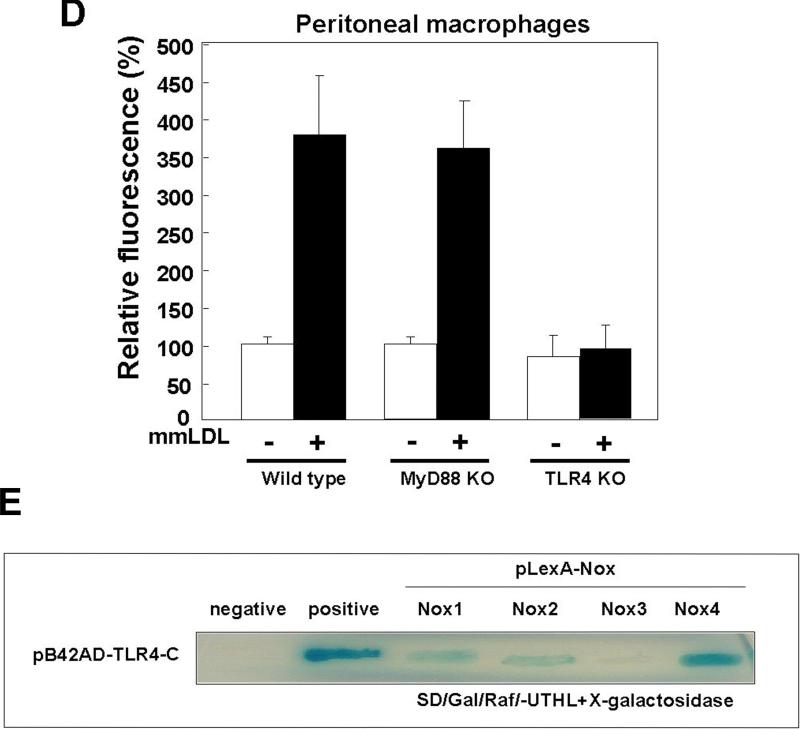
Effect of Nox2 depletion on mmLDL–induced ROS generation. (A) J774 cells were infected with either a retrovirus encoding Nox2-specific siRNA or a retrovirus encoding scrambled siRNA. After 48 hours, cells were stimulated with mmLDL for 10 min, and the generation of H2O2 was monitored by FACS as in Fig. 1. Data are means ± S.E. of mean fluorescence intensity from three independent experiments. *Comparison to the value of control group, p<0.005. (B) Nox2 mRNA expression was assessed in total RNA by RT-PCR as described in Methods. GAPDH served as the loading control. (C and D) Resident peritoneal macrophages were obtained from C57BL/6 wild type, Nox2−/−, MyD88−/− and TLR4−/− mice, stained with a PE-labeled CD11b antibody to select for macrophages and then incubated with mmLDL (50μg/ml) for 10 min, followed by a 10 min incubation with DCF-DA. DCF fluorescence was then measured by FACS in the population of CD11b-positive cells. Data are means ± S.E. of mean fluorescence intensity from three independent experiments. **Comparison to the value of control group, p<0.0001 and ***p<0.01 wild type vs. TLR4−/− macrophages (E) Interactions of the COOH-terminal region of TLR4 with the COOH-terminal domains of Nox1, Nox2, Nox3, or Nox4 were estimated using a yeast two-hybrid assay (see Methods). The intensity of blue color indicates the expression levels of LacZ and corresponds to the affinity of TLR4 binding with Nox isozymes.
MmLDL-induced ROS generation is mediated by TLR4 and Syk
Peritoneal macrophages from TLR4 knockout mice, stimulated with mmLDL, failed to generate ROS, whereas macrophages from MyD88 knockout mice showed a normal ROS response (Fig. 2D), indicating that mmLDL-induced ROS generation depends on the presence of TLR4 but not MyD88.
We previously showed that LPS-induced ROS generation and NF-κB activation in HEK293T cells was mediated by a direct interaction of TLR4 with Nox4 (7). In this study, we found that, unlike Nox4, the COOH-terminal domain of Nox2 displayed only a weak interaction with TLR4 (Fig. 2E), which is unlikely to account for the Nox2 activation. These results suggest that rather than a direct interaction, TLR4 activates Nox2 via a complex signaling pathway.
