In November 2007, a large multi-national trial called STEP, evaluating the lead candidate in what the HIV vaccine field termed T cell based vaccines, was halted at its first interim analyses because of the vaccine’s lack of efficacy 1, 2. The vaccine candidate used in STEP included a replication incompetent Adenovirus type 5 (Ad5) viral vector, and the objectives of the trial were to investigate whether this candidate vaccine was able to reduce HIV acquisition or to modulate viral load 3. This vaccine prototype first entered human clinical trials in 2000. The initial clinical studies resulted in several modifications to the vector’s promoters as well as an attempt to increase the breadth of the host response by the addition of the pol and nef genes to the original gag construct. The final vaccine candidate which contained clade B sequences of gag/pol and nef genes (MRK Ad5 gag/pol/nef) underwent an extensive series of clinical trials between 2003–2006 which demonstrated detectable T cell immunogenicity as measured by the ELISpot assay in about 80% of recipients with a median magnitude ranging 275–300 sfu/million PBMCs, a frequency and magnitude greater than any other T cell based approach at the time of the initiation of the STEP trial in 2005 4. More detailed analyses of the T cell responses indicated that >50% of the persons with detectable ELISpot responses after vaccination exhibited CD8+ T cells to HIV antigens and 50% exhibited CD4 positive responses. Durability of the responses was prolonged (>6 months post last vaccination) 5, 6. Thus, the lack of success of the vaccine, especially on controlling post infection viral load (setpoint viremia) among vaccinated individuals who became infected has sent reverberations throughout the scientific community for its implications on our ability to develop a globally effective HIV vaccine.
The STEP trial involved 3,000 healthy, uninfected volunteers who were randomized to receive a placebo or a vaccination. Vaccine recipients in the STEP trial were stratified by their prior exposure to adenovirus into those who were considered seronegative to type 5 adenovirus (<18), and those with low (18–200), moderate (200–1000) and high (>1,000) antibody titers to Ad5 at enrollment; a stratification based upon the above phase 1 clinical trials that suggested prior immunity to the vector reduced the vaccine’s immunogenicity.7 Vaccination was given at 0, 1 and 6 months and all enrollees were followed for HIV infection over time. Those who became HIV infected had their infection confirmed and several measurements of their viral load measured over the following 3–6 months. At the time of the analysis of the trial in November 2007:8 84 cases of HIV infection had occurred over the course of the study; 82 of the 84 cases occurred in men, about all of whom acquired HIV through sexual activity with other men. One of the disconcerting and unanticipated findings from the trial was that among men who entered the trial with prior adenovirus type 5 infection (AD5 titers >18), the incidence of HIV acquisition was 2-fold higher among vaccinated versus placebo recipients (Table 1). In contrast, the acquisition rate of HIV was identical among men who entered the trial seronegative for Ad5 (relative incidence vaccine to placebo of 1.0). A more recent multivariate analysis has shown that uncircumcised men, especially those who were Ad5 seropositive, had as much as a 4-fold higher rate of acquisition of HIV infection (95% CI 1.3–11); suggesting a statistically increased rate of acquisition in this subgroup of uncircumcised men who were Ad5 seropositive, especially when compared with the lack of increased risk among circumcised men with no pre-existing Ad5 immunity.9
Table 1.
