Abstract
When the two eyes view dissimilar monocular stimuli, the resulting interocular suppression can spread beyond the region of explicit stimulus conflict: portions of one rival target will disappear even though there is no competing stimulation at the corresponding location in the other eye’s view. In a series of experiments we examined whether this spread of suppression is spatially isotropic or governed by the configuration of the stimulus a portion of which is subject to suppression. Observers reported the incidence of stimulus disappearance at different locations along or nearby the contours of a large figure, part of which was suppressed by presentation of a continuous flash-suppression stimulus to a restricted region of the other eye. For all observers, suppression spread over several degrees along the contours of the figure, but tended not to spread to locations nearby but disconnected from the figure. Suppression spread effectively over a smoothly curved contour, and it spread around a sharp corner defined by two abutting contours, albeit less effectively. Suppression tended not to spread to features within the interior of a figure (a face), even if those features formed an integral part of the figure. A gap within a spatially extended stimulus arrested the spread of suppression, unless that gap appeared to arise from occlusion. Spread of suppression was unrelated to sensory eye dominance and was found with a more conventional binocular rivalry configuration, too. These findings implicate the involvement of neural circuitry in which inhibition propagates along paths of excitation beyond spatial regions of explicit interocular conflict.
1 Introduction
When left and right eyes view dissimilar stimuli, those two stimuli compete for dominance rather than merging into a stable binocular impression. Called binocular rivalry, this beguiling phenomenon has a long history of investigation in vision science (Alais and Blake 2005), and in recent years rivalry has been adopted by neuroscientists as a promising means for uncovering neural correlates of visual perception (Leopold and Logothetis 1999; Blake and Logothetis 2002; Ooi and He 2003; van Boxtel et al 2008) and, perhaps, even consciousness (Koch 2004).
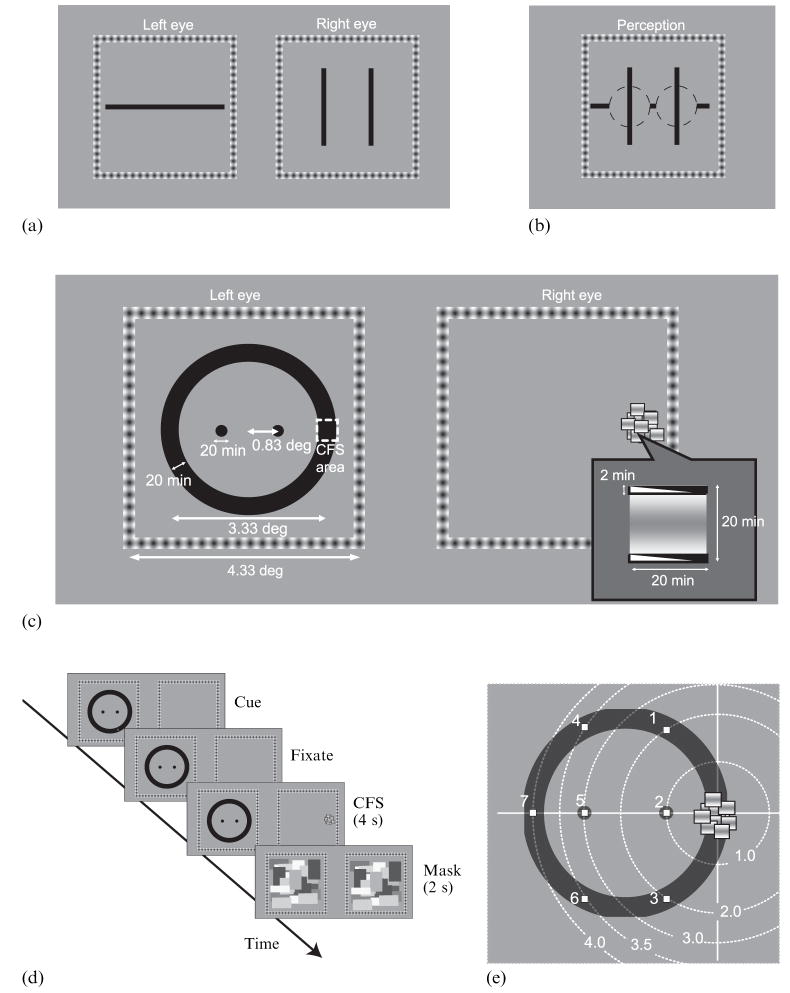
During binocular rivalry, one of two dissimilar stimuli may be suppressed for several seconds at a time. Moreover, that suppression is not strictly confined to the retinal area of rival stimulation but, instead, can spread beyond the boundaries of the suppressed stimulus (Kaufman 1963; Levelt 1965; Blake and Camisa 1979). Kaufman (1963) estimated the spatial extent of suppression by presenting two parallel vertical lines to one eye and a single horizontal line to the other eye (figure 1a). He found that the visibility of the interior region of the horizontal line depended strongly on the lateral separation between the two vertical lines. For separations less than 1 deg, portions of the horizontal line falling between the two vertical lines disappeared entirely, even though that part of the line did not spatially overlap the rival vertical lines in the visual field (figure 1b). Kaufman’s results clearly show that suppression during rivalry expands beyond the regions of explicit conflict between rival stimuli. Subsequent work showed that the spatial extent of the spread of suppression varied depending on the spatial-frequency content of the rival stimuli, with the extent of the spread of suppression inversely related to the peak spatial frequency of the rival patterns (Liu and Schor 1994). From these previous studies it is impossible to know whether the spread of suppression beyond the region of explicit rivalry is isotropic, forming a uniform penumbra in all directions, or, instead, is governed by the configuration of the rival target undergoing suppression. An answer to this question is potentially important for models of binocular rivalry that posit inhibitory connections as the mechanism by which suppression is achieved (eg Lehky 1988; Blake 1989; Laing and Chow 2002; Wilson 2003; Noest et al 2007).
Figure 1.
Conceptual bases of the main experiment. (a) Schematic of stimuli used by Kaufman (1963) to measure the spatial spread of binocular rivalry suppression. (b) Schematic of characteristic perceptual experience by observers in Kaufman’s experiment. (c) Schematic figure of stimuli used in the main experiment. Note that the wedge in the CFS (continuous flash suppression) component above and below the sinusoidal grating represents the dynamic contrast reversal of these bars that flanked the grating. In the actual CFS stimuli, a uniform black bar was rapidly interchanged with a uniform white bar. (d) Diagram of the procedure in the main experiment. (e) Seven possible positions of the ‘indicator’ mark (indicating the position that observers were to monitor on that particular trial; see methods for the main experiment) and the distances of that mark from the centre of CFS stimulus. Distances are here normalized by the distance between the CFS center and the nearby interior disk (0.83 deg).
In this paper we describe experiments that measure the spatial distribution of the spread of suppression. By way of preview, our results show that the spread depends strongly on the spatial configuration of the stimulus undergoing suppression.