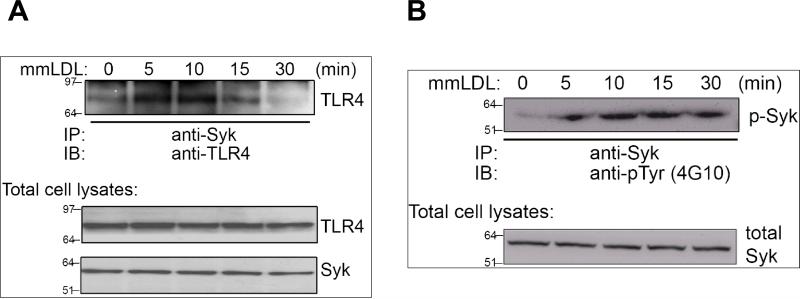
Because MyD88 was not involved in the mmLDL-induced ROS generation, we searched for a kinase that would associate with TLR4 in response to mmLDL and activate a signaling cascade leading to the Nox2-depenent ROS generation. We found that in J774 macrophages stimulated with mmLDL, spleen tyrosine kinase (Syk) co-immunoprecipitated with TLR4 (Fig. 3A and Online Figure IIA). In addition, Syk was phosphorylated in response to mmLDL (Fig. 3B and Online Figure IIB). To confirm the functional connection between Syk and TLR4, TLR4 shRNA was transfected into J774 cells and the phosphorylation of Syk was examined in a FACS assay. The TLR4 knockdown (∼60%) abolished mmLDL-induced Syk phosphorylation (Online Figure III).
Figure 3.
Syk association with TLR4 and phosphorylation of Syk. J774 cells were incubated with mmLDL for the indicated times and the incubations were terminated by the addition of a lysis buffer. Cell lysates were subjected to immunoprecipitation with an antibody against Syk and then the immune complexes were directly subjected to immunoblot analysis with an antibody against TLR4 (A) or phosphotyrosine (4G10) (B). Total cell lysates were used for the control of Syk and TLR4 load. IP and IB designate immunoprecipitation and immunoblot, respectively.
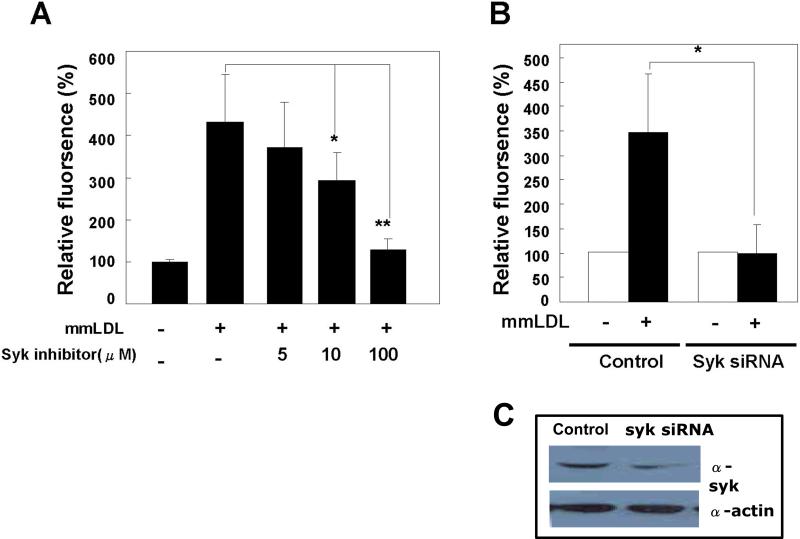
Next, we examined whether Syk regulates gp91phox/Nox2 and ROS generation. Pretreatment of J774 cells with piceatannol, a pharmacological inhibitor of Syk, reduced ROS generation in response to mmLDL in a dose-dependent manner (Fig. 4A). To provide specific evidence for a role of Syk, J774 cells were transfected with a Syk-specific shRNA or a control vector for 48 hours. Syk protein expression was significantly reduced, and the Syk-deficient cells failed to generate ROS in response to mmLDL, whereas cells transfected with control shRNA exhibited normal ROS levels (Fig. 4B). These results indicate that Syk is a key upstream regulatory molecule in mmLDL-induced ROS generation.
Figure 4.
Role of Syk in mmLDL-induced ROS generation. (A) J774 cells were pretreated for 30 minutes with indicated concentrations of the Syk inhibitor, piceatannol, and then incubated with 50 μg/ml mmLDL for additional 10 min. ROS was measured as in Fig. 1. Data are means ± S.E. of mean fluorescence intensities from three independent experiments. *, p<0.02 and **, p<0.001 vs. control. (B) J774 cells were transfected with either Syk-specific shRNA or control shRNA. After 48 hours, the cells were stimulated with mmLDL (50 μg/ml) for 10 min and ROS were measured. Data are means ± S.E. of mean fluorescence intensities from five independent experiments. *Comparison to the value of control group, P<0.01. (C) The Syk knockdown was confirmed in cell lysates subjected to western blot analysis with antibodies against Syk and β-actin (loading control).
Activation of Nox2 requires its interaction with GTP-bound Rac. Indeed, mmLDL induced Rac activation in control J774 cells, but not in Syk knockdown cells (Online Figure IV). These results support the hypothesis that Syk regulates mmLDL-induced Nox2 activation in macrophages.