Incidence (95% CI) per 100 person years of HIV Infection MITT population (males)
| Baseline Ad5 titer | Vaccine V | Placebo P | Relative Incidence (V:P) |
|---|---|---|---|
| ≤ 18 | 4.0 (2.5, 6.3) | 4.0 (2.5, 6.2) | 1.0 (0.5, 2.0) |
| 19–200 | 4.4 (1.9, 8.8) | 2.2 (0.6, 5.5) | 2.1 (0.6, 9.3) |
| 201–1000 | 6.1 (3.3, 10.2) | 3.0 (1.2, 6.2) | 2.0 (0.8, 5.9) |
| >1000 | 4.4 (1.8, 9.1) | 1.2 (0.2, 4.5) | 3.5 (0.7, 35.0) |
|
| |||
| ≤18 | 4.0 (2.5, 6.3) | 4.0 (2.5, 6.2) | 1.0 (0.5, 2.0) |
| >18 | 5.1 (3.4, 7.3) | 2.2 (1.2, 3.8) | 2.3 (1.1, 4.7) |
|
| |||
| ≤ 200 | 4.2 (2.8, 6.0) | 3.5 (2.3, 5.2) | 1.2 (0.7, 2.1) |
| > 200 | 5.4 (3.3, 8.2) | 2.3 (1.0, 4.3) | 2.4 (1.0, 5.8) |
|
| |||
| Overall | 4.6 (3.4, 6.1) | 3.1 (2.1, 4.3) | 1.5 (0.9, 2.4) |
18 is the LOQ for the Ad5 titer assay; includes all HIV cases thru Oct 17, 2007
Recently, the first data from the companion Phambili trial have become available.10 This trial was conducted in South Africa and used the same clade B based vaccine, to investigate whether the MRK Ad5 gag/pol/nef vaccine would demonstrate efficacy in a clade C region.. The trial enrolled 801 participants before it was stopped. Of the 11 acquisitions reported to date, 9 were among those seropositive to Ad5. HIV acquisition occurred in 6 who received the vaccine and 3 who received placebo; the 2 HIV infections in the Ad5 seronegatives at entry were split 1 and 1 in each group; ten of the 11 HIV infections were in women. These very preliminary results from South Africa continue to provide evidence that pre-existing immunity to the Ad5 vector was an independent risk factor in the increased role of HIV acquisition among vaccine recipients. The placebo in these clinical trials was a saline solution, thus limiting the ability to evaluate the impact of the empty Ad5 vector on HIV-1 acquisition. This increased risk for HIV-1 acquisition among persons entering the trial with prior Ad5 immunity was unanticipated and not encountered in any non-human primate (NHP) challenge experiments using adenovirus vectors11–14.
Why didn’t such a highly immunogenic vaccine control post infectious viral load and perhaps more importantly how did prior Ad5 infection increase the frequency of HIV acquisition; an observation that has implications for other vaccines using adenovirus vectors? Were immune responses elicited by the vaccine lower in subjects who became infected as compared to those who did not become infected? Was the quantity, quality, or breadth of the immune responses suboptimal? While definitive answers to these issues are not yet available, several procedures built into the STEP trial have started to provide some data of relevance to these issues.
ELISpot responses were measured in a random sample of 25% of all recipients at week 8, four weeks post second dose of vaccine; the time period at which phase 1 studies had shown near peak ELISpot responses. These analyses indicate that the frequency of responses as measured by ELISpot in PBMC was similar between vaccinated persons who subsequently developed HIV infection versus those who did not. Among Ad5 seronegatives these responses were 74% vs. 76%for gag, 63% vs. 73% for pol, and 74% vs. 70% for nef for infected versus noninfected vaccine recipients, respectively.9 The ELISpot geometric mean titers (GMT) were also similar in the two groups (~350 SFU/million PBMC). Interestingly, ELISpot responses in the STEP trial in persons who had pre-existing immunity to Ad5 were somewhat lower than those in the earlier phase I trials (GMT ELISpot responses ~170/SFC/million PBMCs) 4, 8, 11. However, again no discernible differences were seen between those seropositive vaccinees who developed HIV vs. those who did not, e.g., the ELISpot responses to gag were 46% and 54% among those who subsequently acquired HIV versus those who remained uninfected during follow-up, respectively. Interestingly, over 50% of the HIV specific CD8 T cells elicited after vaccination produced γIFN alone and did not exhibit IL-2 or TNF responses, suggesting they exhibited a less polyfunctional phenotype 9, 15. While these data are still preliminary, they clearly indicate that the MRK gag/pol/nef vaccine was immunogenic, that vaccinated persons who acquired HIV-1 did respond to the vaccine and no measurable differences in the overall quality, quantity or magnitude of the response to vaccination have as yet been defined between vaccine recipients who subsequently developed HIV versus those who did not. Thus, there appears to be no obvious correlate between immune responses to the vaccination and the acquisition of HIV infection. Perhaps this is not surprising, in that most authorities in the field (including these coauthors) felt there was little likelihood that a vaccine containing just gag-pol and nef genes would reduce HIV acquisition. Until an immunogen that elicits high levels of neutralizing antibodies is developed, most authorities feel the most likely effect of T cell based vaccines will be on controlling post infectious viral load and lower mucosal tissue titers of HIV-1.
