Abstract
A growing body of work has documented sex differences in many behavioral, neurochemical, and morphological responses to stress. Chronic stress alters morphology of dendrites in medial prefrontal cortex in male rats. However, potential sex differences in stress-induced morphological changes in medial prefrontal cortex have not been examined. Thus, in Experiment 1 we assessed dendritic morphology in medial prefrontal cortex in male and female rats after chronic stress. Male and female rats underwent either three hours of restraint daily for one week or were left unhandled except for weighing. On the final day of restraint, all rats were euthanized and brains were stained using a Golgi-Cox procedure. Pyramidal neurons in layer II-III of medial prefrontal cortex were drawn in three dimensions, and morphology of apical and basilar arbors was quantified. In males, stress decreased apical dendritic branch number and length, whereas in females, stress increased apical dendritic length. In Experiment 2, we assessed whether estradiol mediates this stress-induced dendritic hypertrophy in females by assessing the effects of restraint stress on female rats that had received either ovariectomy with or without 17-β-estradiol replacement or sham ovariectomy. Brains were processed and neurons reconstructed as described in Experiment 1. Both sham-operated and ovariectomized rats with estradiol implants showed stress-induced increases in apical dendritic material, whereas ovariectomy without estradiol replacement prevented the stress-induced increase. Thus, the stress-induced increase in apical dendritic material in females is estradiol-dependent.
Introduction
Stress can disrupt cognitive and emotional behavior (Lupien and Lepage, 2001) and can precipitate or exacerbate many psychological disorders, most notably depression, schizophrenia, and posttraumatic stress disorder (Brown and Harris, 1989;Ventura et al., 1989). Sex is an important variable influencing many of these stress-related disorders. For example, in women, risk for depression is about double that in men (Nolen-Hoeksema and Girgus, 1994), and women are more susceptible to stress-related disorders such as generalized anxiety and post-traumatic stress disorders (Weissman et al., 1988;Stein et al., 2000). Thus, understanding the basis of this sex difference is an important goal in developing appropriate interventions for stress-related disorders (Pinn, 2005).
Stress has profound effects on the morphology of corticolimbic structures including the hippocampus, amygdala, and prefrontal cortex (Magariños and McEwen, 1995;Vyas et al., 2002;Cook and Wellman, 2004). Relatively little work has examined potential sex differences in the morphological effects of stress. However, sex differences in stress-induced changes in hippocampal morphology have been documented in the CA3 and CA1 regions of the hippocampus (Galea et al., 1997;McLaughlin et al., 2005), and these differences are paralleled by differential stress effects on hippocampally mediated behaviors (Williams and Meck, 1991;Luine et al., 1994;Bowman et al., 2001;Luine, 2002;Conrad et al., 2003).
Prefrontal cortex has been implicated in many psychological disorders, including schizophrenia, depression, and posttraumatic stress disorder (Baxter et al., 1989;Drevets et al., 1992;Carter et al., 2001;Takahashi et al., 2004), is involved in cognitive processes that are influenced by chronic stress (e.g., Dias et al., 1996), and is a target for the hormones involved in the stress response (Meaney and Aitken, 1985). Stress produces profound changes in the morphology of neurons in medial prefrontal cortex (mPFC) of male rats (e.g., Cook and Wellman, 2004;Brown et al., 2005;Izquierdo et al., 2006). In males, chronic restraint stress dramatically remodels apical dendrites of pyramidal neurons in mPFC, producing reductions in apical branch number and length (Cook and Wellman, 2004). Interestingly, dendritic morphology of mPFC appears to be exquisitely sensitive to stress: just one week of brief (10 min) daily restraint reduces mPFC apical dendritic branch number and length in male rats (Brown et al., 2005). Furthermore, chronic stress also alters prefrontally mediated behaviors in male rats. For instance, chronic restraint stress impairs extradimensional set-shifting (Liston et al., 2006) and retrieval of extinction of conditioned fear (Miracle et al., 2006).
Importantly, all of the studies to date examining stress effects on the morphology of prefrontal cortex have focused on males. Given the sex differences in stress-related psychological disorders mentioned above, the absence of information on the impact of stress on the prefrontal cortex of females, as well as the mechanisms underlying potential sex differences, is problematic. A recent study demonstrated a sex difference in the effect of chronic stress on recall of extinction of conditioned fear (Baran et al., 2009), suggesting that stress could also have differential effects on morphology of mPFC in males versus females. Thus, to begin to examine potential sex differences in medial prefrontal cortex stress effects, we assessed dendritic morphology in medial prefrontal cortex in male and female rats after chronic restraint stress.
Experiment 1
Experimental Procedures
Animals and Treatment
Male (n=12; 200-246 g, 51-59 days old at the start of the experiment; Harlan, Indianapolis, IN) and female Sprague-Dawley rats (n=12; 177-222 g, 55 days old at the start of the experiment; Harlan, Indianapolis, IN) were same-sex group-housed in a vivarium with ad libitum access to food and water. The room was temperature- (23-25° C) and humidity-controlled with a 12 h light-dark cycle (lights on at 7 a.m.). All experimental procedures were performed during the light cycle. All procedures were approved by the Bloomington Institutional Animal Care and Use Committee and were conducted in accordance with NIH guidelines.
Half of the rats (n=6 male and 6 female) were placed in a plastic restrainer in their home cages 3 h daily for 7 days. Previous data indicate that this manipulation is stressful, resulting in a significant increase in plasma corticosterone (Cook and Wellman, 2004). The remainder of the rats (n=6 male and 6 female) served as unstressed controls. All rats were weighed every other day.
Histology and Dendritic Analysis
Immediately after the final session of restraint stress, animals were overdosed with urethane and then perfused with 0.9% saline. Brains were removed and processed using Glaser and Van der Loos' modified Golgi stain (Glaser and Van der Loos, 1981). Tissue was immersed in Golgi-Cox solution (a 1:1 solution of 5% potassium dichromate and 5% mercuric chloride diluted 4:10 with 5% potassium chromate) for 12 days. Brains were then dehydrated and infiltrated with a graded series of celloidins before being embedded in 8% celloidin. Coronal sections were cut at 160 μm on a sliding microtome (AO 860; American Optical Company, Buffalo, NY). Free-floating sections were then alkalinized in 18.7% ammonia, developed in Dektol (Kodak), fixed in Kodak Rapid Fix (prepared as per package instructions with Solution B omitted), dehydrated through a graded series of ethanols, cleared in xylene, mounted, and coverslipped.
Pyramidal neurons in layer II–III of the Cg1-3 area of medial prefrontal cortex (anterior cingulate and prelimbic cortex; Fig. 2a) were drawn. Cg1-3 is readily identified by its position on the medial wall of rostral cortex, and its location dorsal to infralimbic cortex, which is markedly thinner than the Cg1-3 area and has fewer, less well-defined layers (Zilles and Wree, 1995). Within Cg1-3, layer II–III is readily identifiable in Golgi-stained material based on its characteristic cytoarchitecture. Its position is immediately ventral to the relatively cell-poor layer I (which also contains the distal dendritic tufts of layer II–III pyramids) and immediately dorsal to layer IV; in medial prefrontal cortex, this boundary is pronounced because of the greater cell-packing density and smaller somata of pyramidal cells in layer II–III relative to layer IV (Cajal, 1995;Zilles and Wree, 1995).
Figure 2.