Activation of PLCγ1 and PKC by mmLDL
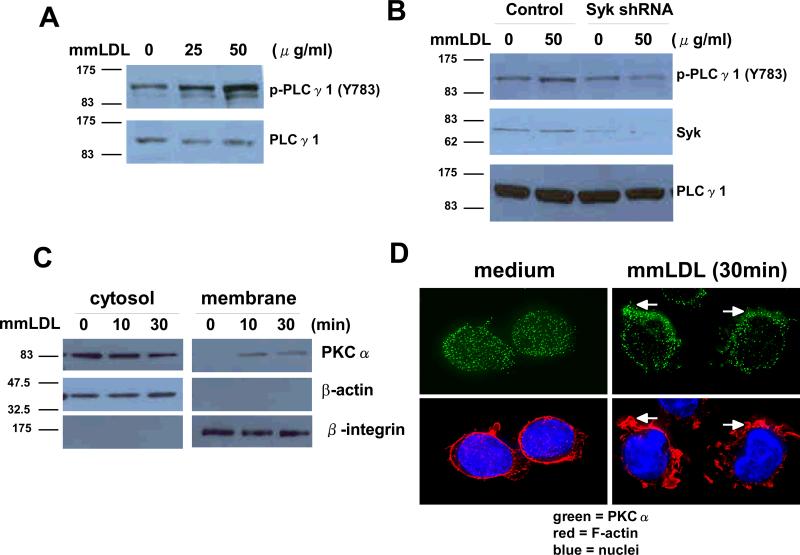
A protein tyrosine kinase activity is known to phosphorylate PLCγ1 at Y783, which, in turn, attracts PKC to the membrane, where it is activated. The sequential activation of PLCγ1 and PKC leads to Nox2 activation and ROS generation 19. Thus, we tested whether mmLDL-induced Syk activation stimulates PLCγ1 phosphorylation. Treatment of J774 cells with mmLDL resulted in a concentration-dependent increase in tyrosine phosphorylation of PLCγ1 (Fig. 5A and Online Figure VA). Transfection of J774 cells with Syk-specific shRNA abolished mmLDL-induced tyrosine phosphorylation of PLCγ1 compared to that seen with control shRNA (Fig. 5B and Online Figure VB).
Figure 5.
MmLDL-induced activation of PLCγ1 and membrane translocation of PKCα. (A) J774 cells were stimulated with mmLDL (25 or 50 μg/ml) for 10 min. Cell lysates from each sample were then prepared and subjected to western blot analysis with antibodies to PLCγ1 or phospho-specific PLCγ1 (Y783). (B) J774 cells were transfected with either Syk-specific shRNA or control shRNA. After 48 hours, the cells were stimulated with mmLDL (50 μg/ml) for 10 min. Cell lysates from each sample were then subjected to western blot analysis with antibodies against Syk, PLCγ1 or phospho-specific PLCγ1 (Y783). (C) J774 cells were stimulated with mmLDL (50 μg/ml) for indicated times and subjected to cellular fractionation (see Methods). Density fractions were immunoblotted with antibodies against PKCα, β-actin (cytosolic marker) and β2-integrin (membrane marker). (D) J774 cells were incubated with medium alone or medium plus mmLDL (50 μg/ml) for 30min. PKCα localization was visualized with a primary anti-PKCα antibody and a secondary FITC-labeled antibody. Cells were also stained with TRITC-phalloidin and Hoechst 33258 to visualize F-actin and nuclei, respectively.
A PKC-dependent phosphorylation of p47phox leads to its association with and activation of gp91phox/Nox2. We analyzed mmLDL-dependent activation of PKCα in terms of its membrane translocation using a subcellular fractionation assay and an immunocytochemical analysis. J774 cells were stimulated with mmLDL and then the cytosol and plasma membranes were separated (see Methods). MmLDL stimulated translocation of PKCα from cytosol to the plasma membrane in a time-dependent manner (Fig. 5C). This result was confirmed by examining PKCα distribution in cells stimulated with mmLDL, which resulted in PKCα translocation from the cytosol to the membrane and its concentration in areas of actin polymerization (Fig 5D). Thus, mmLDL-induced activation of Syk and PLCγ stimulates PKCα activation in macrophages, leading to Nox2-dependent ROS generation.