1.1 Stimulus configuration shapes the spatial spread of suppression
Figure 1c shows a schematic of the dichoptic display used to study the spread of suppression. One eye viewed a black ring that contained within its interior two small dark disks, and the other eye viewed a small array of horizontally oriented sinusoidal gratings that rapidly and repetitively changed positions. With these dichoptic stimuli we could reliably induce continuous flash suppression (CFS) on a restricted region of the ring: the flickering gratings provided a potent suppressing stimulus that dominated the corresponding portion of the other eye’s view continuously for many seconds (Tsuchiya and Koch 2005). Using these rival stimuli, we measured the spatial spread of interocular suppression around the contours defining the ring and within the interior of the ring. If suppression spreads uniformly in all directions from the region of conflict between the ring and the CFS target, the incidence of suppression should depend on the angular distance from the explicitly suppressed area of the ring. Hence, suppression should spread as readily to a given disk in the interior as to a region around the ring located an equivalent distance from the suppressed area. But if suppression tends to spread along the boundaries of a stimulus subjected to suppression, the incidence of suppression should be greater around the ring than on a disk, even when the angular distance is larger around the ring.
Upon viewing this display, one initially perceives a dark ring whose right-hand portion is suppressed by the CFS stimulus. Then, almost immediately, regions of the ring outside the boundaries of the CFS stimulus are also suppressed from visibility, and the spatial extent of this suppression of the ring can be large. Sometimes suppression extends symmetrically in both directions, occasionally encompassing the entire ring; other times, suppression extends further in one direction than in the other. The time course of the spread of suppression also varies considerably with repeated observation of this dichoptic display: sometimes a wave of suppression is clearly evident, propagating at the rate of several degrees per second, but other times large portions of the ring seem to disappear at the same time, implying very rapid spread of suppression. Eventually, the suppressed regions of the ring begin to reappear, usually starting at a location far removed from the CFS stimulus and expanding back toward that stimulus. At the same time, the disks within the interior of the ring seldom disappear when the CFS stimulus is displayed, and, when they do fade, their invisibility is nearly always accompanied by invisibility of large parts of the ring. It is obvious that suppression spreads around the ring to a much greater extent than it does within the interior of the ring. This fading around the ring, occasioned by presentation of the CFS stimulus, is conspicuously different from the spontaneous fading that can occur with extended monocular viewing of the ring alone. This latter fading, most likely a manifestation of Troxler’s effect (Clarke 1960), takes many seconds before occurring, and when it does happen the fading tends first to arise on portions of the ring far removed from the point of fixation.
To quantify this dependence of interocular spread of the suppression on stimulus configuration we performed a series of experiments.
2 Measuring the spread of suppression
2.1 Method
2.1.1 Apparatus
Dichoptic stimuli were displayed on a calibrated CRT monitor (1280×1024 pixels resolution; 60 Hz frame rate) controlled by a Macintosh G4 computer; left-eye and right-eye stimuli were viewed through a custom-built mirror stereoscope with the head stabilized by a chin-and-head rest.
2.1.2 Observers
Five observers participated in the main experiment (four naive, and the first author of the paper). All had normal or corrected-to-normal acuity and good stereopsis, and all observers had extensive prior experience in observing and tracking binocular rivalry. All observers gave signed consent after being informed of the nature of the experiment.
2.1.3 Display
Left-eye and right-eye half-images were presented against a gray uniform background (24.1 cd m−2) on the left and right halves of the video monitor (figure 1c). Each half-image was surrounded by a square frame (4.33 deg×4.33 deg) composed of regularly spaced dark spots on a white background; these frames, one viewed by each eye, served to promote and maintain stable binocular alignment.
One eye’s image consisted of a black ring and two disks displayed inside the ring. The ring’s outer diameter was 3.33 deg, and its width was 20 min of arc. The diameter of each disk was 20 min of arc, and the two disks were located 0.83 deg to the left and to the right of the center of the ring. The other eye viewed an array of small rectangles each containing 1 cycle of a horizontal, sinusoidal bar and each subtending 20 min of arc×20 min of arc. Framing the top and bottom of each rectangle were thin bars that reversed contrast at 30 Hz. The small rectangles were partially overlapping, and the entire array of rectangles covered a virtual area 40 min of arc×40 min of arc. Within this virtual area, the positions of individual rectangles changed locations at 16.7 Hz. This dynamic array of high-contrast elements appeared centered on an area corresponding to the right-hand portion of the ring seen by the other eye.
2.1.4 Procedure
Figure 1d schematically shows the sequence of events comprising a test trial. Each trial began with the appearance of the two monocular fusion frames, and centered within the frame viewed by the left eye were the black ring and the two black disks. Also presented to that eye was a small red ‘indicator’ dot that appeared in any one of seven possible positions including either of the disk locations or any one of five, evenly spaced positions around the ring (see figure 1e). During the initial phase of the trial, the right eye viewed only the fusion frame. The observer noted the position of the indicator dot (whose position varied randomly over trials) and then pressed a key to extinguish the indicator dot.
Next, the observer fixated the right-hand side of the ring and pressed a key to trigger presentation of the CFS stimulus to the other eye. This suppressor stimulus remained present for 4 s after which the ring and dynamic suppressor were replaced by dynamic, checkerboard masks presented to both eyes for 2 s (to erase any after-images of the ring, disks, or suppressor). During the 4 s period of rival stimulation, the observer monitored that spatial location previously designated by the indicator dot and specifically noted whether the spread of suppression reached that location (ie either one of the disks or a portion of the ring perceptually disappeared).
Following each 4 s presentation, the observer indicated whether the stimulus at the monitored location disappeared at any time during the 4 s period of rival stimulation. Because all observers had experience observing rivalry, they understood that nature of the judgment and the importance of maintaining a fixed criterion for stimulus disappearance. The experiment was conducted in two sessions, and in a given session each of the seven indicator positions was tested 20 times, the order of trials being random.