NHP data has indicated that T cell based vaccines are more likely to control the post infection course of HIV infection 13–16. The analyses evaluating the association between the magnitude or quality of the T cell response and post infection viral load is just being initiated in STEP trial participants. While it is apparent that on a group basis there was little difference in setpoint viremia and post acquisition viral load, data analyses are proceeding to see if a subgroup of persons, e.g., those with very high responses to the vaccines had overall lower loads of setpoint viremia. As control of setpoint viremia was hypothesized to be the most likely endpoint for the study, we must await these analyses before one can determine if the STEP trial provides us with some leads on whether T cell responses after vaccination influenced viral load or CD4 cell count depletion post HIV acquisition.
Epitope Specific Responses
One of the central hypotheses of the STEP trial was that T cell responses to the conserved regions of the HIV gag-pol and nef genes would be elicited, and that the immune responses elicited by the vaccine would “match” the strains circulating in the population and result in reduced HIV acquisition or disease progression 17, 18. At present, data defining whether the epitopes elicited by vaccination are commonly found in the infecting strains are not available. In other words, is the lack of vaccine efficacy related to the lack of epitope coverage from the vaccine? Available data from phase 1 studies indicated an average of one epitope per gene product in most vaccine recipients.16 This in itself is of interest in that biometric analysis of the sequence data from the gag, pol and nef genes in the MRK vaccine indicates that there are potentially over 150 epitopes contained in the gag vaccine insert and over 50 in pol and nef.19 Yet, immunodominance is displayed in nearly all people. 20 The initial data on epitope mapping from the phase 1/2 trials suggest that most of the gag epitopes elicited from vaccination are present in circulating strains in Clade B populations, but for multiple epitopes the coverage may be as low as 30% of circulating strains. 19, 21 This narrowness of the immune response may be an important factor in the vaccine’s overall lack of efficacy. Studies on full length sequences of infecting isolates from the 82 cases are underway by Drs. Francine McCutchen and James Mullins, and the sequences of the infecting isolates will be analyzed with those epitopes that were elicited prior to and after infection. Additional evaluations will focus on the viral load levels among persons who made significant numbers of CD8+ T cells that “matched” the infecting strain of HIV-1 versus those who did not. Designing and developing T cell vaccines that elicit T cell responses to multiple conserved regions of HIV should be a major area of emphasis for subsequent T cell based vaccines.
Several biometric proposals to design inserts that are more likely to elicit a diverse set of epitopes to circulating strains have, in the last 12 months, been described 22–24. Whether such an approach will overcome the immunodominance one sees with the current viral vector vaccines is unclear. What is clear is that strategies must be developed to elicit greater epitope breadth to provide more optimal T-cell responses to subdominant responses which may provide more optimal matching of vaccine elicited T-cell responses to the diverse array of circulating strains of HIV.21 Immunization with a variety of peptides matching defined CD8 epitopes would be an attractive approach for accomplishing such a goal. However, to date, peptide based vaccines have not been shown to be immunogenic in humans, either when given as protein or with a viral vector such as a poxvirus.25 It may be that alternative approaches to achieve breadth, e.g., protein subunits conjugated to adjuvants capable of eliciting CD8+ T cell responses may be necessary.26 Recent evidence has shown that continued boosting with homologous vectors changes the T cell response to vaccination and appears to also enhance immunodominance.27 Strategies to use more complex heterologous vectors with heterologous inserts given at separate sites and times should be investigated. Such approaches require more complex vaccination regimens and combinations of vectors; but data are present that such an approach will likely be required to improve T cell breadth. 28
Assessing the “quality” of Vaccine Induced T Cell Responses
Recently several groups have stressed that T cells that exhibit a variety of functions (polyfunctional cells) appear to be more effective in controlling experimental viral infections than cells that exhibit a more selective phenotype 29. Some studies on the clinical course of HIV-1 have associated T cells, especially CD4+ T cells exhibiting both IL-2 and IFNγ responses with better control of disease 30, 31. Polyfunctional T cells have been associated with protection from Leishmania infection and have been elicited by vaccination with vaccinia and yellow fever vaccination 32–34. The techniques for describing these polyfunctional cells are based on surface marker expression, and at present studies define “associations” between such cells and do not provide mechanistic proof of phenotypic function. Unfortunately, an adoptive transfer model to presently define what type of polyfunctional cells are most likely to control HIV replication has not been devised. Thus, quantitative methods to define what types of immune functions are associated with control of HIV replication are needed. Complicating this issue are data to show that innate immune responses influence both the qualitative and quantitative features of adoptive immunity 12. Hence, even if the required responses are well defined, eliciting such responses may require considerable empiricism. NHP studies may be useful in advancing knowledge about these questions and provide direction for future immunogen design and testing. However, at present, only human clinical trials are likely to provide insight into defining whether a particular type of response is effective in modifying HIV-1 replication.