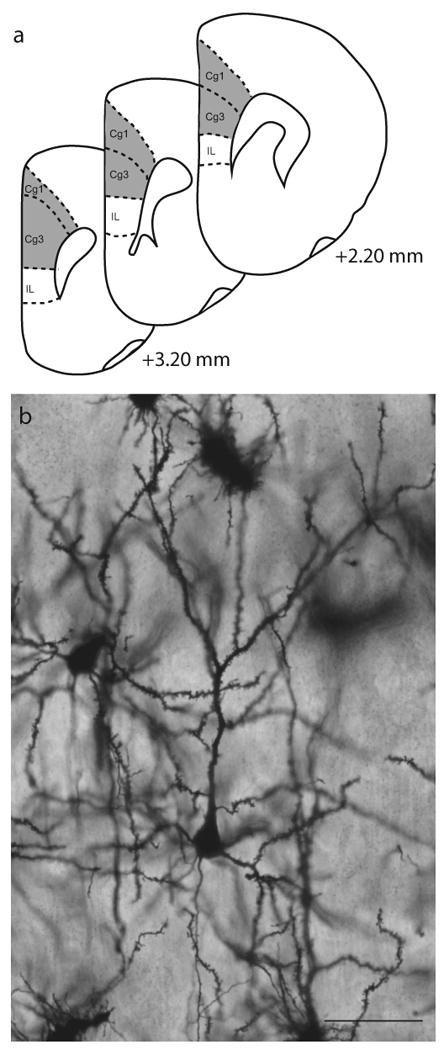
a. Schematic diagram of coronal sections through rat prefrontal cortex. The portions of areas Cg1 and Cg3 (anterior cingulate and prelimbic cortex, respectively) from which neurons were sampled is shown (shaded areas). Adapted from Paxinos & Watson (1998). Coordinates indicate position relative to bregma. b. Digital light micrograph of Golgi-stained neuron in layer II-III of medial prefrontal cortex. Scale bar = 50 μm.
Pyramidal neurons were defined by a distinct, single apical dendrite extending from the apex of the soma toward the pial surface of the cortex, two or more basilar dendritic trees extending from the base of the soma, and dendritic spines (see Fig. 2b). Neurons selected for reconstruction did not have truncated branches and were unobscured by neighboring neurons and glia, with dendrites that were easily discriminable by focusing through the depth of the tissue. In 5 sections evenly spaced through the rostral-caudal extent of Cg1-3, all pyramidal neurons meeting these criteria were identified. Using a random number generator (www.randomizer.org), ten neurons per animal (5 from each hemisphere) were then randomly selected from all of these identified neurons and reconstructed. This yielded a within-animal error of approximately 10% [mean within-animal SEM for total branch length = 12.56 ± 0.58% (males), 11.79 ± 0.39% (females)], and thus was considered to provide a representative sample. All neurons were drawn at 600× and morphology of apical and basilar arbors was quantified in three dimensions using a computer-based neuron tracing system (Neurolucida; MBF Bioscience, Williston, VT) with the experimenter blind to condition.
Several aspects of dendritic morphology were examined. To assess overall changes in dendritic morphology, the number and length of apical and basilar dendrites were analyzed using 2-way ANOVAs (sex × stress) followed by appropriate planned comparisons. To assess differences in the amount and location of dendritic material, a three-dimensional version of a Sholl analysis (Sholl, 1956) was performed. A Sholl analysis estimates the amount and distribution of dendritic material by counting the number of intersections of dendrites with an overlay of concentric rings centered on the soma. In the present study, the total length of dendrites between concentric spheres at 20 μm intervals was assessed. These data were compared for males and females using 3-way repeated-measures ANOVAs (sex × stress × distance from soma) followed by appropriate planned comparisons. For all analyses, planned comparisons consisted of F tests done within the context of the overall ANOVA (Hays, 1994), comparing either male versus female unstressed rats or unstressed versus stressed rats.
Results
Weight Data
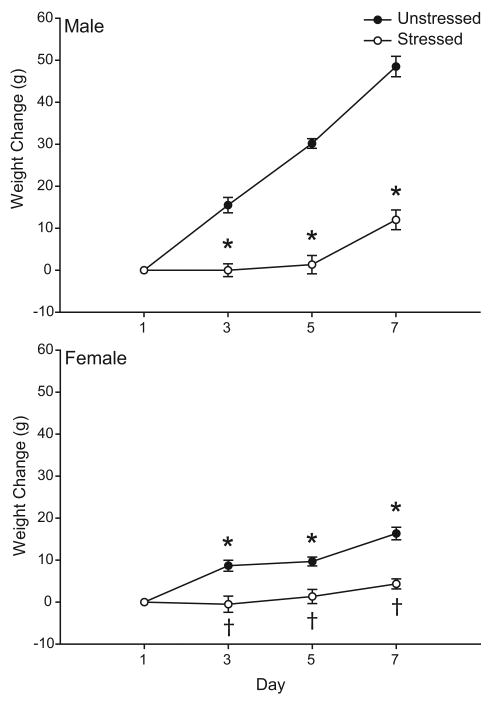
Unsurprisingly, weight gain differed significantly between males and females [Fig. 1; main effect of sex, F(1,20) = 48.64, p<0.05]. Likewise, consistent with work demonstrating that chronic stressors such as restraint or immobilization attenuate normal weight gain (Martí et al., 1994;Cook and Wellman, 2004), both male and female stressed rats showed an attenuation of weight gain [main effect of stress, F(1, 20) = 129.31, p<0.05]. However, the effects of stress varied with both sex and day [interaction of sex and stress, F(1,20) = 27.99, p<0.05; day × sex × stress interaction, F(1,60) = 31.62, p<0.05]. Planned comparisons indicated that on days 3, 5, and 7, both female and male stressed rats showed significantly less weight gain compared to their respective unstressed controls [all F's(1,10) ≥ 15.48, p<0.05]. By the final day of restraint, stressed male rats showed a weight gain that was only 25% that of male controls, while the mean weight gain of stressed female rats was only 27% as much as that of female controls.
Figure 1.
Mean weight change in male (above) and female (below) rats that either were unhandled except for weighing (Unstressed) or received 3 h of daily restraint stress for one week (Stressed). Stressed rats gained less weight than unstressed controls. Vertical bars represent SEM values. Asterisks (*) indicate significant differences relative to unstressed males; daggers (†) indicate significant differences relative to unstressed females.
Dendritic Analyses
In all treatment groups, complete impregnation of numerous cortical pyramidal neurons was apparent, and both Cg1-3 and layer II–III were readily identifiable. To rule out potential artifactual differences in dendritic morphology due to differential sampling in layer II and III, the distance from the soma to the pial surface of cortex was measured in each neuron (range=180 μm − 582 μm). Average distance to the cortical surface was then compared across groups using 2-way ANOVA. Average distance to the cortical surface did not vary by sex [unstressed male rats, M = 323.13 ± 10.32 μm; unstressed female rats, M = 342.78 ± 11.26 μm; for main effect of sex, F(1, 20) = 0.68, ns]. Similarly, the average distance to the cortical surface did not vary by stress and this did not differ between sexes [stressed males, M = 329.04 ± 11.03 μm; stressed females, M = 334.27 ± 20.37 μm; for stress, F(1, 20) = 0.63, ns; for the interaction, F(1, 20) = 0.37, ns]. Thus, neurons were sampled from equivalent laminar depths across groups.