2.2 Results
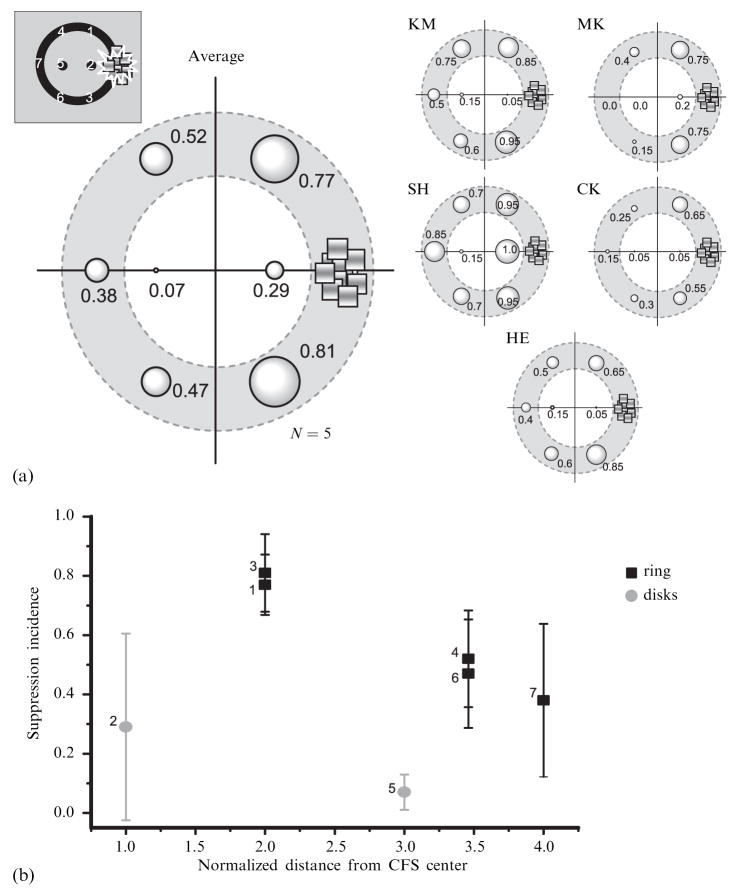
To quantify the spread of suppression at different locations within the display, we computed the incidence of suppression at each of the seven monitored positions around and within the ring. Data for individual observers and average results across observers are shown in figures 2a and 2b. Here it can be seen that for all observers the incidence of suppression was generally higher on the ring than on the disks within the ring. For example, the nearest monitored locations on the ring (positions 1 and 3 in figure 1e) are twice as far from the CFS target as the nearby central disk (position 2). Nevertheless, incidence of suppression at the ring locations was substantially higher (~2×) than at the disk location. Likewise, the incidence of suppression at the most remote portion of the ring (positions 4, 6, and 7) averaged 40% – 50%, whereas suppression was rarely observed at the second disk position, which was much nearer to the CFS target (position 5). We performed a paired t-test to evaluate the difference in suppression incidence for the two disk locations compared to the equivalent locations on the ring (these latter values were estimated from regression lines fitted to each observer’s data). That analysis confirmed that these incidence values were significantly different (t9 = 6.32, p < 0.001).
Figure 2.
Results of the main experiment. (a) The incidence of suppression at each position is expressed by the size of the bubble at that location. (b) The mean suppression incidence as a function of the distance from the center of the CFS (continuous flash suppression) area. The distance is normalized by the distance between the CFS center and the nearby interior disk (0.83 deg). Data for black rectangles show incidence of suppression at the positions on the outer ring. Data for gray circles show incidence of suppression on the interior disks. Prefix numbers near symbols indicate the positions indicated in figure 1e. Error bars show 95%-confidence intervals.
2.2.1 Control experiments
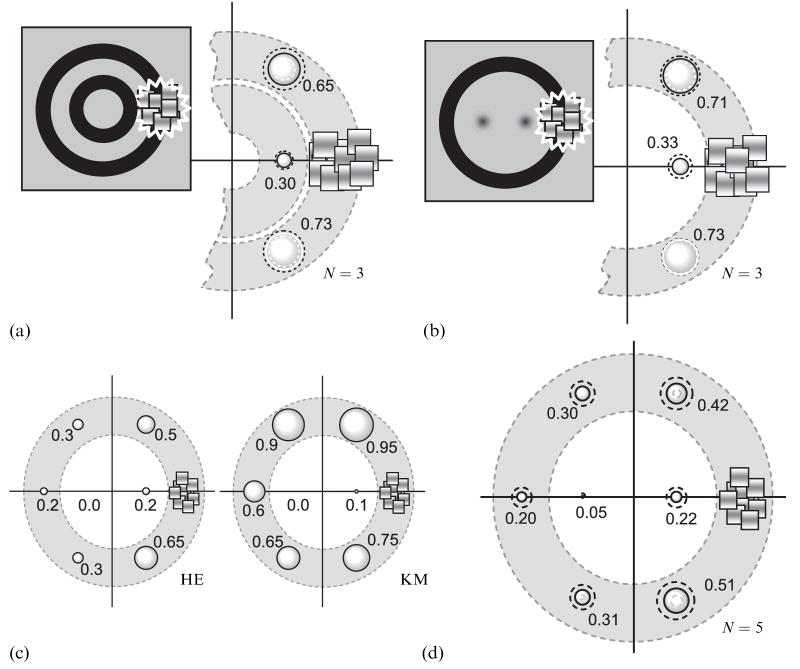
Could these differences in suppression incidence arise because the disks are intrinsically more difficult to suppress compared to a region of the ring? A disk is defined by a sharp, continuous edge around its entire perimeter, whereas the ring is defined by two parallel contours. Hence, the spatial-frequency content of these two stimuli is different, and this difference could make the ring more susceptible to suppression than the disk. To test this possibility, we repeated our measurements with the central disks replaced by one of two stimuli. On some trials the interior of the ring contained a pair of 2-D Gaussian blobs (blurred disks without sharp edges, full-width at half-height of which equaled 20 min of arc), and on other trials the interior contained a smaller diameter ring of the same width as the outer ring (see insets in figures 3a and 3b). We measured the incidence of suppression for three locations (left-eye stimulus) relative to the CFS stimulus (right-eye stimulus): at two locations on the large ring (equally spaced in either direction from the CFS stimulus) and at a single, interior location very near the CFS stimulus (ie the nearest Gaussian blob or the right-hand edge of the small diameter ring). Note that, again, the measurement locations on the outer ring are twice as far from the CFS as is the interior location. We retested three of the participants from the previous experiment, and the procedure used to measure the incidence of suppression was identical to that used previously. Figures 3a and 3b summarize the incidence of suppression at the three locations tested. Again, observers experienced a higher incidence of suppression on the large ring relative to a location nearer to the CFS within the interior of the ring. This implies that differences in the incidence of suppression of the disk compared to a region of the ring (figure 2) do not arise because the disks are intrinsically more difficult to suppress.
Figure 3.
Results of control experiments. (a) and (b) The results of measurements with the central disks replaced by one of two stimuli: a small diameter ring (a) or blurred disks (b). Data are shown as in figure 2a. The dotted gray and black circles show the 95%-confidence intervals. (c) The results of measurements with large fuse frames. Data are shown as in figure 2a. The dotted gray and black circles show the 95%-confidence intervals. (d) Results from experiment in which suppression was induced by a static Mondrian pattern. Data are shown as in figure 2a. The dotted gray and black circles show the 95%-confidence intervals.
Could the close proximity of the fusion frame to the ring, but not to the disks, increase the incidence of suppression of regions around the ring, without influencing the incidence of suppression of a disk? To answer this question, we repeated the measurements illustrated in figures 1d and 1e using fusion frames that were expanded to subtend 5.33 deg on a side, without changing the sizes of the ring, disks, and CFS stimulus. Doubling the distance between the ring and fusion frame had no effect on the pattern of results of the two observers tested in this ancillary experiment (figure 3c): once again, suppression spread more readily around the ring than to the interior disk, confirming that the fusion frame is not responsible for the high incidence of suppression around the ring.