Gp91phox/Nox2-dependent expression of pro-inflammatory cytokines in response to mmLDL
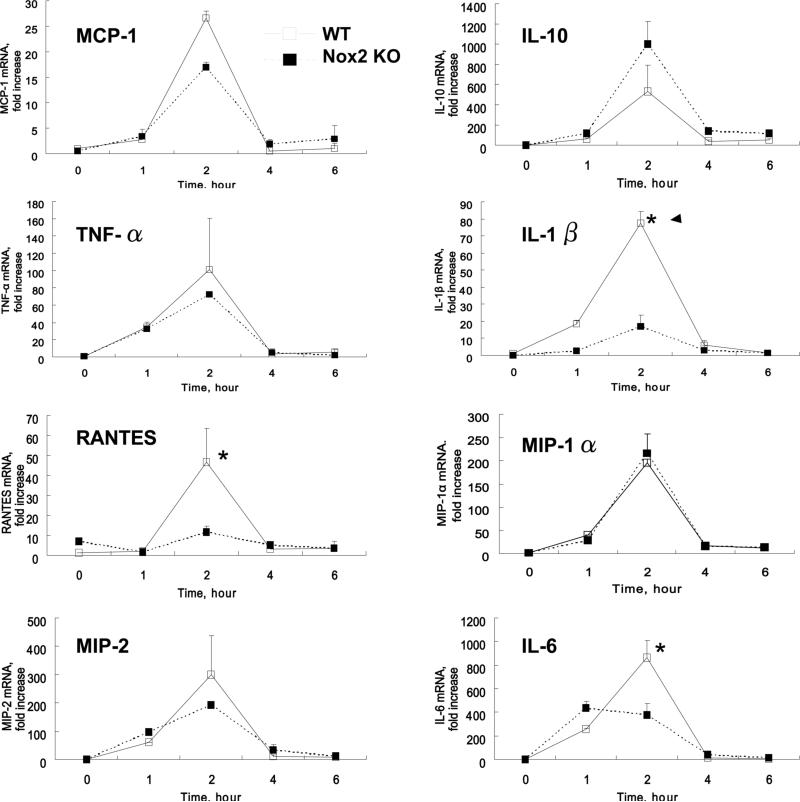
Because proinflammatory cytokines play an important role in atherogenesis, we next analyzed the time-dependent cytokine expression in peritoneal macrophages from wild type or Nox2 knockout mice (Fig. 6). Quantitative real-time PCR demonstrated that mmLDL induced expression of MCP-1, MIP-2, MIP-1α, IL-1β, RANTES, TNF-α, IL-10 and IL-6, which all peaked 2 hours post stimulation. Remarkably, mmLDL-induced expression of RANTES, IL-1β, and IL-6 was significantly reduced in Nox2−/− macrophages compared to wild type, whereas the expression of MCP-1, MIP-1, MIP-2, and TNFα was unchanged or non-significantly reduced. In contrast, the expression of anti-inflammatory IL-10 in Nox2−/− macrophages tended to increase, but the difference was not statistically significant. To corroborate the results with primary Nox2−/− macrophages, we tested the cytokine expression in Nox2 knockdown J774 cells stimulated with mmLDL and found that the expression of IL-6, IL-1β and RANTES was Nox2-dependent, although the expression levels in J774 cells were significantly lower than that in primary macrophages (Online Figure VI). These experiments with Nox2 deficient macrophages suggest that mmLDL stimulates both Nox2-dependent and -independent expression of cytokines, and that the cellular redox state selectively regulates specific macrophage functions.
Figure 6.
MmLDL-induced expression of cytokines in peritoneal resident macrophages. Peritoneal resident macrophages were isolated from wild type and Nox2−/− mice and stimulated with mmLDL (50 μg/ml) or media only for indicated times. Total RNA was isolated, reverse transcribed, and quantified by real-time PCR with respective primers specific for each cytokine and GAPDH. The data are presented as a fold increase in mRNA levels in mmLDL-stimulated cells over the levels in non-stimulated cells. *, p<0.05 wild type vs. Nox2−/− macrophages.
Stimulation of mouse aortic smooth muscle cell migration by RANTES
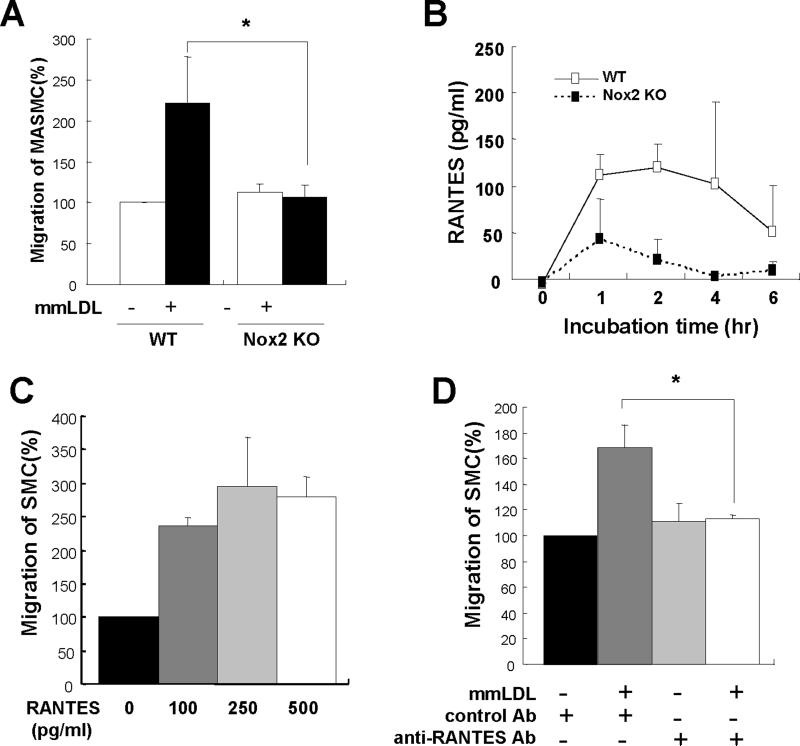
Peritoneal macrophages from wild type or Nox2−/− mice were plated in the bottom chamber of transwells and stimulated with mmLDL. Upper chambers with mouse aortic smooth muscle cells (MASMC) were subsequently assembled with the bottom chambers to induce migration of MASMC. Wild type macrophages stimulated by mmLDL led to a significantly higher level of MASMC migration compared to Nox2−/− (Fig. 7A), suggesting that mmLDL stimulated macrophage secretion of a potent chemokine in an Nox2 dependent manner. Because our gene expression data above demonstrated that RANTES expression was Nox2-dependent (Fig 6 and Online Figure VI), we examined if RANTES was responsible for the MASMC migration. First, we demonstrated that the secretion of RANTES protein was induced by mmLDL in wild type, but not Nox2−/− macrophages (Fig. 7B). Addition of recombinant RANTES to the culture system induced MASMC migration (Fig. 7C). Further, when a neutralizing antibody against RANTES was added to the lower chamber with mmLDL-stimulated macrophages, the migration of MASMC was suppressed to the control level (Fig 7D). These data collectively suggest that the mmLDL-induced, Nox2-mediated RANTES expression in macrophages is a likely mechanism leading to SMC migration.