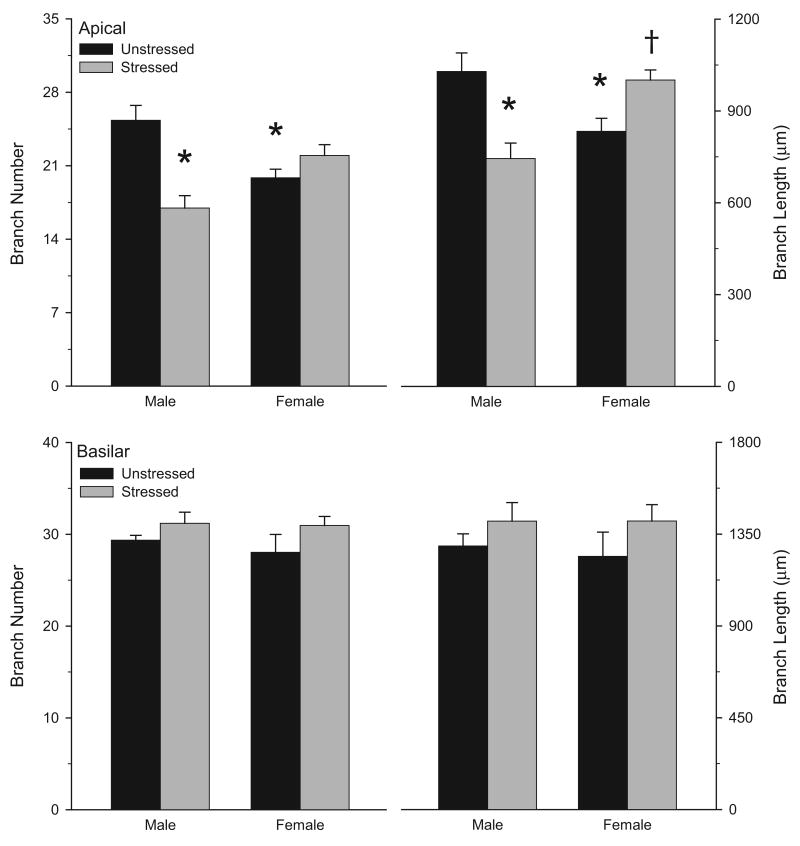
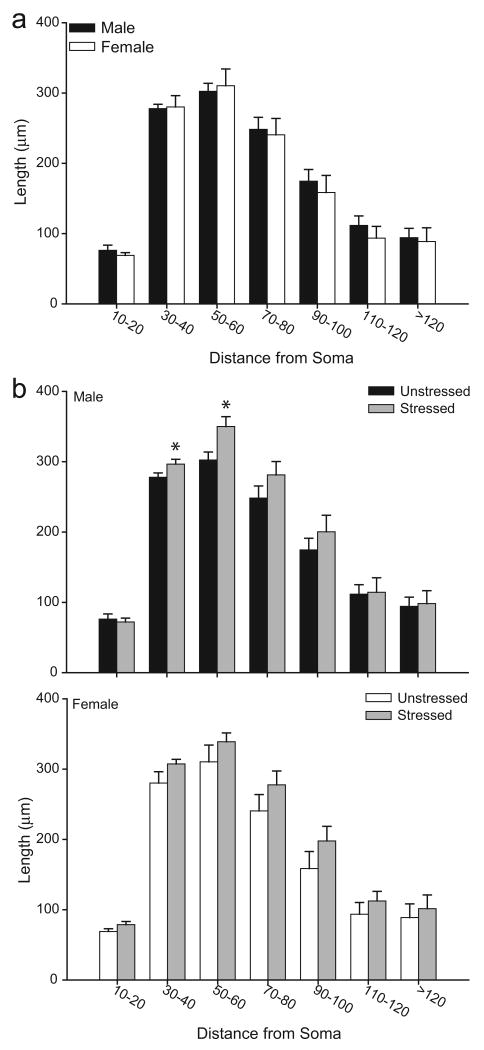
Two-way ANOVAs revealed that the effect of stress on overall apical branch number and length varied significantly for male versus female rats [see Figs. 3 and 4; interaction of sex and stress, F(1, 20) = 21.57 and 22.37, respectively, p <0.05; main effect of sex, F(1,20) = 0.04 and 0.41, respectively, ns]. Planned comparisons revealed a sex difference in unstressed rats, with 22% fewer apical dendritic branches in unstressed female rats relative to unstressed males [F(1,10) = 11.21, p <0.05]. Similarly, apical dendritic length was 11% less in unstressed females relative to unstressed males [F(1,10) = 6.90, p <0.05]. Furthermore, in males, stress significantly reduced apical branch number [33%; F(1,10) = 20.71, p <0.05] and length [28%; F(1,10) = 12.96, p <0.05] relative to unstressed controls. On the other hand, in female rats, stress significantly increased apical branch length [20%; F(1,10) = 9.71, p <0.05] but not number [F(1,10) = 2.63, ns] relative to unstressed controls.
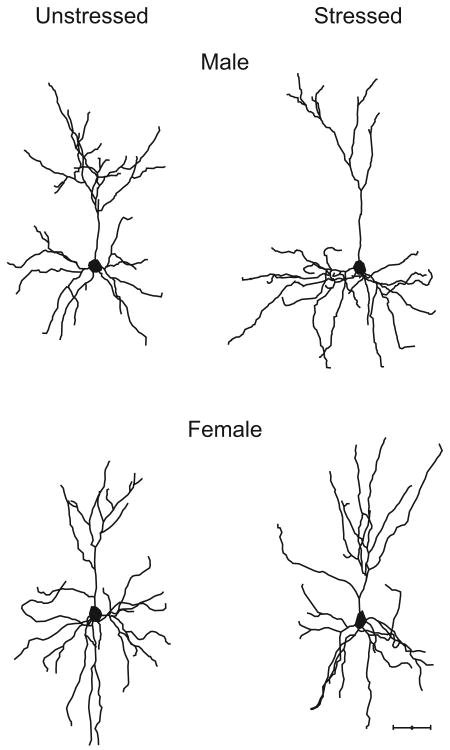
Figure 3.
Computer-assisted reconstructions of Golgi-stained neurons in layer II-III of medial prefrontal cortex in unstressed (left) and restraint-stressed (right) male (top) and female (bottom) rats. Scale bar = 50 μm. These neurons were selected because they are representative of apical dendritic lengths near their respective group means.
Figure 4.
Mean apical (above) and basilar (below) branch number and length for unstressed versus stressed male and female rats. Unstressed females had shorter apical dendrites than unstressed males. Stress reduced apical dendritic branching and length in males, but increased apical dendritic length in females. Vertical bars represent SEM values. Asterisks (*) indicate significant differences relative to unstressed males; daggers (†) indicate significant differences relative to unstressed females.
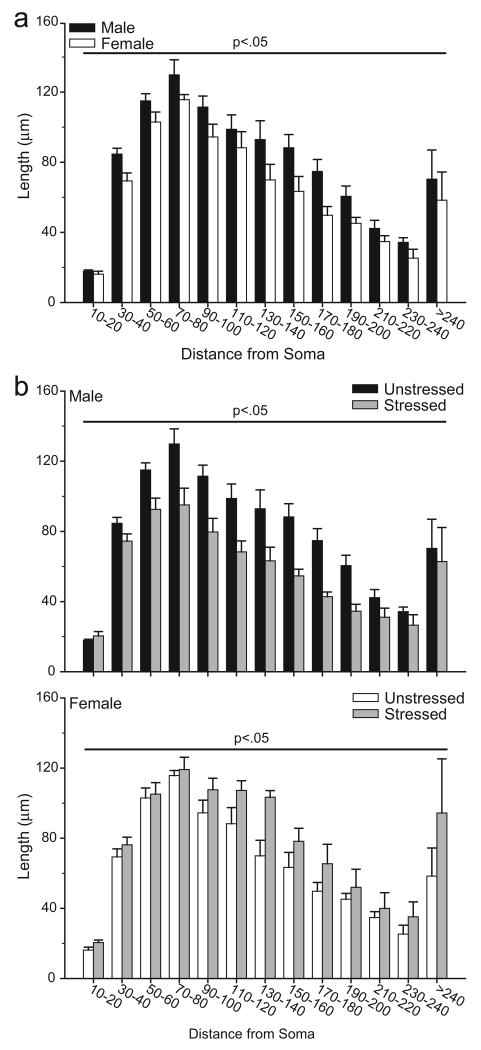
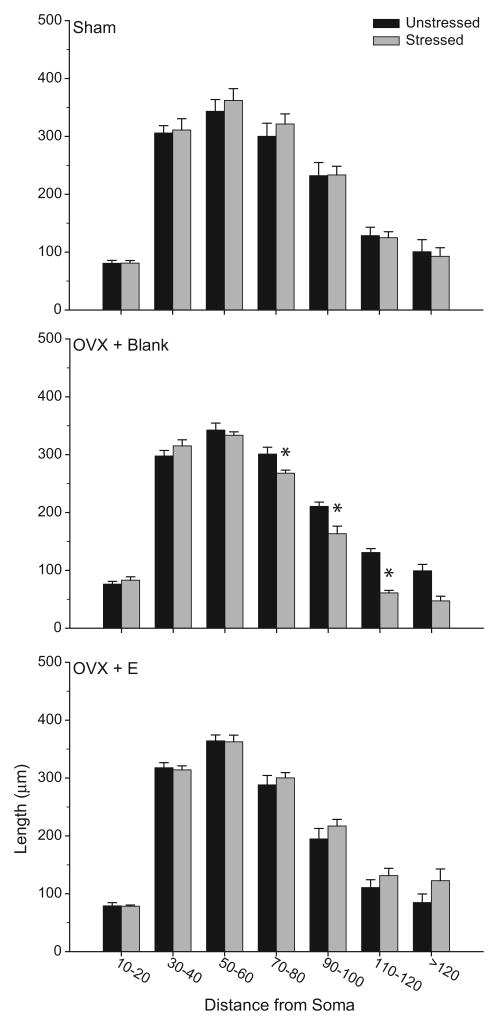
To more closely examine stress-induced changes in the distribution of apical dendritic material, Sholl analyses were performed (see Fig. 5). Three-way repeated-measures ANOVA revealed the effect of stress varied between males and females [main effect of sex, F(1,20) = 0.56, ns; main effect of stress, F(1,20) = 1.22, ns; interaction of sex and stress, F(1,20) = 22.65, p<0.05], and that this interactive effect was uniform across the apical arbor [interaction of either sex or stress with distance from soma, F(12,240) = 0.48 and 0.94, respectively, both ns; interaction of sex, stress, and distance from soma, F(12,240) = 1.18, ns). Given the lack of a three-way interaction, follow-up analyses consisted of 2-way repeated-measures ANOVAs comparing unstressed males versus females, unstressed versus stressed males, and unstressed versus stressed females. Analyses revealed that unstressed females had significantly less apical dendritic material compared to unstressed males [main effect of sex, F(1,10) = 6.78, p<0.05], and that this difference was consistent across the arbor [interaction of sex and distance from soma, F(12, 120) = 0.54, ns]. In males, stress significantly decreased apical dendritic material [main effect of stress, F(1,10) = 12.96, p<0.05], and this effect was again distributed throughout the arbor [interaction of stress and distance from soma, F(12, 120) = 1.73, ns]. Finally, in females, consistent with alterations in overall apical branch length, stress significantly increased apical dendritic material [main effect of stress, F(1,10) = 9.93, p<0.05], and this effect was again relatively uniform across the arbor [interaction of stress and distance from soma, F(12, 120) = 0.69, ns].