The spatial extent of the suppression reported here—up to 3 deg from the nearest border of the CFS stimulus—is quite large compared to that in previous studies (Kaufman 1963; Liu and Schor 1994). Part of this increased spread is attributable to the configural effect we have documented. However, we used CFS to induce interocular suppression, and it has been shown that CFS produces more potent suppression within the boundaries of rival targets than does regular flash suppression or conventional rivalry (Tsuchiya et al 2006). We thus felt it worthwhile to assess spread of suppression using a monocular stimulus more like those used in previous studies.
To accomplish this, we replaced the dynamic CFS stimulus with a single frame from the CFS stimulus that remained unchanged during the 4 s test period; in all other respects, the stimuli and procedures were the same as those used previously (figures 1d and 1e). Figure 3d shows the incidence of suppression of the two disks within the interior of the ring and the incidence of suppression at various locations around the ring. This display produced the same pattern of results as those obtained with the dynamic CFS stimulus, except that the observed incidence of suppression was generally lower. We conclude that the spread of interocular suppression induced by conventional binocular rivalry is also shaped by the overall configuration of the stimulus, a part of which is engaged in rivalry.
2.3 Conclusion
The results reveal reliable differences in the incidence of interocular suppression on locations around the ring compared to the incidence of suppression of small disks within the interior of the ring. These differences cannot be attributed to artifacts in the stimulus displays. The results imply that interocular suppression does not spread uniformly over space but, instead, depends on the configuration of the stimulus within which suppression originates locally.
3 Spread of suppression within other stimulus configurations
3.1 Spread of suppression within a grouped object
In the experiments described so far, the components of the rival stimuli were relatively simple geometric figures defined by closed boundaries; the disks within the interior bore no obvious relation to the large ring surrounding them. Would suppression more readily spread to the disks within the interior of the ring if those disks and the ring were perceived as components of a single, grouped object? In the following experiments we tested this possibility.
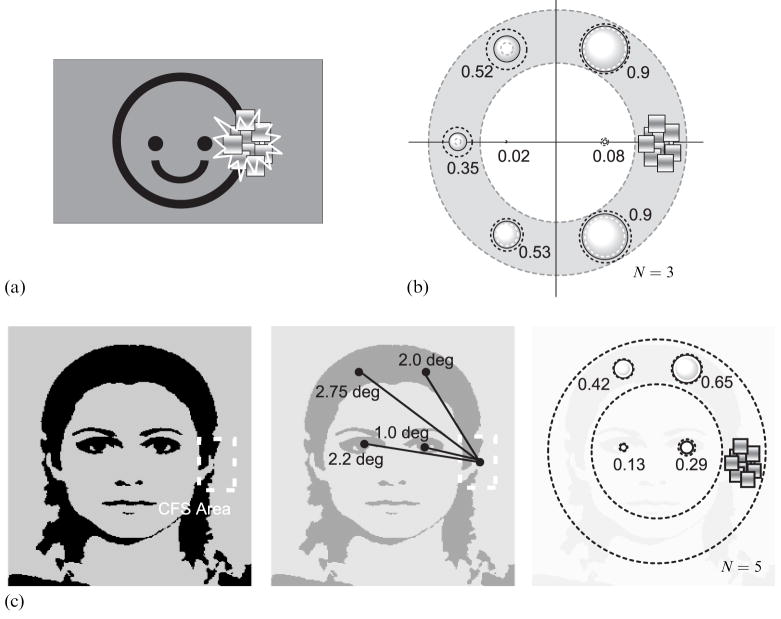
To begin, we modified the disk/ring stimulus by adding a single, upturned arc that promoted grouping of all these stimulus elements into one configuration, namely a ‘smiley face’ (figure 4a). Does interocular suppression spread throughout this grouped configuration when a restricted region of the object (the right-hand edge of the ‘face’) is suppressed by CFS? Three observers including the first author participated in this experiment. The procedure and stimuli were the same as for the first experiment, except for the addition of the ‘mouth’ component to the target stimulus.
Figure 4.
Spread of suppression with an iconic face and with a digitized face image. (a) and (b) Stimuli and results for the ‘iconic face’ experiment. Data are shown as in figure 2a. The dotted gray and black circles show the 95%-confidence intervals. (c) Stimuli and results for the ‘digitized face’ experiment. Left panel shows the digitized face image used as the target stimulus. Center panel schematizes distances between the CFS (continuous flash suppression) center and four monitored positions. Right panel shows mean suppression incidences. Data are shown as in figure 2a.
This revised configuration produced the same pattern of results as that obtained in the first experiment (figure 4b; cf figure 3). The incidence of suppression was very low at the location of the ‘eyes’, but much higher at more remote locations around the outline of the figure. Grouping, in other words, did not alter the spatial spread of suppression. Of course, one could argue that this simple geometric configuration, while iconically signifying a face, is ineffective for engaging high-level object representations of an actual face. This consideration led us to repeat this experiment with a digitized image of a real human face (figure 4c). In all other respects, the procedures were the same as those used before, with monitored locations corresponding to locations around the circumference of the head or to locations occupied by the eyes. Despite the realism of the face, suppression still spread much more readily around the continuous contour defining the oval shape of the head and not to the eyes within the interior regions of the face.
We therefore conclude that the spread of suppression is guided by explicit contour information without regard to the high-level object interpretation implied by those contours.
3.2 Effect of stimulus discontinuity on the spread of suppression
We have seen that suppression tends not to spread from part of one stimulus (eg a ring) to another nearby stimulus (eg a disk) when the two stimuli are separated by a blank uncontoured region. But what if that blank region is created by placing a gap in an otherwise continuous figure? Can the suppression bridge a gap in a rival stimulus and thus propagate to other parts of that stimulus located beyond the gap? In the following experiment we demonstrate that suppression can indeed spread over a gap if the implied cause of that gap is partial occlusion of a continuous stimulus.
3.2.1 Method
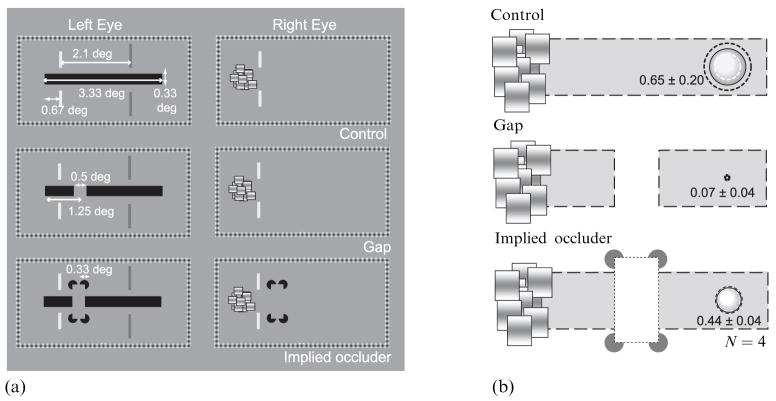
Four observers, including the first author, participated in this experiment. One eye viewed a horizontal target bar (3.33 deg × 0.33 deg) and the other viewed a dynamic CFS target (figure 5a). The CFS target was centered on the left side of the bar when the dichoptic images were appropriately aligned binocularly. Both images were surrounded by fusion frames identical to those used in the previous experiments.
