Abstract
Purpose
In vitro, in vivo, and epidemiologic studies support a role for selenium in reducing the risk of prostate cancer. Our group previously demonstrated a strong interaction between plasma selenium and the manganese superoxide dismutase (SOD2) gene and incident prostate cancer risk. We now hypothesized that SOD2 modifies the association between selenium level and risk of aggressive prostate cancer at diagnosis.
Patients and Methods
We assessed SOD2 variants and plasma selenium in 489 patients with localized/locally advanced prostate cancer from an ongoing retrospective cohort. Cross-sectional associations with aggressive prostate cancer (ie, stage T2b-3, prostate-specific antigen > 10 ng/mL, or biopsy Gleason score ≥ 7) were evaluated using the χ2 test, Cochran-Armitage test for trend, and estimations of relative risk (RR) and 95% CIs.
Results
SOD2 genotype alone was not associated with disease aggressiveness, whereas higher versus lower selenium levels were associated with a slightly increased likelihood of presenting with aggressive disease (RR = 1.35; 95% CI, 0.99 to 1.84). There was evidence of an interaction between SOD2 and selenium levels such that among men with the AA genotype, higher selenium levels were associated with a reduced risk of presenting with aggressive disease (RR = 0.60; 95% CI, 0.32 to 1.12), whereas among men with a V allele, higher selenium levels were associated with an increased risk of aggressive disease (for VV or VA men, RR = 1.82; 95% CI, 1.27 to 2.61; P for interaction = .007).
Conclusion
These data suggest that the relationship between circulating selenium levels at diagnosis and prognostic risk of prostate cancer is modified by SOD2 genotype and indicate caution against broad use of selenium supplementation for men with prostate cancer.
INTRODUCTION
Experimental and epidemiologic studies indicate that selenium may reduce prostate cancer risk, potentially through inhibition of cellular proliferation, apoptosis, antiangiogenesis, or antioxidant pathways.1–22 A protective effect of higher versus lower endogenous selenium levels on incident prostate cancer risk has been reported in large observational prospective studies.1,2,4,5,7–10,14,17 In the Nutritional Prevention of Cancer Trial, secondary data analyses revealed a 50% reduction in incident prostate cancer risk with daily supplementation of 200 μg of selenium (mean follow-up time = 7.4 years).6,23 A few studies have reported that the inverse association for endogenous selenium and prostate cancer risk was more pronounced among smokers, multivitamin users, and men with high vitamin E intake or circulating level1,8,17 or who had higher diagnostic stage or prostate-specific antigen (PSA) level.9,14
In contrast, the Selenium and Vitamin E Cancer Prevention Trial (SELECT) recently ended early (5.5 years of median follow-up) based on convincing interim analyses that indicated that neither selenium nor vitamin E, alone or in combination, was effective (at the tested doses and formulations) for the primary prevention of prostate cancer.24,25 Although this trial provided strong evidence for a null association for oral supplementation with l-selenomethionine 200 μg/d and overall prostate cancer risk, it did not address the questions of whether higher selenium levels affect the risk of incident advanced-stage prostate cancer or risk of progression in men who already have prostate cancer and how genetics may modify associations between selenium and prostate cancer. On the basis of this current report and our previous reports,26 it is possible that selenium intervention for prostate cancer may still be important for men of specific genotypes or with specific tumor phenotypes, and further understanding of gene-diet interactions may help elucidate findings from SELECT.
Our collaborative group previously demonstrated a strong interaction between plasma antioxidants and a gene variant in manganese superoxide dismutase (SOD2) and incident prostate cancer risk.26 SOD2 is an endogenous mitochondrial enzyme that metabolizes reactive oxygen species and superoxide anions to oxygen and hydrogen peroxide. The latter is further converted to water via catalase, glutathione peroxidase (GPX), or peroxiredoxin, and GPX and peroxiredoxin are selenium dependent. In a nested case-control study within the Physicians' Health Study (PHS), our group reported a strong interaction between the valine (V)/alanine (A) polymorphism (rs4880) in SOD2 and plasma selenium and risk of total and aggressive prostate cancer.26 Men who were in the highest quartile of selenium and had the AA genotype had more than a 50% reduction in risk of total (odds ratio = 0.47; 95% CI, 0.26 to 0.85; P for interaction = .05) and aggressive (odds ratio = 0.35; 95% CI, 0.15 to 0.82; P for interaction = .01) prostate cancer compared with men who were in the lowest quartile of selenium and had a V allele. Among V allele carriers, the effect of high versus low selenium level on prostate cancer risk was in the protective direction but weaker and not statistically significant. Although the exact biologic mechanism of the variant is unknown, the valine variant of SOD2 may result in less efficient transport of SOD2 into the mitochondrial matrix, which compromises the cell's ability to neutralize superoxide radicals.27 To expand on this work, we examined a distinct cohort of 489 men with prostate cancer and hypothesized that SOD2 would modify the association between plasma selenium level and risk of presenting with aggressive prostate cancer at diagnosis.