Figure 5.
a. Mean length of apical dendrites between 20-μm concentric spheres in male versus female unstressed rats. Increased dendritic material in male relative to female rats was distributed relatively uniformly throughout the arbor. b. Mean length of apical dendrites between 20-μm concentric spheres in unstressed and stressed male (above) and female (below) rats. In both male and female rats, the stress-induced changes in apical dendritic length were relatively uniform throughout the arbor. Vertical bars represent SEM values.
Overall basilar branch number and length did not significantly differ between males and females [Fig. 4; main effect of sex, F(1,20) = 0.38 and 0.08, respectively, both ns]. Furthermore, stress did not significantly alter overall basilar branch number and length [Fig. 4; main effect of stress, F(1,20) = 3.45 and 2.69, respectively, both ns], and this did not vary across sexes [interaction of sex and stress, F(1,20) = 0.18 and 0.08, respectively, both ns]. However, 3-way repeated-measures ANOVA revealed that, although overall basilar material did not vary with sex or stress [main effect of sex, F(1,20) = 0.06, ns; main effect of stress, F(1,20) = 2.78, ns; interaction of sex and stress, F(1,20) = 0.06, ns], the effect of stress varied significantly across the basilar arbor [Fig. 6; interaction of stress with distance from soma, F(6,120) = 2.20, p<0.05; all other interactions with distance from soma, Fs(6,120) ≤ 0.62, ns). Planned comparisons revealed that in male rats, stress resulted in a modest (6 to 15%) but significant increase in basilar length at 30-40 and 50-60 μm from the soma [Fs(1,10) ≥ 6.82, p<0.05; all other Fs(1,10) ≤ 1.73, ns]. Although the same trend was present in female stressed rats, with increases ranging from 14 to 25%, this difference did not reach significance at any distance from the soma [for female unstressed versus stressed rats, all Fs(1,10) ≤ 2.61, ns].
Figure 6.
a. Mean length of basilar dendrites between 20-μm concentric spheres in male versus female unstressed rats. Basilar dendritic length was not significantly different in male and female rats. b. Mean length of basilar dendrites between 20-μm concentric spheres in unstressed and stressed male (above) and female (below) rats. In male rats, stress produced a small but significant increase in basilar dendritic length proximal to the soma. In female rats, a nonsignificant trend towards increased basilar dendritic length was present. Vertical bars represent SEM values. Asterisks (*) indicate significant differences relative to unstressed males.
Experiment 2
Chronic stress resulted in very different patterns of alterations in the morphology of apical dendrites in layer II-II pyramidal neurons in prelimbic cortex of male and female rats: whereas one week of daily restraint resulted in retraction and debranching of apical dendrites in males, this same stressor produced hypertrophy of apical dendrites in females. Previous studies suggest that, in females, estrogens may play a role in protecting neurons from stress-induced morphological alterations (Tanzer and Jones, 1997;Huppenbauer et al., 2005;Hoffman et al., 2006).
Estradiol mediates some of the sex differences in stress effects on hippocampal morphology. For example, after acute stress, CA1 spine density is enhanced in males, but reduced in females. These changes in spine density are correlated with levels of estradiol and testosterone (Shors et al., 2001). In addition, estradiol mediates the sex difference in stress effects on CA3 apical arbors: ovariectomized female rats show apical dendritic retraction in CA3 neurons after 3 weeks of daily restraint stress similar to that seen in males (McLaughlin et al., 2005). Likewise, chronic restraint stress reduces neuronal density in area CA3 in ovariectomized rats, and this effect is prevented with estradiol replacement (Takuma et al., 2007) Thus, estrogens may contribute to sex differences in medial prefrontal cortex stress effects as well. In Experiment 2, we tested this hypothesis by assessing the effect of chronic restraint stress on dendritic morphology in medial prefrontal cortex in ovariectomized rats with and without estradiol replacement.
Experimental Procedures
Animals
Female Sprague-Dawley rats (n=36; 160-183 g, 52-61 days old at the start of the experiment; Harlan, Indianapolis, IN) were housed as described in Experiment 1. All procedures were approved by the Bloomington Institutional Animal Care and Use Committee and were conducted in accordance with NIH guidelines.
Hormonal Manipulations and Restraint Stress
Rats received either sham ovariectomy (sham), ovariectomy plus blank implant (OVX+Blank), or ovariectomy plus estradiol implant (OVX+E). Surgeries were performed under ketamine-acepromazine-xylazine anesthesia 9 days prior to stress manipulations. Bilateral incisions of approximately 2 cm were made approximately 1 cm lateral to the spine and at the same anterior-posterior position as the kidneys. The underlying muscle was cut and the ovaries were located and excised. An empty 20 mm Silastic capsule (1.58mm i.d., 3.18mm o.d., Dow Corning) or a capsule containing 17-β-estradiol (10% estradiol in cholesterol; Steraloids, Inc.; Newport, RI) was implanted subcutaneously anterior to one of the incisions (see Smith et al., 1977). These capsules generate serum levels of estradiol slightly higher than average peak estradiol levels in intact cycling females in the proestrous phase (Sfikakis et al., 1977;Overpeck et al., 1978). Sham surgeries were performed to control for potential dendritic changes due to the stress of surgery alone.
As in the first experiment, one-half of the rats from each group (sham, n=6; OVX+Blank, n=6; OVX+E, n=6) underwent 3 h of daily restraint stress for 7 days as described in Experiment 1. The remainder of the rats in each group (sham, n=6; OVX+Blank, n=6; OVX+E, n=6) served as unstressed controls. All rats were weighed every other day.
Histology and Dendritic Analyses
On the final day of restraint, vaginal lavages were performed in the gonadally intact animals to characterize estrous cycle phase. Samples were collected into Boerner well slides and exfoliate cytology examined immediately (unstained) under light microscopy. Estrous phase was determined based on the morphology of the cells present in the smear. Metestrous smears were characterized by the high density of leucocytes with the occasional cornified cell; diestrous smears consist mainly of leucocytes and occasional nucleated epithelial cells; proestrous was characterized by the high frequency of nucleated epithelial cells; and estrous was characterized by presence of a large number of cornified cells (Fox and Laird, 1970).
All rats were then overdosed and perfused as in Experiment 1. Uteri were removed and weighed to verify the efficacy of the hormonal manipulations. Brains were processed for Golgi histology, and 10 pyramidal neurons in layer II-III of area Cg1-3 were randomly selected, reconstructed, quantified, and compared as described in Experiment 1. In addition, body weight was compared across groups using 3-way ANOVA (hormonal status × stress × day), while uterine weights were compared across groups using 2-way ANOVA (hormonal status × stress). For all analyses, significant ANOVAs were followed up with planned comparisons as described in Experiment 1.