Figure 5.
Stimuli and results in the ‘gap’ experiment. (a) Stimuli in the ‘gap’ experiment. Top panel shows a schematic of stimuli in the ‘control’ condition. Middle panel shows a target configuration in the ‘gap’ condition. Bottom panel shows a target configuration in the ‘implied occlusion’ condition. In all conditions, observers fixated on the part of target horizontal bar indicated by a pair of red vertical line segments (shown here in light gray) and monitored the part of target bar indicated by a pair of green vertical line segments (shown here in dark gray). The monitored position was located 2.1 deg right of the fixation position. (b) Results from four observers. Mean incidence of suppression for each condition is expressed by the size of the bubble. The dotted gray and black circles show the 95%-confidence intervals.
On one-third of the trials, the entire, uninterrupted horizontal bar was displayed to the left eye (‘control condition’). On another one-third of trials, the horizontal bar was interrupted by a 0.5 deg gap located 1.25 deg to the right of the left end of the bar (‘gap’ condition). On the remaining one-third of trials, the horizontal bar with the 0.5 deg gap was presented together with four sectored disks (0.33 deg × 0.33 deg) presented to both eyes at locations above and below the edges of the gap in the bar; the illusory contours induced by these sectored disks created the strong impression of a vertically oriented rectangle that was occluding a small part of a long horizontal bar (‘implied occlusion’ condition). These three trial types were randomly intermixed within a session.
Observers fixated the location on the horizontal bar 0.67 deg from the left end of the bar; this fixation region was indicated by a pair of thin vertical red lines (shown as light gray in figure 5a) presented to both eyes at locations immediately above and below the bar. The CFS stimulus was triggered by observer’s key-press and it remained present for 4 s. At stimulus offset, the observer judged whether a region at the right end of the horizontal bar, 2.1 deg from the border of the CFS stimulus, disappeared during the 4 s presentation; this monitoring location was denoted by a pair of thin vertical green lines (shown as dark gray in figure 5a) located above and below that region of the horizontal target bar. The experiment was conducted in two sessions; in a given session, each of the three stimulus conditions was tested 20 times in a randomized order.
3.2.2 Results
We calculated the incidence of suppression for each of the three stimulus conditions. As can be seen in figure 5b, suppression spread readily over the entire extent of the horizontal bar when no gap was present. In the presence of the gap, however, the incidence of suppression was significantly smaller than that observed in the control condition (t3 = 6.99, p < 0.01); suppression, in other words, rarely spread to the monitored location beyond the gap. A similar result has been recently reported by Takase et al (2008). Suppression did tend to spread over the entire horizontal bar, however, in the implied occlusion condition: the incidence of suppression in the presence of the gap plus sectored disks was not significantly different from that observed in the control condition (t3 = 2.38, p < 0.10) and was significantly greater than that observed with the gap alone (t3 = 19.7, p < 0.001). The spread of suppression, in other words, was restored when the gap was construed as part of the surface of an occluding object defined by the illusory contours induced by the sectored disks. We will consider the implications of this finding in section 5, but it is worth noting here that the present results are not incompatible with earlier work showing that suppression disrupts the formation of illusory contours when the inducers are placed in direct rivalry competition with dissimilar stimulation presented to the other eye (Sobel and Blake 2003): in our experiment, the inducers were outside the spatial zone of suppression and, therefore, maintained their effectiveness regardless of the state of rivalry of the horizontal bar.
3.3 Spread of suppression around corners
The results presented so far show that suppression spreads around a smoothly curved contour in which local orientation changes gradually over space. Does suppression also readily spread within a stimulus configuration with sharp corners? An answer to this question could have implications for the nature of the neural circuitry underlying spread of suppression. As reviewed by Hess et al (2003), converging lines of evidence implicate cooperative interactions mediated by lateral connections among cortical neurons whose receptive fields exhibit similar orientation preferences but different visual-field locations. Among other functions these interactions are believed to be involved in promotion of contour integration over extended regions of visual space, and it is natural to wonder whether they provide a substrate for the spread of suppression. This led us to perform the following two experiments.
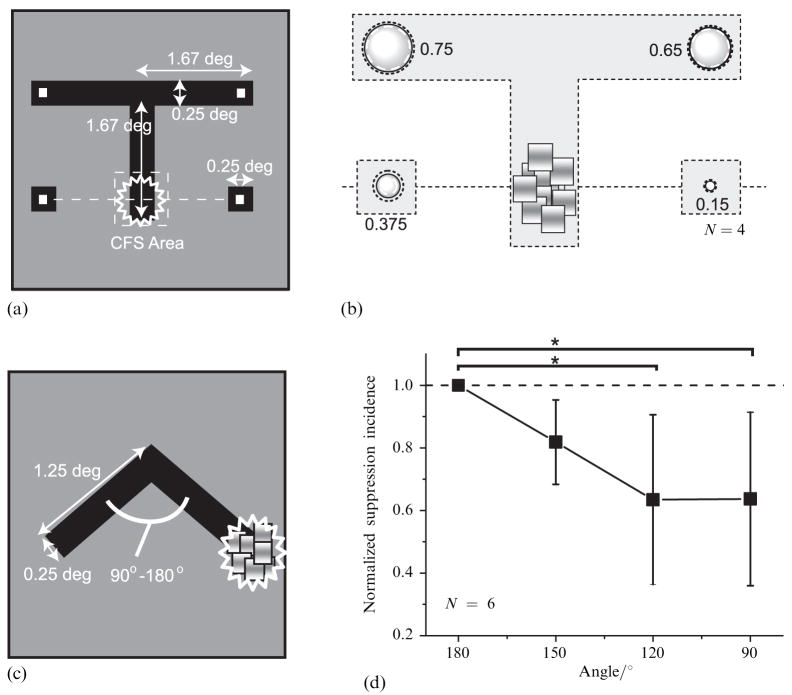
In the first of this pair of experiments, we modified the target stimulus from a ring to a T-shaped figure (figure 6a), with the CFS stimulus viewed by the other eye presented at the bottom part of the vertical arm of the figure. On each trial the observer was cued to monitor one of four locations as indicated in figure 6a. Four observers were tested, two naive about the purpose of the experiment.
Figure 6.
Stimuli and results in the ‘corner’ experiments. (a) The target stimulus configuration in ‘T-shape’ experiments. (b) Results for the ‘T-shape’ experiment. The incidence of suppression is shown as in figure 2a. (c) The target stimulus configuration in ‘inverted V-shape’ experiment. (d) Results for the ‘inverted V-shape’ experiment. The suppression incidence is plotted as a function of the angle subtended by two arms of the inverted V. To remove the influence of individual variation as for the overall incidence level, the incidences were normalized for each observer with the incidence in 180° condition and then averaged. Error bars show 95%-confidence intervals. CFS = continuous flash suppression. Asterisks denote that significant differences were observed by ANOVA (see text).