Increased Acquisition of HIV in Persons Who Entered the STEP Trial with Proven Ad5 Immunity
The most surprising finding from the STEP trial was the 2-fold increased risk of HIV acquisition among Ad5 seropositive persons, an effect not seen in any prior NHP studies of Ad virus vectors. Whether this risk is biologic or confounded by behavior or other factors such as geographic clustering of cases or infection with strains of unusual virulence is as yet unclear. There were distinct differences in Ad5 seroprevalence between sites; Ad5 seronegative subjects were two times more common in North America than in South America or the Caribbean. However, among placebo recipients, infection rates were higher in Ad5 seronegative than seropositive persons, albeit not statistically different (Table 2).
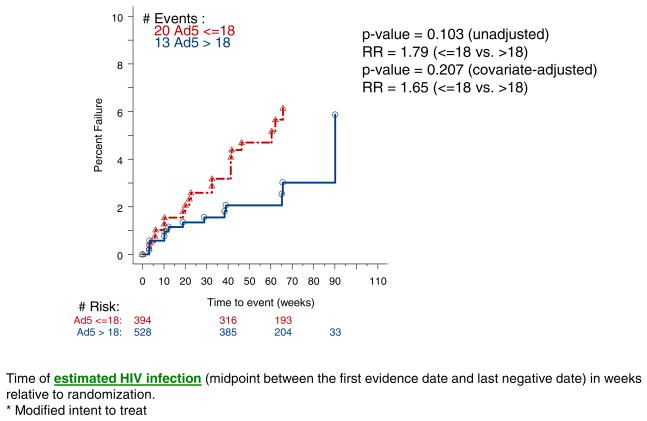
At present, some degree of uncertainty still exists about whether the increased acquisition in Ad5 seropositives who received the vaccine has an underlying biologic mechanism. However, as shown in Figure 1, among placebo recipients there was no statistically significant increase in HIV acquisition between Ad5 seropositive versus Ad5 seronegative men and that in both univariate and multivariate analyses. No increase in HIV acquisition was seen among Ad5 seronegative men or women who received the vaccine. These latter data indicate that trials of adenovirus vector vaccines for HIV (as well as other diseases in HIV at risk populations) can be safely conducted in Ad5 seronegative persons.
Figure 1.
Kaplan-Meier Plot (% Failure) For Subjects who Received Placebo: MITT* population (males)
Several hypotheses for explaining the increased susceptibility to infection after vaccination among Ad5 seropositive versus seronegative persons have been put forth; these include.
Receipt of the vaccine boosted adenovirus specific T cells (especially CD4+ T cells and especially at mucosal sites) among persons with previous immunity to the vector. This increase in activated target cells resulted in increased susceptibility to infection following high risk sexual behavior.
Prior Ad5 immunity “skewed” the immune response to the vector and reduced innate immune response to HIV-1 infection and hence led to higher acquisition rates in sexually exposed men.
Prior immunity produced some “enhancing” antibodies which led to increased susceptibility to infection.