Results
Manipulation Validation
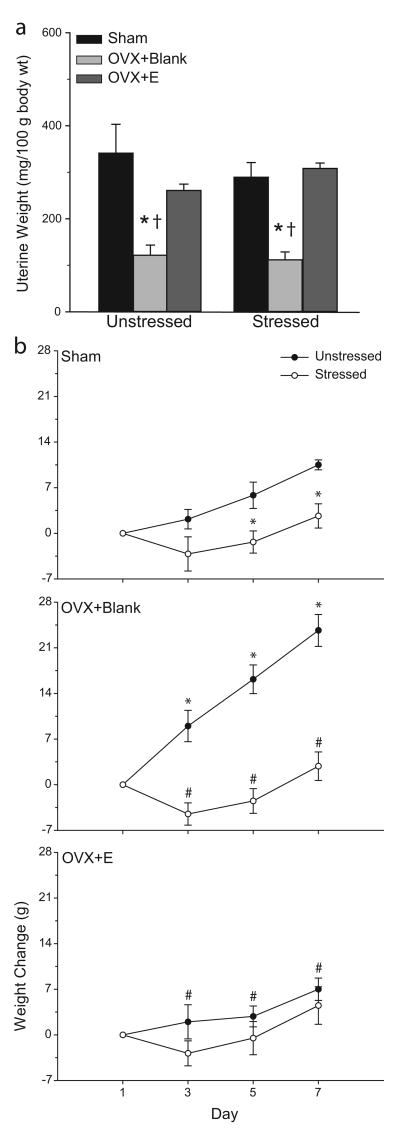
Uterine weight correlates well with concentration of circulating estradiol (White et al., 1978). Two-way ANOVA revealed uterine weight varied significantly across groups [main effect of hormonal status, F(2, 30) = 23.10, p<0.05], and this effect was not altered by stress [main effect of stress, F(1,30) = 0.04, ns; hormonal status × stress interaction, F(2,30) = 1.25, ns]. Planned comparisons revealed that for both unstressed and stressed rats, ovariectomy without estradiol replacement significantly decreased uterine weight relative to both sham and OVX+E rats [see Fig. 7a; all Fs(1,10) ≥ 11.14, p<0.05), while estradiol replacement prevented this effect (sham versus OVX+E, all Fs(1,10) ≤ 1.60, ns].
Figure 7.
a. Mean uterine weights for unstressed or stressed sham, ovariectomized (OVX+Blank), and ovariectomized with estradiol replacement rats (OVX+E). Ovariectomy reduced uterine weight, and estradiol prevented this effect. Vertical bars represent SEM values. Asterisks (*) indicate significant differences relative to sham rats; daggers (†) indicate significant differences relative to OVX+E rats. b. Mean weight change in unstressed or stressed sham (above), ovariectomized (middle), and ovariectomized with estradiol replacement (below) rats. In unstressed rats, ovariectomy increased weight gain, while estradiol replacement suppressed weight gain. Stress decreased weight gain, and weight of stressed rats did not vary significantly across hormonal conditions. Vertical bars represent SEM values. Asterisks (*) indicate significant differences relative to sham unstressed rats; pound signs (#) indicate significant differences relative to unstressed OVX+Blank rats.
Inspection of vaginal lavages indicated that 10 out of 12 of the sham rats were in the diestrous stage at the time of perfusion; one rat was in proestrous and one rat was in estrous. Given the extremely limited representation of animals in different estrous phases, we did not analyze morphology based on estrous cycle.
Body weight also varied significantly with hormonal status [Fig. 7b; main effect of stress, F(2, 30) = 4.62, p<0.05]. Furthermore, as in Experiment 1, by the end of the stress manipulation, the overall average weight gain of unstressed rats was significantly different from that of stressed rats [main effect of stress, F(1, 30) = 36.21, p<0.05]. In addition, the effect of both stress and hormonal status varied across days [interaction of hormonal status and stress, F(2, 30) = 7.58, p<0.05; interaction of hormonal status and day, F(6, 90) = 3.84, p<.05; interaction of stress and day, F(3,90) = 20.52, p<.05; interaction of hormonal status, stress, and day, F(6,90) = 5.30, p<.05]. As demonstrated by planned comparisons, ovariectomy significantly increased weight relative to either sham or OVX+E rats [Fig. 7b; for unstressed OVX+Blank rats versus either sham or OVX+E rats on each day, all Fs(1, 10) ≥ 3.87, p<0.05; for unstressed OVX+E versus sham rats, all Fs(1,10) ≤ 3.48, ns]. For both sham and OVX+blank rats, unstressed animals had greater weight gain than their stressed counterparts [see Fig. 7b; on days 5 and 7, all Fs(1, 10) ≥ 7.40, p<0.05]. Although unstressed versus stressed OVX+E rats demonstrated the same tendency, this did not reach significance at any day [all Fs(1,10) ≤ 2.21, ns]. However, this was due to the attenuated weight gain in the unstressed OVX+E rats rather than to a lack of stress effect: Body weights of stressed rats did not vary significantly across hormonal conditions [all Fs(1,10) ≤ 0.42, ns].
Dendritic Analyses
As in Experiment 1, in all treatment groups, complete impregnation of numerous cortical pyramidal neurons was apparent, and both Cg1-3 and layer II–III were readily identifiable. Likewise, the distance from the soma to the pial surface of the cortex was measured in each neuron (range = 192 μm - 495 μm) and compared across groups using 2-way ANOVA. Average distance to the cortical surface did not vary by hormonal status [F(2, 30) = 0.50, ns] or stress condition [for main effect of stress, F(1, 30) = 0.01, ns; for the interaction of treatment and stress, F(2,30) = 0.73, ns]. Thus, neurons were sampled from equivalent laminar depths across groups.
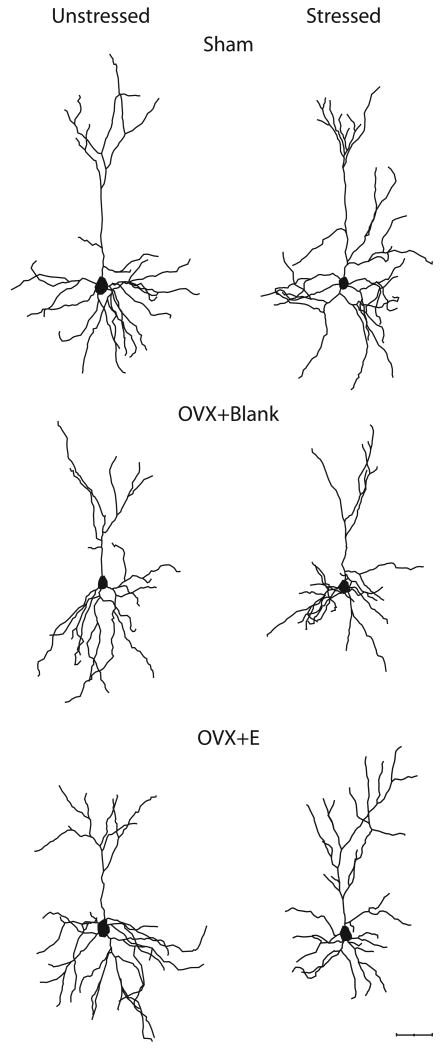
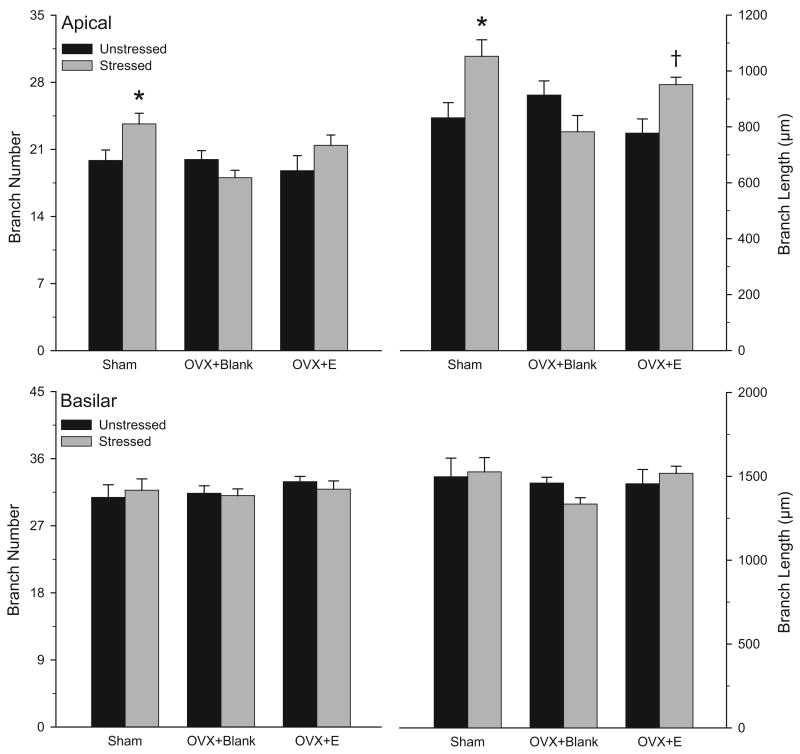
Two-way ANOVAs revealed that the effect of stress on overall apical branch number and length varied significantly across hormonal conditions [see Figs. 8 and 9; apical branch number: main effect of hormonal condition, F(2,20) = 3.12, ns; main effect of stress, F(1,20) = 2.79, ns; interaction of hormonal condition and stress, F(2,30) = 3.69, p<0.05; apical branch length: main effect of hormonal condition, F(2,20) = 1.95, ns; main effect of stress, F(1,20) =4.40, p<0.05; interaction of hormonal condition and stress, F(2,30) = 6.96, p<0.05]. Planned comparisons revealed a 19% increase in apical dendritic branch number in stressed, sham rats relative to unstressed, sham rats [F(1,10) = 6.03, p <0.05], whereas stress failed to significantly alter apical branch number in either OVX+Blank or OVX+E rats [F(1,10) = 2.52 and 1.96, respectively, ns]. Stress increased apical dendritic length by 26% in sham rats and 23% in OVX+E rats [F(1,10) = 7.53 and 9.16, respectively, p <0.05], but failed to significantly alter apical branch length in OVX+Blank rats [F(1,10) = 2.88, ns].