Figure 7.
RANTES-dependent MASMC migration. (A) Mouse aortic smooth muscle cells (MASMC) (8 × 103 cells in 100 μl media) were added to the upper chamber and peritoneal macrophages from wild type or Nox2−/− mice in the lower chamber. First, macrophages were activated with media alone or 50 μg/ml mmLDL for 2h, and then upper and lower chambers were assembled. After 18h incubation, the membrane in the upper chamber was recovered, fixed, stained and the number of migrated cells was counted. Data are from five independent experiments. *, p<0.05 mmLDL/WT vs. mmLDL/Nox2−/−. (B) Analysis of RANTES protein secretion into cell culture media following macrophage activation with mmLDL (50 μg/ml) for 0, 1, 2, 4 or 6 hours. (C) Mouse aortic smooth muscle cells (MASMC) (8 × 103 cells in 100 μl media) and RANTES (0, 100, 250 or 500 pg/ml) were added to the upper chamber and lower chamber, respectively, and incubated for 18h, and the numbers of migrated cells were counted, (D) Blocking experiments were performed by incubating the mmLDL-stimulated macrophages (wild type) with 100 ng/ml of the neutralizing antibody against RANTES (R&D systems) or with the isotype-matched control antibody in the lower chamber. The number of migrated cells was determined as above. Data are from three independent experiments. *, p<0.05 vs. mmLDL/anti-RANTES antibody.
Discussion
Although the minimal degree of oxidation that occurs in our mmLDL preparation is insufficient to trigger its binding to scavenger receptors 14, we and others have demonstrated that mmLDL has numerous biological effects14, 15, 20, 21. Furthermore, we have shown that a number of these biological effects depends, at least in part, on CD14 binding and TLR4-mediated signaling 14, 15. In addition, we have recently shown that oxidized cholesteryl esters found in mmLDL and in murine atherosclerotic lesions are responsible in part for its impact on macrophage function 22. In this study, we report an important new biological effect of mmLDL, namely the ability to stimulate ROS generation in macrophages via activation of Nox2, and that this was TLR4-dependent, but MyD88-independent. Furthermore, we identify Syk as a necessary component linking the TLR4 activation by mmLDL with the intracellular activation of PLC, PKC, Rac and Nox2 (Figs. 2-6 and Online Figure II-V).
Previously, we reported that TLR4 directly interacts with and activates the Nox4 isozyme in human aortic endothelial cells (HAEC) and HEK293 cells leading to the generation of ROS 4, 7. Nox4 is mainly expressed in fibroblasts, epithelial and endothelial cells, whereas expression of Nox2 is restricted mainly to hematopoietic cells. In this report, we confirm that peritoneal macrophages and J774 cells predominantly express Nox2. Whereas TLR4 directly binds to Nox4 in HAEC, a yeast two–hybrid study detected only a weak binding of Nox2 to TLR4 in macrophages (Fig. 2E), which is, based on our experience with the yeast two-hybrid system, unlikely to have any biological importance. Thus, TLR4 mediated activation of Nox2 in macrophages likely occurs by a pathway distinct from that observed for TLR4 and Nox4 in EC.