Additional explanations or a combination of all three hypotheses are also possible. One of the intriguing factors about the increased rate of acquisition is that 100% of the HIV acquisition in the STEP study occurred after the second dose of vaccine. Thus, most of the adenovirus seronegative persons had likely seroconverted to adenovirus and exhibited evidence of adenovirus specific CD4+ and CD8+ T cell responses by the time of HIV-1 acquisition. Any mechanistic explanations for the difference in acquisition rate of HIV between Ad5 seropositive versus Ad5 seronegative must account for this observation. One suggested explanation may be due to “imprinting” of a certain phenotype, e.g., homing to mucosal sites from natural mucosal Ad5 infection rather than from IM injection of the replication incompetent vector. Regardless of the eventual explanation, one of the lessons learned from the STEP trial is the need to assess mucosal and tissue specific immune responses to both the vector and HIV vaccine inserts in future clinical trials.
The collaborators in the STEP and Phambili trials (HVTN and Merck research labs) have initiated several modifications to the ongoing STEP and Phambili trials to attempt to answer the above questions. Biopsies of mucosal tissue sites among vaccinees are underway to evaluate whether the quantity or quality of T cells present in such sites differ between those with naturally acquired Ad5 infection versus those with Ad5 immunity induced only by vaccination. As most of the STEP recipients had received their last vaccination 6–11 months prior to these mucosal studies, this approach, while necessary, may not be optimal. Ideally, a prospective trial to evaluate such issues should be conducted to provide optimal information regarding the tissue specific T cell responses after adenovirus vaccination. As defining a potential mechanism for acquisition is essential for the continued development of adenovirus based vectored vaccines, we hope the availability of the immunogens to perform such investigations will be forthcoming. It is clear that for the T cell vaccine field answering these questions are of fundamental importance. As such, the trial sponsors, Merck Research Labs, NIAID and the HVTN have also established a scientific review committee to lead the scientific program being developed to evaluate these issues. The outside investigative community has been asked to participate in a process to submit ideas for funding. The application process and review is described on the HVTN website (www.HVTN.org).
Summary
The STEP trial has been a milestone event in the area of T cell based vaccines. It has resulted in several unique scientific contributions to the HIV field. These include the use of test of concept trial for defining vaccine efficacy for prototype vaccines; evidence that HIV 89.6P challenge in genetically sensitive animals (MAMU-A*01 or B*08) should not be used to gauge vaccine effectiveness and that vector induced immunity should be tightly evaluated in the course of immunogen development. While the STEP trial clearly did not produce an effective vaccine, the critical isolates and immunologic assays that will define if there are correlates between host responses to vaccination and post infection control of viremia are just being analyzed. These data will be central to resetting the immunological measurements that should be made on future vaccine candidates.
Acknowledgments
Supported in part by NIH grant AI-46747
References Cited
- 1.Press release from Merck and the HIV Vaccine Trials Network. Whitehouse Station NJ; Seattle, WA: Sept 21, 2007. Vaccination and enrollment are discontinued in phase II trials of Merck’s investigational HIV vaccine candidate. Interim Analysis of STEP Study Shows Vaccine was not Effective. [Google Scholar]
- 2.Cohen J. AIDS research. Promising AIDS vaccine’s failure leaves field reeling. Science. 2007 Oct 5;318(5847):28–29. doi: 10.1126/science.318.5847.28. [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra DV, Li X, Gilbert PB. A comparison of eight methods for the dual-endpoint evaluation of efficacy in a proof of concept HIV vaccine trial. Biometrics. 2006;62:893–900. doi: 10.1111/j.1541-0420.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 4.Priddy F, Brown D, Kublin J, et al. Safety and immunogenicity of a replication-incompetent adenovirus type-5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 5.Fu TM, Dubey SA, Mehrotra DV, et al. Evaluation of cellular immune responses in subjects chronically infected with HIV type 1. AIDS Res Hum Retroviruses. 2007;23(1):67–76. doi: 10.1089/aid.2006.0114. [DOI] [PubMed] [Google Scholar]
- 6.Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 7.Isaacs R. AIDS Vaccine. Lausanne; 2004. Impact of pre-existing immunity on the immunogenicity of adenovirus serotype 5-based vaccines. [Google Scholar]
- 8. [Accessed 4/24/08];HIV Vaccine Trials Network 2007 Full Group Meeting. Nov 7; http://www.hvtn.org/science/1107.html.