Figure 8.
Computer-assisted reconstructions of Golgi-stained neurons in layer II-III of medial prefrontal cortex in unstressed (left) and stressed (right) sham (top), ovariectomized (OVX+E, middle), and ovariectomized with estradiol replacement (OVX+E, bottom) rats. Scale bar = 50 μm. These neurons were selected because they are representative of apical dendritic lengths near their respective group means.
Figure 9.
Mean number and length of apical (above) and basilar (below) dendritic branches for unstressed or stressed sham, ovariectomized (OVX+Blank), and ovariectomized with estradiol replacement (OVX+E) rats. Ovariectomy prevented the stress-induced increase in apical dendritic length, and estradiol replacement reversed this effect. Vertical bars represent SEM values. Asterisks (*) indicate significant differences relative to unstressed sham rats; dagger (†) indicates significant difference relative to unstressed OVX+E rats.
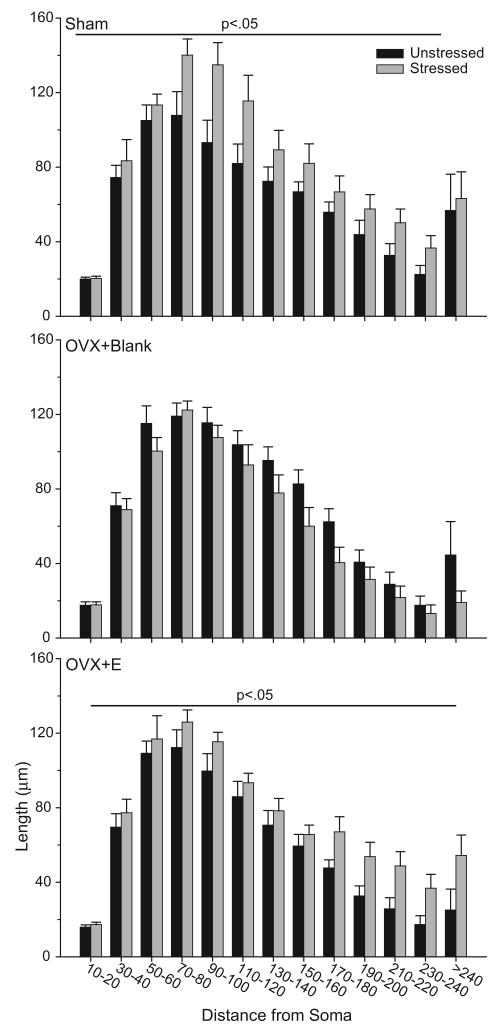
To more closely examine stress-induced changes in the distribution of apical dendritic material, Sholl analyses were performed (Fig. 10). Three-way repeated-measures ANOVA revealed that stress significantly altered the distribution of apical dendritic material, and that this effect varied across hormonal conditions [main effect of stress, F(1,30) = 4.27, p<0.05; main effect of hormonal condition, F(2,30) = 2.15, ns; interaction of hormonal condition and stress, F(2,30) = 7.46, p<0.05]. This interactive effect was uniform across the apical arbor [interaction of hormonal condition with distance from soma, F(24,360) =1.01, ns; interaction of stress with distance from soma, F(12,360) = 0.89, ns; interaction of hormonal condition, stress, and distance from soma, F(24,360) = 0.83, ns). Given the absence of an overall effect of hormonal status or a three-way interaction, follow-up analyses consisted of 2-way repeated-measures ANOVAs comparing unstressed to stressed rats within each hormonal condition. Analyses revealed that stress increased dendritic material in both sham and OVX+E rats [main effect of stress, F(1,10) = 7.51 and 10.87, respectively, p<0.05], and that this reduction was consistent across the arbor [interaction of hormonal condition and distance from soma, F(12, 120) = 0.91 and 0.69, respectively, ns]. On the other hand, in OVX+Blank rats, stress failed to significantly alter apical dendritic material [main effect of stress, F(1,10) = 3.15, ns], and this was again consistent throughout the arbor [interaction of stress and distance from soma, F(12, 120) = 0.92, ns].
Figure 10.
Mean length of apical dendrites between 20-μm concentric spheres in unstressed versus stressed sham (above), ovariectomized without estradiol replacement (middle), and ovariectomized with estradiol replacement rats (below). Stress-induced increases in dendritic material in sham and OVX+E rats were distributed relatively uniformly throughout the arbor. Vertical bars represent SEM values.
Overall basilar branch number and length did not significantly differ with hormonal status [see Fig. 9; main effect of hormonal status, F(2,30) = 0.63 and 1.40, respectively, both ns]. Furthermore, stress did not significantly alter overall basilar branch number and length [main effect of stress, F(1,30) = 0.01 and 0.04, respectively, both ns], and this did not vary with hormonal status [interaction of hormonal status and stress, F(1,20) = 0.35 and 0.96, respectively, both ns]. However, for the Sholl analysis, 3-way repeated-measures ANOVA revealed that, although overall basilar material did not vary with hormonal status [Fig. 11; main effect of hormonal status, F(2,30) = 1.47, ns], the effect of hormonal status varied significantly across the basilar arbor [interaction of hormonal status with distance from soma, F(12,180) = 1.86, p<0.05]. Furthermore, overall basilar material did not vary with stress [Fig. 11; main effect of stress, F(1,30) =0.01, ns; interaction of hormonal status and stress, F(2,30) = 1.28, ns], but the effect of stress varied with hormonal status at particular parts of the arbor [interaction of hormonal status, stress, and distance from soma, F(12,180) = 2.49, p<0.05]. Planned comparisons revealed that this significant interaction was due to a stress-induced decrease in basilar dendritic length distal to the soma in OVX+Blank rats [for 70-80 μm, 90-100 μm, and 110-120 μm from the soma, Fs(1,10) ≥ 6.62, p<0.05; all other Fs(1,10) ≤ 3.71, ns].
Figure 11.