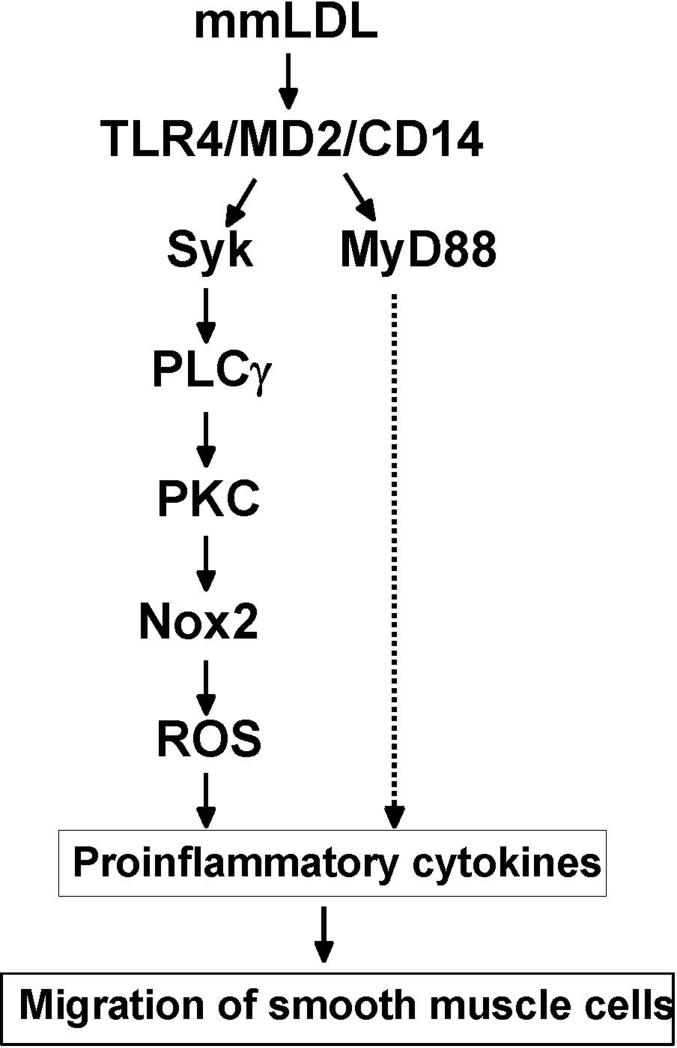
Because mmLDL-induced Nox2 activation in macrophages was MyD88-independent (Fig. 2D) and because the TLR4 and Nox2 direct interaction was insignificant (Fig. 2E), we sought a signaling pathway that would link TLR4 with Nox2. A canonical mechanism of Nox2 activation requires activated PKC. In turn, PKC activation is regulated by a PLCγ-catalyzed hydrolysis of PIP2 into IP3 and DAG, the latter being a specific PKC activator19, 21, 23. Syk is one of the tyrosine kinases that phosphorylates Tyr783 of PLCγ. Syk has been mainly implicated in lymphocyte development, integrin signaling pathways and in regulation of phagocytosis24-27. Recent studies suggested that Syk may be constitutively associated with TLR4 in monocytic cells and that the TLR4-Syk interaction can be stimulated by LPS in neutrophils 25, 26. Remarkably, our data demonstrated that the mmLDL induced activation of TLR4 in macrophages led to a similar recruitment of Syk to TLR4 and Syk phosphorylation (Fig. 3). In turn, Syk was required for mmLDL-induced PLCγ activation and ROS generation (Figs. 4 and 5). In support of this pathway, we demonstrated that mmLDL induced the membrane translocation of PKC (Fig. 5C and D), a necessary step in Nox2 activation. Thus, our results suggest that the ROS generation by mmLDL involves the sequential activation of TLR4, Syk, PLCγ, PKC, and Nox2 (Fig. 8).
Figure 8.
A proposed signaling pathway of mmLDL-induced, TLR4/Syk-dependent ROS generation and proinflammatory cytokine expression.
Several groups have reported that ROS play an important role in NF-κB-dependent inflammatory processes28, 29. For example, we demonstrated that TLR4/Nox4-mediated ROS generation in HAEC was necessary for LPS-induced NF-κB activation and expression of IL-8, MCP-1, and ICAM-1 7. Likewise, in macrophages, mmLDL induced the expression of a number of cytokines, such as MCP-1, MIP-2, TNF-α and IL-6, but only the MIP-2 expression was clearly MyD88-dependent 15. In the current study, we used primary peritoneal macrophages from wild type and Nox2−/− mice to determine the ability of mmLDL to induce cytokine expression. In these studies, mmLDL induced 8 different cytokines (MCP-1, MIP-2, TNF-α, IL-6, IL-1β, RANTES, and IL-10) (Fig. 7). From this set, three of them - IL-1β, RANTES, and IL-6 were significantly downregulated in Nox2−/− macrophages, implying that their regulation was redox-dependent. The results showing that the MIP-2 expression was not significantly affected in Nox2−/− cells (Fig. 6) and that the ROS generation was MyD88-independent (Fig. 2D) agree with our previous data showing that MIP-2 expression was MyD88-dependent 15. These data suggest that the mmLDL engagement of TLR4 stimulates at least two independent pathways, one via MyD88 and another mediated by Syk, the latter leading to Nox2 activation and the redox-sensitive expression of IL-1β, RANTES, and IL-6 (Fig. 8).