As with the ring stimulus, suppression spread much more readily along the contours defining the rival figure, in this case turning the corner to spread along the horizontal portion of the T (figure 6b). The incidence of suppression at the corners of the T was significantly higher than at the two isolated squares (t3 = 3.52, p < 0.05). From this we conclude that the spread of suppression is not limited to a contour that is defined by a smooth, continuous change in orientation; suppression can spread around sharp corners. But is the incidence of the spread of suppression weakened by this configuration? Because we cannot directly compare the data from the ring with those collected from the T figure, we performed an additional experiment in which the acuteness of the angle formed by two adjoining contours was varied.
In this experiment, the target stimulus was an inverted V-shaped figure, with the CFS stimulus presented to the other eye imaged at a location corresponding to the rightmost part of that figure. We varied the angle subtended by two arms of the inverted V in four steps from 90° to 180° (figure 6c). On each trial, the observer judged whether the suppression induced at the extreme right of the figure spread to the opposite location at the extreme left of the figure, as evidenced by complete suppression of the entire V figure for at least some portion of the 2 s viewing period. Six observers were tested with this configuration, five of whom were naive about the purpose of the experiment.
Results showed that the suppression spread more readily along the straight contours relative to the condition where the target contained a sharp corner. We normalized incidences for each observer with an incidence in the 180° condition to emphasize the influence of the sharpness of the corner. The normalized incidence of suppression at the left end of the figure was reduced as the angle between the two arms increased (figure 6d). This decrement was significant statistically as revealed by ANOVA performed on data without normalization (F3,5 = 4.94, p < 0.05). An a posteriori test with the LSD method revealed that the suppression incidence in the 90° and the 120° conditions was less than that in the 180° condition (MSE = 0.011, p < 0.05). This finding is particularly striking in that the left-hand portion of the V figure is actually considerably closer to the CFS stimulus in the 90° condition than it is in the 180° condition. Despite this spatial proximity, the spread of suppression is weaker, again indicating that the spread of suppression is not isotropic.
Evidently the strength of suppression is weakened when suppression encounters a large, abrupt change in contour orientation. This observation is what would be predicted on the basis of the association-field concept that has usefully been invoked to account for other aspects of contour perception (Hess et al 2003). It is noteworthy that this weakening is relatively modest; suppression is certainly not abolished by the presence of a sharp corner.
4 Spread of suppression and eye dominance
In the experiments reported so far, we found that the spread of suppression was more extensive for some observers than for others; compare, for example, observers SH and HE in figure 3. Because we always tested for spread of interocular suppression within the rival target presented to the left eye, it is possible that these individual differences are attributable to differences in eye dominance (Ooi and He 2001). To assess this possibility, we measured sensory eye dominance in a group of observers and correlated that measure with the incidence of the spread of suppression measured for the left eye and for the right eye in each of those observers.
4.1 Method
4.1.1 Observers
In this experiment we tested nine observers, including four individuals who participated in the first experiment. All had normal or corrected-to-normal acuity and good stereopsis.
4.1.2 Spread of suppression
The apparatus and procedure for these measurements were identical to those employed in the first experiment (figures 1d and 1e), with two exceptions. First, we estimated the spread of suppression of two, not seven, locations around the ring figure; those locations were equidistant from the right-hand portion of the ring where suppression was instigated by the CFS stimulus. Second, over a block of 40 trials we randomized which eye received the CFS stimulus and, therefore, which eye viewed the ring. In all other respects, the sequence of events on each trial was the same as in the original experiment.
4.1.3 Sensory eye dominance
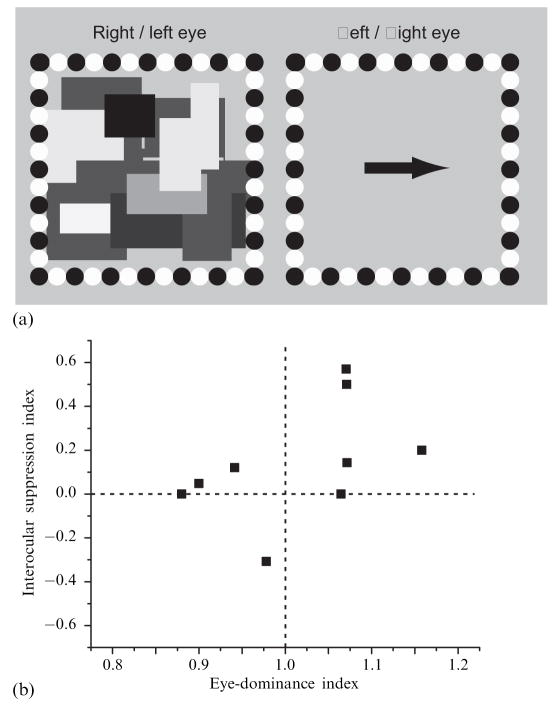
For these measurements we employed a modified version of the balancing technique developed by Ooi and He (2001), in which binocular rivalry is used to gauge the degree of interocular imbalance. With their technique, the luminance intensity of pairs of briefly flashed rival gratings was manipulated to find the interocular intensity difference where the gratings presented to the two eyes were equally likely to be dominant. In our implementation of their procedure, observers dichoptically viewed a CFS stimulus with one eye and a black arrow pointing either to the left or to the right with the other eye (figure 7a). At the start of each trial, the contrast of the CFS stimulus was 50% and the contrast of the arrow was 0%. Over time the contrast of the CFS was ramped down slowly and steadily and the contrast of the arrow was ramped up slowly and steadily; for both monocular stimuli, contrast changed at the rate of 10% s−1. On each trial the observer pressed one of the two arrow keys on a computer keyboard as soon as the arrow viewed by one eye achieved visibility sufficient to judge confidently the direction in which the arrow pointed. Upon this key-press, both displays disappeared and the computer recorded the correctness of the response and the time that had elapsed between the onset of the trial and the observer’s response.
Figure 7.
Stimuli and results in ‘eye-dominance’ experiment. (a) Monocular stimulus on the left shows one frame of the continuous flash suppression sequence; the monocular stimulus on the right shows a schematic of the target arrow that, over trials, could point either left or right. (b) Results of ‘eye-dominance’ experiment. Suppression index for each observer is plotted as a function of eye-dominance index.
Observers viewed these dichoptic stimuli through liquid-crystal stereo-goggles synchronized to the 120 Hz frame rate of the video monitor used for these measurements. Observers were given 10 practice trials to familiarize themselves with the procedure, followed by 50 test trials in which the eye receiving the arrow was randomized, with the stipulation that each eye be tested 25 times.