Mean length of basilar dendrites between 20-μm concentric spheres in unstressed versus stressed sham (above), ovariectomized without estradiol replacement (middle), and ovariectomized with estradiol replacement rats (below). The distribution of basilar dendritic material did not differ significantly with stress or hormonal condition. Vertical bars represent SEM values. Asterisks (*) indicate significant differences relative to unstressed OVX+Blank rats.
Discussion
In Experiment 1, we found that the morphology of layer II-III pyramidal neurons in mPFC is sexually dimorphic: apical dendritic arbors of unstressed female rats are smaller than those of unstressed males, with females having both fewer branches and decreased branch length. This difference is consistent with a previous report of smaller apical dendritic arbors in layer V pyramidal cells in anterior cingulate cortex (Markham and Juraska, 2002; Zilles & Wree's Cg1; see Fig. 2a).
In addition to being sexually dimorphic, layer II-III pyramidal neurons in mPFC also responded to chronic stress in a sexually dimorphic fashion. Stress decreased apical dendritic branch number and length in males but increased these parameters in females. Although the mechanisms for this sex difference remain to be elucidated, several possibilities exist. For instance, sex differences in the corticosterone response to stress could be responsible. Female rats have higher baseline levels of corticosterone than males (Galea et al., 1997), and this does not vary across the estrous cycle (Viau and Meaney, 1991;Conrad et al., 2004a). After one hour of restraint, naïve males have a 7-10 fold increase in plasma corticosterone levels (Watanabe et al., 1992;Cook and Wellman, 2004), while females show only a 2-3 fold increase in corticosterone levels (Galea et al., 1997;Bowman et al., 2001). Additionally, female rats may habituate more quickly to restraint compared to males (Bowman et al., 2001;Luine, 2002). However, differences in corticosterone levels alone are unlikely to account for the different pattern of stress effects in males and females, as we have previously shown that a milder stressor that produces only moderate elevations in corticosterone or an acute stressor both produce apical dendritic retraction in males, albeit to a lesser extent (Brown et al., 2005; Izquierdo et al., 2006). Furthermore, stressed males and females showed comparable attenuation of weight gain, suggesting comparable stressfulness of the manipulation.
The stress-induced increase in apical dendritic length in females was dependent on estradiol: females with either sham ovariectomy or ovariectomy plus estradiol replacement demonstrated the stress-induced increase in apical dendritic length, whereas ovariectomy without estradiol replacement prevented the stress-induced increase in apical dendritic length. Vaginal lavages performed at the time of euthanasia indicated that the majority (approximately 83%) of the sham rats were in the diestrous stage at the time of perfusion. Thus, even the low levels of circulating estradiol present during this phase are enough to support the stress-induced dendritic hypertrophy seen in females. Importantly, ovariectomy failed to produce the male pattern of stress effects (a marked reduction in apical branch number and length). Thus, while the stress-induced hypertrophy in females is estradiol-dependent, removal of estradiol is not permissive of the male pattern of stress-induced changes.
The sex difference in stress-induced dendritic changes in medial prefrontal cortex is consistent with the sex-dependent stress effects described in other brain regions. For instance, several studies have shown that chronic stress produces retraction of apical dendrites of pyramidal cells in hippocampal area CA3 (Galea et al., 1997) and decreased spine density on pyramidal cells in hippocampal area CA1 (McLaughlin et al., 2005) in male rats. The same chronic stressor does not affect apical or basilar dendritic length of neurons in the CA3 region of the hippocampus in females (Galea et al., 1997), and results in altered spine morphology but no change in spine density on CA1 pyramidal cells (McLaughlin et al., 2005). Just as the preceding studies indicate that stress effects vary across hippocampal regions in both male and female rats, recent studies suggest both regional and hemispheric variations in the magnitude and patterns of stress-induced dendritic alterations in mPFC subregions, and such variations may have implications for psychopathology (Perez-Cruz et al., 2007;Czéh et al., 2008;Perez-Cruz et al., 2009). Although beyond the scope of the present study, future studies should assess the potential for sex-dependent differences is these regional and hemispheric variations in mPFC stress effects on dendritic morphology.
Furthermore, Experiment 2 demonstrated that estradiol is permissive of the stress-induced dendritic hypertrophy in mPFC of female rats, an effect that is again consistent with the hippocampal literature. For example, the sex difference in chronic stress-induced dendritic retraction in hippocampal area CA3 is estradiol-dependent (McLaughlin et al., 2005). In addition, acute stress-induced reductions in spine density in area CA1 of the hippocampus in females are correlated with circulating levels of estradiol, with acute stress occurring during the low-E diestrus 2 phase producing greater reductions in spine density (Shors et al., 2001). This could be seen as contrasting with the present findings, in which animals, most of which were in diestrous at the time of euthanasia, showed stress-induced dendritic proliferation. This difference could be due to a number of factors, including differential sensitivity to gonadal hormones in hippocampus versus medial prefrontal cortex, differential effects of chronic versus acute stress, or differential regulation of dendritic length versus spine morphology by gonadal hormones. In our study, the extremely limited representation of animals in non-diestrous phases precluded analyses of morphology across estrous phases; thus, future studies should investigate potential differential effects of chronic stress on dendritic morphology in mPFC across the estrous cycle.
Interestingly, unlike in hippocampus (McLaughlin et al., 2005), in mPFC, removal of gonadal estrogens did not produce the dramatic reduction in apical dendritic length seen in stressed males. This raises at least two possibilities: estrogens are permissive of the female pattern of stress-induced mPFC alterations, whereas the presence of androgens (either alone or in conjunction with estrogens) results in the male pattern of stress-induced mPFC alterations; alternatively, the same gonadal hormone—E— mediates both effects via different mechanisms in males and females. Assessing the effects of androgen manipulations in unstressed versus stressed rats could discriminate between the possibilities.
Alternatively, nongonadal mechanisms may also contribute to the sex difference in mPFC stress effects. Indeed, several neurotransmitter systems have been shown to be altered in a sex-dependent manner as a result of stress. For instance, while males show increased GABA release in CA3 following stress, females have increases in norepinephrine and serotonin in CA3 (Bowman et al., 2003). Likewise, males, but not females, have decreased concentrations of dopamine metabolites in prefrontal cortex and amygdala after chronic stress (Bowman et al., 2003). In addition, ovariectomy potentiates the stress-induced decrease in BDNF in hippocampus of female rats, and estradiol replacement prevents this effect (Franklin and Perrot-Sinal, 2006). Thus, differences in alterations in the activity of any of these neurotransmitter systems could play a role in differential effects of stress on dendritic morphology in mPFC in males versus females, and sex differences in alterations in these neurochemicals could be dependent in part on gonadal hormones.
Furthermore, estrogens and androgens can be neurosteroids, and as such can be synthesized de novo from cholesterol in the hippocampus (Baulieu, 1997;Rune and Frotscher, 2005). In Experiment 2, we removed gonadal hormones without altering potential neuronal synthesis of estrogens. Thus, it is possible that estrogens synthesized in the brain—either in mPFC or elsewhere—are providing a neuroprotective effect, preventing the stress-induced dendritic retraction seen in males. However, this is unlikely, as estradiol, as a major metabolite of testosterone, is present in males. Nonetheless, a follow-up study using a receptor antagonist such as tamoxifen could rule out this possibility.
In addition, it is possible that the differential effect of stress seen in the present experiments represents an alteration of the timing rather than pattern of stress-induced dendritic remodeling. For instance, it is possible that dendrites undergo an initial retraction followed by lengthening in response to chronic stress. Such bi- or multi-phasic plasticity has precedence. For example, after denervation, the dendritic arbors of cortical and subcortical neurons undergo initial regression followed by proliferation (Sumner and Watson, 1971;Standler and Bernstein, 1982;Caceres and Steward, 1983). Thus, changing the duration or intensity of restraint, or examining dendritic morphology some time after the cessation of stress, could demonstrate a pattern of mPFC morphological alterations in females similar to that seen here in males. In fact, Bowman and colleagues (2001) showed that while 21 days of restraint stress impaired performance on the radial arm maze in males, it enhanced performance in females. However, after 28 days of stress, the enhancement in females was not present, suggesting that the duration of stress necessary to produce impairment may be shifted in females.