Our data demonstrate that mmLDL activates TLR4, which in turn initiates an intracellular signaling cascade leading to Nox2-mediated ROS generation, culminating in the secretion of IL-1β, IL-6 and RANTES, which are proinflammatory and likely proatherogenic. IL-6 has been implicated in atherogenesis. IL-6 expression is significantly reduced in carriers bearing a TLR4 ASP299GLY polymorphism, which is associated with a significantly lower frequency of myocardial infarction compared to the control population 30. Both MyD88-dependent and MyD88-independent pathways may stimulate IL-6 expression31. In this study, we provide evidence that the Nox2-mediated ROS generation is MyD88-independent (Fig. 2) and that this Nox2 activity regulates the IL-6 expression (Fig. 6). RANTES and IL-1β has also been shown to have a functional role in atherogenesis. Kirii et al. have demonstrated that atherosclerotic lesion size of IL-1β−/− apoE−/− mice is significantly smaller than that in IL-1β+/+apoE−/− mice 32. RANTES through its binding to CCR5 stimulates both the recruitment and transendothelial migration of leukocytes to inflammation sites, thereby facilitating the development of inflammatory vascular lesions 33. We demonstrated that mmLDL-induced Nox2 activity regulated the production of RANTES in macrophages (Fig. 6 and Fig. 7B). Remarkably, RANTES antagonists prevent progression of even established atherosclerotic lesions in LDLR−/− mice 34, 35. This underscores the relevance and importance of our findings that mmLDL-induced Nox2 activity stimulated the production of RANTES by macrophages (Fig. 6 and Fig. 7B), which, in turn, induced MASMC migration (Fig. 7A). The specificity of this mmLDL/Nox2 effect was shown in the experiments with Nox2−/− macrophages and with an anti-RANTES antibody, which both blocked the MASMC migration (Fig. 7A and D). Recently, Yamada et al. reported that expression of RANTES was regulated by Syk in nasal fibroblast 36, which would agree with our results placing Syk upstream of the RANTES expression. Taken together, these results suggest that the mmLDL-initiated redox signaling may be involved in the processes of vascular inflammation and atherosclerosis.
Although one would predict that Nox2 would be proinflammatory, and thus proatherogenic, conclusive experimental data to support such a role in vivo is not currently available. The role of p47phox or gp91phox have been evaluated in various murine models by examining whole body knockouts in the background of apoE deficiency 37-39. In one study, the knockout of p47phox decreased lesion formation in the whole aorta, but not at the aortic root 37. In a second study, disruption of p47phox led to no changes in lesion formation at the aortic ring 38. Yet, in a third study, gp91phox knockout led to lowered plasma cholesterol levels, yet no measured decreases in atherosclerosis 39, which in the setting of lowered plasma cholesterol, might even be interpreted as enhanced lesion formation. The reasons for these differences are not clear, but could be related to a differential impact of NADPH oxidase on different stages of lesion development, or to its role in different cell types 40. Tissue specific knockouts will be required to evaluate the latter possibility.
In conclusion, we present a signaling mechanism by which mmLDL stimulates ROS generation in macrophages. The interaction of mmLDL with TLR4 results in the activation of Syk tyrosine kinase leading to phosphorylation of PLCγ1, which in turn induces PKC, activation of Rac, and Nox2 activation, resulting in the expression of proinflammatory cytokines IL-1β, IL-6 and RANTES (Fig. 8). Because mmLDL is likely a component of atherosclerotic lesions, these data define mechanisms by which endogenous ligands, such as mmLDL, can activate TLR4-dependendent, signaling mechanisms in macrophages that lead to proatherogenic effect.
Sources of funding
This work was supported by the NCRC program of MOST/KOSEF (grant # R15−2006−020−00000−0) through the Center for Cell Signaling & Drug Discovery Research at Ewha Womans University, by the Korea Health 21 R&D Project (grant number A06−00043579), Ministry of Health & Welfare, by Seoul R&D program (grant number 10527), by NRL program (grant number ROA-2007−000−20004−00), by NIH grant HL081862 (Y.I.M.), by a grant from the AHA (0530159N) (Y.I.M.) and a grant from the Leducq Fondation (J.L.W.). Y.S.B. is a recipient of International Exchange Program of LG YeonAm Foundation and S.K. is a recipient of BK21 scholarship.