4.2 Results and discussion
With this procedure for estimating eye dominance, we assume that the image of the arrow presented to the dominant eye of someone with strong eye dominance will become visible more quickly, yielding briefer response times than when the arrow is presented to the nondominant eye. (This is the same rationale behind Ooi and He’s rivalry test, except that they used briefly flashed rival targets to estimate interocular imbalance.) To derive an index of eye dominance for each observer, we computed the ratio of the average left-eye response time to the average right-eye response time. Thus, an index value less than 1.0 denotes left-eye dominance; a value greater than 1.0 right-eye dominance; and a value of unity denotes approximately equal dominance. For each observer we were able to use the data from all 50 trials since the observers made no mistakes when judging the direction of the arrow. In the nine observers tested, eye-dominance index values ranged from 0.88 to 1.16.
For the spread-of-suppression task, we pooled the data for the two locations tested since the incidence values for those locations were essentially the same for a given observer and eye-viewing condition; this is not surprising, given that those two locations were equidistant from the region where suppression originated. For each observer we derived an interocular suppression index for the spread of suppression by subtracting the left-eye incidence from the right-eye incidence and normalizing this difference by dividing by the sum of those two incidence values. This interocular-suppression index ranged from −0.31 to 0.57 for different observers, although for six of the nine this index value was small (0.2 or less) or zero.
Figure 7b shows a scatterplot of interocular-suppression index plotted against eye-dominance index. There is a tendency for eye-dominance index to increase with interocular-suppression index, but the correlation between these two indices, 0.47, failed to achieve statistical significance (t9 = 1.40, p > 0.20). This failure stems, in part, from the fact that interocular differences in the spread of suppression were very small for the majority of observers. These results indicate that individual differences in eye dominance, as assessed by a version of the balancing technique introduced by Ooi and He (2001), are not strongly related to the spread of suppression.
5 General discussion
Our results clearly show that the spatial spread of interocular suppression is governed by the configuration of the stimulus undergoing suppression, even when the target explicitly inducing suppression is confined to a small portion of the stimulus succumbing to suppression. The spread of suppression, in other words, is not isotropic over space but, instead, behaves as if it were dynamically shaped by the patterns of neural activity associated with a given stimulus. What conclusions can be drawn about the nature and locus of those neural events? The following paragraphs address this central question.
Previous work on binocular rivalry led to the notion of zones of suppression the size of which scale with retinal eccentricity (Blake et al 1992) and with spatial frequency (Liu and Schor 1994). While not wrong, that conceptualization clearly needs to be defined to incorporate the dependence of the spatial extent of suppression on stimulus configuration. This refinement may usually be guided by modeling work that addresses another, possibly related, aspect of binocular rivalry that has to do with perceptual transitions from suppression to dominance. Called traveling waves, these transitions typically resemble spreading waves of dominance that originate locally and spread along the boundaries of rival stimulation (Wilson et al 2001; Knapen et al 2007). Accompanying those perceptual waves of dominance are fluctuations in neural responses within retinotopically organized visual areas (Lee et al 2005, 2007). Wilson et al (2001) developed a dynamical model to account for traveling waves of dominance during binocular rivalry. According to that model, excitatory connections among neurons with spatially neighboring receptive fields promote cooperative interactions among those neurons which, at the same time, are in competition via inhibitory connections with other neurons responsive to rival orientations. The effects of these excitatory and inhibitory influences spread within the network of neurons, promoting orderly transitions from one perceptual state to the other. Knapen et al (2007) refined this dynamical model to include asymmetric inhibitory interactions among direction-selective neurons, to account for the tendency of stimulus motion to propel traveling waves of dominance during state transitions of rivalry.
The traveling-wave model was designed to explain the spatial spread of dominance within explicit regions of rivalry. Can that model also account for the spatial spread of suppression documented in our experiments? To account for our results, the traveling-wave model would have to be modified to promote the spread of activity into portions of the neural network in which explicit competition is not transpiring, ie into portions of the neural representation of a stimulus that falls outside the region in which rivalry is instigated. Moreover, the spread of inhibition that triggers a wave of suppression would have to be guided by the pattern of activity associated with the initially dominant stimulus. The model of Wilson et al, and its extension developed by Knapen et al, does embody network interactions that could accomplish these requirements, although the network parameters in the current formulations are insufficient to promote the spatial extent of suppression spread found in the present experiments. Moreover, the traveling-wave model incorporates lateral excitatory connections whose strengths are related to the similarity in orientation selectivity of neighboring neurons—a feature of the model designed to account for the propensity of traveling waves to propagate more effectively around stimulus configurations containing collinear contours. We, too, found some evidence that the spread of suppression is dependent on the degree of collinearity (recall that suppression spread around corners less effectively than it did around smoothly curved contours). We did not measure the speed with which suppression spreads throughout a stimulus, and it is possible that sharp corners also could affect the speed of suppression spread (just as it affects the speed of traveling waves). While further theoretical work clearly is required, we are encouraged by the possibility that the spatial spread of suppression documented in this paper might be a natural consequence of the same network responsible for the temporal spread of dominance associated with transitions in rivalry state. Unfortunately, the considerable trial-to-trial variability in the speed of the spread of suppression makes it challenging to obtain reliable estimates of this aspect of the phenomenon.
What conclusions can be drawn about the level of visual processing at which spreading interocular suppression transpires? We found no indication that the spread of suppression is governed by high-level grouping mechanisms, as evidenced by the failure of suppression to spread to stimulus elements within the interior of a configuration readily identified as a face, even though those stimulus elements clearly constituted the eyes of the face. On the basis of this finding, we are disinclined to think that the spread of suppression induced by CFS is transpiring within high-level object representations. It is possible, of course, that suppression might behave differently if one were to induce interocular suppression using a meaningful figure, not CFS. Indeed, Alais and Melcher (2007) have found that the depth and coherence of binocular rivalry suppression vary depending on the nature of the stimuli inducing rivalry. In particular, rival targets comprising coherent visual objects (eg faces and houses) yielded deeper suppression, as indexed by elevations in identification thresholds, and less piecemeal rivalry than did simple, rival grating targets (see also Nguyen et al 2003). It is possible that the CFS stimulus used by us engages an early suppression mechanism that is insensitive to the global configuration of the stimulus undergoing suppression. This is a possibility that could be explored in future work.
In contrast, however, the spread of suppression induced by CFS was influenced by implied occlusion, as demonstrated by our gap experiment. Suppression induced at one end of a horizontal rectangle tended to spread over a discontinuity in that rectangle if—but only if—that discontinuity appeared to arise from occlusion. In our experiment, we created implied occlusion using a subjective figure created by illusory contours. Unlike faces, illusory contours are explicitly registered within early stages of the visual hierarchy. There is abundant physiological evidence from studies of non-human primates that V2 neurons are responsive to illusory contours of the sort used in our experiment (von der Heydt et al 1984; Ramsden et al 2001). Moreover, one study shows that V1 neurons also are responsive to illusory contours, with those V1 responses plausibly arising from V2 feedback signals because the V1 responses occur about 100 ms after neural responses in V2 (T S Lee and Nguyen 2001). Brain-imaging studies in humans also point to early cortical activations in response to stimulus configurations that generate illusory contours, although there is a controversy concerning just how early in the visual pathways those activations are seen (see review by Seghier and Vuilleumier 2006). Thus it seems reasonable to assume that the neural events promoting formation of illusory contours arise from interactions among cortical areas within early stages of visual processing, possibly including primary visual cortex. In turn, this suggests that the spread of suppression promoted by the presence of implied occlusion of a figure defined by illusory contours may transpire within early cortical areas thought to be involved in other aspects of binocular rivalry (Tong et al 2006).