Nonetheless, we have shown a sex-dependent effect of stress on dendritic morphology in medial prefrontal cortex, and have demonstrated that this sex difference is in part dependent on estrogens. The dendritic hypertrophy after stress that we have observed in females could provide a potential explanation for the greater incidence of stress-related psychological disorders in women. It has been hypothesized that the stress-induced dendritic atrophy observed in males serves a protective function, preventing stress-induced excitotoxicity (McEwen, 2001). The stress-induced dendritic hypertrophy in females could put them at greater risk for excitotoxicity, providing a potential explanation for the sex difference observed in stress-related psychological disorders. However, recent data suggest that the stress-induced dendritic retraction in the hippocampus of males may not afford protection from excitotoxicity: Ibotenic acid lesions produce more extensive damage in hippocampus of chronically stressed male compared to unstressed controls, but this increased excitotoxicity is not seen in female stressed rats (Conrad et al., 2004b;Conrad et al., 2007). Alternatively, the hypertrophy seen in mPFC of females could result in dysfunction. For example, dendritic hypertrophy in the infralimbic cortex in serotonin transporter knockout mice is associated with deficits in recall of extinction, a prefrontally mediated behavior (Wellman et al., 2007). Regardless, it is important to further understand the mechanisms of the sex difference seen in mPFC stress effects, as the different patterns of stress-induced dendritic alterations in males and females could ultimately suggest different treatment strategies for men and women.
Acknowledgments
The authors thank Javed Sayed for assistance with data analysis and Dr. Dale R. Sengelaub for guidance on hormonal manipulations and helpful comments on the manuscript. Support provided by MH067607, Indiana University FRSP, and Indiana METACyt Initiative of Indiana University, funded in part through a major grant from the Lilly Endowment, Inc.
Abbreviations
- mPFC
medial prefrontal cortex
- OVX+blank
ovariectomy plus blank implant
- OVX+E
ovariectomy plus 17-β-estradiol implant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiology of Learning and Memory. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Progress in Hormone Research. 1997;52:1–32. [PubMed] [Google Scholar]
- Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Sumida RM. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiat. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Hormones Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Depression. In: Brown GW, Harris TO, editors. Life Events and Illness. New York: Guilford; 1989. pp. 49–93. [Google Scholar]
- Brown SM, Henning S, Wellman CL. Short-term, mild stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Caceres A, Steward O. Dendritic reorganization in the denervated dentate gyrus of the rat following entorhinal cortical lesions: a Golgi and electron microscopic analysis. J Comp Neurol. 1983;214:387–403. [Google Scholar]
- Cajal SRy. Histology of the nervous system. New York: Oxford University Press; 1995. [Google Scholar]
- Carter CS, MacDonald AW, III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatr. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learning Memory. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, et al. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacology Biochemistry & Behavior. 2004a;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wise LS. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neurosci. 2004b;125:759–767. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Czéh B, Perez-Cruz C, Fuchs E, Flügge G. Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: Does hemisphere location matter? Behav Brain Res. 2008;190:1–13. doi: 10.1016/j.bbr.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RR, Laird CW. Sexual cycles. In: Hafez ESE, editor. Reproduction and breeding techniques for laboratory animals. Philadelphia: Lea & Febiger; 1970. pp. 114–115. [Google Scholar]
- Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006;31:38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neurosci. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high-quality Golgi-Nissl stain. J Neurosci Methods. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Hays WL. Statistics. Fort Worth, TX: Harcourt Brace; 1994. [Google Scholar]
- Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;29:217–231. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- Huppenbauer CB, Tanzer L, DonCarlos LL, Jones KJ. Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: equal efficacy of testosterone, dihydrotestosterone, and 17-beta estradiol. J Neurosci. 2005;25:4004–4013. doi: 10.1523/JNEUROSCI.5279-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Rapid dendritic retraction in medial prefrontal neurons and impaired fear extinction following exposure to uncontrollable stress. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- Luine VN, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can't live with it, can't live without it. Behav Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neurosci. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23:579–588. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- Martí O, Martí J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiology and Behavior. 1994;55:747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann NY Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: Possible involvement of CA1 neurons. Neurosci. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. [3H]Dexamethasone binding in rat frontal cortex. Brain Res. 1985;328:176–180. doi: 10.1016/0006-8993(85)91340-x. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learning Memory. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychological Bulletin. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Overpeck JG, Colson SH, Hohmann JR, Applestine MS, Reilly JF. Concentrations of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkeys, rats, mice, and hamsters: a literature survey. J Toxicol Environ Health. 1978;4:785–803. doi: 10.1080/15287397809529700. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Perez-Cruz C, Muller-Keuker JIH, Heilbronner U, Fuchs E, Flugge G. Morphology of pyramidal neurons in the rat prefrontal cortex: lateralized dendritic remodeling by chronic stress. Neural Plasticity 2007. 2007 doi: 10.1155/2007/46276. Article ID 46276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cruz C, Simon M, Czéh B, Flügge G, Fuchs E. Hemispheric differences in basilar dendrites and spines of pyramidal neurons in the rat prelimbic cortex: activity- and stress-induced changes. European Journal of Neuroscience. 2009;29:738–747. doi: 10.1111/j.1460-9568.2009.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinn VW. Research on women's health: progress and opportunities. JAMA. 2005;294:1407–1410. doi: 10.1001/jama.294.11.1407. [DOI] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: Role in synaptic plasticity. Neurosci. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Sfikakis A, Spyraki C, Sitaras N, Varonos D. Implication of the estrous cycle on conditioned avoidance behavior in the rat. Physiol Behav. 1977;21:441–446. doi: 10.1016/0031-9384(78)90105-1. [DOI] [PubMed] [Google Scholar]
- Sholl DA. The Organization of the Cerebral Cortex. London: Methuen; 1956. [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;27:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Damassa DA, Davidson JM. Hormone administration: peripheral and intracranial implants. In: Meyer RD, editor. Methods in Psychobiology. Vol. 3. New York: Academic Press; 1977. pp. 259–279. [Google Scholar]
- Standler NA, Bernstein JJ. Degeneration and regeneration of motoneuron dendrites after ventral root crush: computer reconstruction of dendritic fields. Experimental Neurology. 1982;75:600–615. doi: 10.1016/0014-4886(82)90028-0. [DOI] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behavioral Research and Therapeutics. 2000;38:619–628. doi: 10.1016/s0005-7967(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Sumner BEH, Watson WE. Retraction and expansion of the dendritic tree of motor neurones of adult rats induced in vivo. Nature. 1971;233:273–275. doi: 10.1038/233273a0. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Koeda M, Oda K, Matsuda T, Matsushima E, Matsuura M, Asai K, Okubo Y. An fMRI study of differential neural response to affective pictures in schizophrenia. NeuroImage. 2004;22:1247–1254. doi: 10.1016/j.neuroimage.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Takuma K, Matsuo A, Himeno Y, Hoshina Y, Ohno Y, Funatsu Y, Arai S, Kamei H, Mizoguchi H, Nagai T, Koike K, Inoue M, Yamada K. 17[beta]-estradiol attenuates hippocampal neuronal loss and cognitive dysfunction induced by chronic restraint stress in ovariectomized rats. Neurosci. 2007;146:60–68. doi: 10.1016/j.neuroscience.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Tanzer L, Jones KJ. Gonadal steroid regulation of hamster facial nerve regeneration: effects of dihydrotestosterone and estradiol. Experimental Neurology. 1997;146:258–264. doi: 10.1006/exnr.1997.6529. [DOI] [PubMed] [Google Scholar]
- Ventura J, Neuchterlein KH, Lukoff D, Hardesty JD. A prospective study of stressful life events and schizophrenic relapse. J Abnormal Psychol. 1989;98:407–411. doi: 10.1037//0021-843x.98.4.407. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Leaf PJ, Tischler GL, B DG, Karno M, Bruce ML, et al. Affective disorders in five United States communities. Psychological Medicine. 1988;18:41–153. doi: 10.1017/s0033291700001975. [DOI] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knockout mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JO, Thrower S, Lim L. Intracellular relationships of the oestrogen receptor in the rat uterus and hypothalamus during the oestrous cycle. Biochemistry Journal. 1978;172:37–47. doi: 10.1042/bj1720037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Zilles K, Wree A. Cortex: areal and laminar structure. In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 1995. pp. 649–685. [Google Scholar]