The abbreviations used are
- LDL
low-density lipoprotein
- mmLDL
minimally oxidized LDL
- OxLDL
extensively oxidized LDL
- TLR
toll-like receptor
- NADPH
nicotinamide adenine dinucleotide phosphate
- Nox
NADPH oxidase
- ROS
reactive oxygen species
- Syk
spleen tyrosine kinase
- PLC
phospholipase C
- PKC
protein kinase C
Footnotes
Disclosures
None
References
- 1.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 2.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. TLR signaling. Seminars in Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkbacka H. Multiple roles of Toll-like receptor signaling in atherosclerosis. Curr Opin Lipidol. 2006;17:527–533. doi: 10.1097/01.mol.0000245258.25387.ec. [DOI] [PubMed] [Google Scholar]
- 7.Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovascular Research. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 9.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 10.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nature Medicine. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 12.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 15.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 16.Purdue PE, Lazarow PB. Targeting of human catalase to peroxisomes is dependent upon a novel COOH-terminal peroxisomal targeting sequence. J Cell Biol. 1996;134:849–862. doi: 10.1083/jcb.134.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai J, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem. 1999;274:26217–26224. doi: 10.1074/jbc.274.37.26217. [DOI] [PubMed] [Google Scholar]
- 18.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Choi H, Leto TL, Hunyady L, Catt KJ, Bae YS, Rhee SG. Mechanism of angiotensin II-induced superoxide production in cells reconstituted with angiotensin type 1 receptor and the components of NADPH oxidase. J Biol Chem. 2008;283:255–267. doi: 10.1074/jbc.M708000200. [DOI] [PubMed] [Google Scholar]
- 20.Patricia MK, Kim JA, Harper CM, Shih PT, Berliner JA, Natarajan R, Nadler JL, Hedrick CC. Lipoxygenase products increase monocyte adhesion to human aortic endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19:2615–2622. doi: 10.1161/01.atv.19.11.2615. [DOI] [PubMed] [Google Scholar]
- 21.Boullier A, Li Y, Quehenberger O, Palinski W, Tabas I, Witztum JL, Miller YI. Minimally oxidized LDL offsets the apoptotic effects of extensively oxidized LDL and free cholesterol in macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1169–1176. doi: 10.1161/01.ATV.0000210279.97308.9a. [DOI] [PubMed] [Google Scholar]
- 22.Harkewicz R, Hartvigsen K, Almazan F, Dennis EA, Witztum JL, Miller YI. Cholesteryl ester hydroperoxides are biologically active components of minimally oxidized LDL. J Biol Chem. 2008;283:10241–10251. doi: 10.1074/jbc.M709006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Ann Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 25.Arndt PG, Suzuki N, Avdi NJ, Malcolm KC, Worthen GS. Lipopolysaccharide-induced c-Jun NH2-terminal kinase activation in human neutrophils: role of phosphatidylinositol 3-Kinase and Syk-mediated pathways. J Biol Chem. 2004;279:10883–10891. doi: 10.1074/jbc.M309901200. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhary A, Fresquez TM, Naranjo MJ. Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol Cell Biol. 2007;85:249–256. doi: 10.1038/sj.icb7100030. [DOI] [PubMed] [Google Scholar]
- 27.Palacios EH, Weiss A. Distinct roles for Syk and ZAP-70 during early thymocyte development. J Exp Med. 2007;204:1703–1715. doi: 10.1084/jem.20070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Candore G, Aquino A, Balistreri CR, Bulati M, Di Carlo D, Grimaldi MP, Listi F, Orlando V, Vasto S, Caruso M, Colonna-Romano G, Lio D, Caruso C. Inflammation, longevity, and cardiovascular diseases: role of polymorphisms of TLR4. Ann N Y Acad Sci. 2006;1067:282–287. doi: 10.1196/annals.1354.037. [DOI] [PubMed] [Google Scholar]
- 31.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 2007;178:1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 32.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 33.Duma L, Haussinger D, Rogowski M, Lusso P, Grzesiek S. Recognition of RANTES by extracellular parts of the CCR5 receptor. J Mol Biol. 2007;365:1063–1075. doi: 10.1016/j.jmb.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 34.Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 35.Braunersreuther V, Steffens S, Arnaud C, Pelli G, Burger F, Proudfoot A, Mach F. A novel RANTES antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscler Thromb Vasc Biol. 2008;28:1090–1096. doi: 10.1161/ATVBAHA.108.165423. [DOI] [PubMed] [Google Scholar]
- 36.Yamada T, Fujieda S, Yanagi S, Yamamura H, Inatome R, Sunaga H, Saito H. Protein-tyrosine kinase Syk expressed in human nasal fibroblasts and its effect on RANTES production. J Immunol. 2001;166:538–543. doi: 10.4049/jimmunol.166.1.538. [DOI] [PubMed] [Google Scholar]
- 37.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(-/-) mice. J Clin Investi. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, Hoyt RF, Holland SM, Finkel T. Vascular effects following homozygous disruption of p47(phox) : An essential component of NADPH oxidase. Circulation. 2000;101:1234–1236. doi: 10.1161/01.cir.101.11.1234. [DOI] [PubMed] [Google Scholar]
- 39.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2000;20:1529–1535. doi: 10.1161/01.atv.20.6.1529. [DOI] [PubMed] [Google Scholar]
- 40.Cathcart MK. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]