Acknowledgments
This research was supported by grants from the Japan Society for the Promotion of Science and from the National Institutes of Health (EY13358). We thank Anna Roe for helpful discussion and the anonymous referees for their constructive criticisms. We also thank Eunice Yang for help with the eye-dominance experiment, and Min-Suk Kang for discussion about conclusions.
References
- Alais D, Blake R. Binocular Rivalry. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Alais D, Melcher D. Strength and coherence of binocular rivalry depends on shared stimulus complexity. Vision Research. 2007;47:269–279. doi: 10.1016/j.visres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Blake R. A neural theory of binocular rivalry. Psychological Review. 1989;96:145–167. doi: 10.1037/0033-295x.96.1.145. [DOI] [PubMed] [Google Scholar]
- Blake R, Camisa J. On the inhibitory nature of binocular rivalry suppression. Journal of Experimental Psychology: Human Perception and Performance. 1979;5:315–323. doi: 10.1037//0096-1523.5.2.315. [DOI] [PubMed] [Google Scholar]
- Blake R, Logothetis NK. Visual competition. Nature Reviews Neuroscience. 2002;3:4–11. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Blake R, O’Shea RP, Mueller TJ. Spatial zones of binocular rivalry in central and peripheral vision. Visual Neuroscience. 1992;8:469–478. doi: 10.1017/s0952523800004971. [DOI] [PubMed] [Google Scholar]
- Boxtel JJA, van Alais D, van Ee R. Retinotopic and non-retinotopic stimulus encoding in binocular rivalry and the involvement of feedback. Journal of Vision. 2008;8:1–10. doi: 10.1167/8.5.17. [DOI] [PubMed] [Google Scholar]
- Clarke FJJ. A study of Troxler’s effect. Journal of Modern Optics. 1960;7:219–236. [Google Scholar]
- Hess RF, Hayes A, Field DJ. Contour integration and cortical processing. Journal of Physiology (Paris) 2003;97:105–119. doi: 10.1016/j.jphysparis.2003.09.013. [DOI] [PubMed] [Google Scholar]
- von der Heydt R, Peterhans E, Baumgartner G. Illusory contours and cortical neuron responses. Science. 1984;224:1260–1262. doi: 10.1126/science.6539501. [DOI] [PubMed] [Google Scholar]
- Kaufman L. On the spread of suppression and binocular rivalry. Vision Research. 1963;3:401–415. doi: 10.1016/0042-6989(63)90092-0. [DOI] [PubMed] [Google Scholar]
- Knapen T, van Ee R, Blake R. Stimulus motion propels traveling waves in binocular rivalry. PLoS ONE. 2007;8:1–7. doi: 10.1371/journal.pone.0000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. The Quest for Consciousness: A Neurobiological Approach. Greenwood Village, CO: Roberts & Company; 2004. [Google Scholar]
- Laing CR, Chow CC. A spiking neuron model for binocular rivalry. Journal of Computational Neuroscience. 2002;12:39–53. doi: 10.1023/a:1014942129705. [DOI] [PubMed] [Google Scholar]
- Lee SH, Blake R, Heeger D. Traveling waves of activity in primary visual cortex during binocular rivalry. Nature Neuroscience. 2005;8:22–23. doi: 10.1038/nn1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Blake R, Heeger D. Hierarchy of cortical responses underlying binocular rivalry. Nature Neuroscience. 2007;10:1048–1054. doi: 10.1038/nn1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Nguyen M. Dynamics of subjective contour formation in the early visual cortex. Proceedings of the National Academy of Sciences of the USA. 2001;98:1907–1911. doi: 10.1073/pnas.031579998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehky SR. An astable multivibrator model of binocular rivalry. Perception. 1988;17:215–228. doi: 10.1068/p170215. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Multistable phenomena: changing views in perception. Trends in Cognitive Sciences. 1999;3:254–264. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. On Binocular Rivalry. Soesterberg, The Netherlands: Institute for Perception RVO-TNO; 1965. [Google Scholar]
- Liu L, Schor CM. The spatial properties of binocular suppression zone. Vision Research. 1994;34:937–947. doi: 10.1016/0042-6989(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Nguyen V, Freeman AW, Alais D. Increasing depth of binocular rivalry suppression along two visual pathways. Vision Research. 2003;43:2003–2008. doi: 10.1016/s0042-6989(03)00314-6. [DOI] [PubMed] [Google Scholar]
- Noest AJ, van Ee R, Nijs MM, van Wezel RJA. Percept-choice sequences driven by interrupted ambiguous stimuli: A low-level neural model. Journal of Vision. 2007;7:1–14. doi: 10.1167/7.8.10. [DOI] [PubMed] [Google Scholar]
- Ooi TL, He ZJ. Sensory eye dominance. Optometry: Journal of the American Optometric Association. 2001;72:168–178. [PubMed] [Google Scholar]
- Ooi TL, He ZJ. A distributed intercortical processing of binocular rivalry: psychophysical evidence. Perception. 2003;32:155–166. doi: 10.1068/p3467. [DOI] [PubMed] [Google Scholar]
- Ramsden BM, Hung CP, Roe AW. Real and illusory contour processing in area V1 of the primate: a cortical balancing act. Cerebral Cortex. 2001;11:648–665. doi: 10.1093/cercor/11.7.648. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Vuilleumier P. Functional neural imaging findings on the human perception of illusory contours. Neuroscience and Biobehavioral Reviews. 2006;30:595–612. doi: 10.1016/j.neubiorev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sobel KV, Blake R. Subjective contours and binocular rivalry suppression. Vision Research. 2003;43:1533–1540. doi: 10.1016/s0042-6989(03)00178-0. [DOI] [PubMed] [Google Scholar]
- Takase S, Yukumatsu S, Bingushi K. Local binocular fusion is involved in global binocular rivalry. Vision Research. 2008;48:1798–1803. doi: 10.1016/j.visres.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends in Cognitive Sciences. 2006;10:502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nature Neuroscience. 2005;8:1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C, Gilroy LA, Blake R. Depth of interocular suppression associated with continuous flash suppression, flash suppression, and binocular rivalry. Journal of Vision. 2006;10:1068–1078. doi: 10.1167/6.10.6. [DOI] [PubMed] [Google Scholar]
- Wilson HR. Computational evidence for a rivalry hierarchy in vision. Proceedings of the National Academy of Sciences of the USA. 2003;100:14499–14503. doi: 10.1073/pnas.2333622100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR, Blake R, Lee SH. Dynamics of traveling waves in visual perception. Nature. 2001;412:907–910. doi: 10.1038/35091066. [DOI] [PubMed] [Google Scholar]