- 9.Buchbinder S. Efficacy Results from the Step Study (Merck V520 Protocol 023/HVTN 502) A Phase II Test-of-Concept Trial of the MRKAd5 HIV-1 Gag/Pol/Nef Trivalent Vaccine. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 10.NIAID Bulletin: An Update Concerning the HVTN 503/Phambili HIV Vaccine Study. http://www3.niaid.nih.gov/news/newsreleases/2008/hvtn503_update.htm.
- 11.Shiver JW, Fu TM, Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002 Jan 17;415(6869):331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 12.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008 Jan 21;205(1):7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casimiro DR, Wang F, Schleif WA, et al. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J Virol Dec. 2005;79(24):15547–15555. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch DH, Yang ZY, Kong WP, et al. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol Jul. 2005;79(14):8828–8834. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther Feb. 2005;16(2):149–156. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- 16.Emini EA. Keystone. Banff; 2003. A Potential HIV-1 Vaccine Using a Replication-Defective Adenoviral Vaccine Vector. [Google Scholar]
- 17.Johnston MI, Fauci AS. An HIV vaccine--evolving concepts. N Engl J Med. 2007 May 17;356(20):2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- 18.Duerr A, Wasserheit JN, Corey L. HIV vaccines: new frontiers in vaccine development. Clin Infect Dis. 2006;43:500–511. doi: 10.1086/505979. [DOI] [PubMed] [Google Scholar]
- 19.Li F, McKenney DM, Malhotra U, et al. Towards prediction of degenerate CTL epitope recognition. Hum Vaccin. 2007;4(2) doi: 10.4161/hv.4.2.5215. (epub ahead of print October 26) [DOI] [PubMed] [Google Scholar]
- 20.De Rosa SC, Thomas EP, Huang Y, et al. Kinetics of T cell responses to vaccination for HIV and heterologous DNA primar-rAd5 boost contrasted with homologous rAd5 prime-boost. Keystone Symposia; Banff, Alberta, Canada. 2008. (Abstract #161) [Google Scholar]
- 21.Frahm N, Kiepiela P, Adams S, et al. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat Immunol Feb. 2006;7(2):173–178. doi: 10.1038/ni1281. [DOI] [PubMed] [Google Scholar]
- 22.Nickle DC, Rolland M, Jensen MA, et al. Coping with viral diversity in HIV vaccine design. PLoS Comput Biol. 2007 Apr 27;3(4):e75. doi: 10.1371/journal.pcbi.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, Horton H, Gilbert PB, et al. HIV-1 CTL-based vaccine immunogen selection: antigen diversity and cellular response features. Curr HIV Res. 2007;5:97–107. doi: 10.2174/157016207779316260. [DOI] [PubMed] [Google Scholar]
- 24.Letourneau S, Im EJ, Mashishi T, et al. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS ONE. 2007;2(10):e984. doi: 10.1371/journal.pone.0000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorse GJ, Baden LR, Wecker M, et al. Safety and Immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 2008;26:215–223. doi: 10.1016/j.vaccine.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 26.Wille-Reece U, Wu CY, Flynn BJ, et al. Immunization with HIV-1 gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 gag-specific TH1 and CD8+ T cell responses. J Immunol. 2005;174:7676–7683. doi: 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
- 27.Smith CL, Mirza F, Pasquetto V, et al. Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. J Immunol. 2005 Dec 15;175(12):8431–8437. doi: 10.4049/jimmunol.175.12.8431. [DOI] [PubMed] [Google Scholar]
- 28.Koopman G, Mortier D, Hofman S, et al. Vaccine protection from CD4+ T-cell loss caused by simian immunodeficiency virus (SIV) mac251 is afforded by sequential immunization with three unrelated vaccine vectors encoding multiple SIV antigens. J Gen Virol Oct. 2004;85(Pt 10):2915–2924. doi: 10.1099/vir.0.80226-0. [DOI] [PubMed] [Google Scholar]
- 29.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 30.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J Immunol. 2005;174:1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 32.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med Jul. 2007;13(7):843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 33.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006 Feb 24;124(4):849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Querec T, Bennouna S, Alkan S, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006 Feb 20;203(2):413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]