PATIENTS AND METHODS
Study Population
This work was conducted within an ongoing cohort comprised of patients with localized/locally advanced prostate cancer (ie, stage T3 or less, N0, and M0) with banked biospecimens at the Dana-Farber Cancer Institute, Boston, MA. Patients had to have consented and donated blood for research before undergoing any type of local therapy and consented to clinical research follow-up.28 Of 778 patients who fulfilled these criteria, 764 had sufficient DNA available, and 499 had sufficient plasma for selenium assessment. Four hundred eighty-nine had complete clinical, selenium, and genotype data and were included in this analysis. These men were diagnosed with prostate cancer between January 1994 and March 2001. Median time from diagnosis to blood draw was 44 days (interquartile range, 31 to 60 days), and four patients had blood drawn more than 180 days after diagnosis.
Genomic DNA
Genomic DNA was prepared from peripheral blood using QIAamp DNA Blood mini kit (Qiagen Inc, Valencia, CA). DNA concentration was assayed using PicoGreen (Invitrogen, Carlsbad, CA) and adjusted to 5 ng/μL for genotyping.
Genotyping
Genotyping of rs4880 (C or T) on SOD2 was performed by Increased Plexing Efficiency and Flexibility for MassARRAY System Assay (Sequenom, San Diego, CA) through single base primer extension with mass-modified terminators. This polymorphism locates on exon 2, at nucleotide position 47 (counting from the initial methionine codon).29 C(GCT) type encodes alanine (A), and T(GTT) encodes valine (V). This polymorphism is shown as Ala16Val in a precursor form and Ala-9Val in a mature form.30 Six percent of samples were analyzed twice with blind numbering as internal quality control samples, and there was complete concordance. Hereafter, we refer to these gene variants as AA, VA, and VV.
Plasma Selenium Assessment
Selenium assay methods of this lab (I.B.K.) have been published previously.31 Briefly, selenium concentration was assessed by flameless atomic absorption (Perkin-Elmer 800; Perkin-Elmer, Norwalk, CT) using an electrodeless discharge lamp operating at λ = 196.0 nm and l'Vov platform graphite furnace (Perkin-Elmer) with Zeeman background correction.32 Samples were run bracketed by quality control samples (ie, certified standard reference material no. 66816 from Utak Laboratory, Valencia, CA) with criteria for acceptance coefficient of variation less than 10%; 5% of study samples were analyzed in blinded duplicates (coefficient of variation = 5.3%). This research was approved by the institutional review boards of all collaborating institutions.
Statistical Analysis
Patient disease characteristics at diagnosis and SOD2 genotype (rs4880) frequencies were summarized as the number and percentage of patients or as the median, range, and interquartile range of levels. Plasma selenium levels were categorized according to quintile cutoff values (108.3, 118.0, 125.5, and 139.8 μg/L, equivalent to 1.08, 1.18, 1.26, and 1.40 ppm, respectively).
The primary outcome of interest was presentation of aggressive prostate cancer at diagnosis. Aggressive disease was defined as clinical stage T2b-3, PSA more than 10 ng/mL, or biopsy Gleason sum ≥ 7 (corresponding to D'Amico intermediate-/high-risk categories33). Although central pathology review was not conducted solely for the current study, 94% of participants had their slides reviewed by a single pathology group, Brigham and Women's Hospital Department of Pathology (Boston, MA). Associations of disease aggressiveness with SOD2 and selenium levels were evaluated using the χ2 test or the Cochran-Armitage test for trend. Relative risk (RR) and 95% CIs were estimated using a generalized linear model for binomial data with a log link rather than a logit link function. We also conducted similar analyses using logistic regression models to compute prevalence odds ratios as estimates of the RR. Results were similar, and we present here the results of the log-binomial models only because this method may be superior with regard to providing estimates of 95% CIs from cross-sectional data.34 The likelihood ratio test from the generalized linear model was used to test for an interaction between SOD2 and selenium on disease aggressiveness. There was insufficient variation in this population to examine effects separately by race. All analyses were conducted using SAS version 9 (SAS Institute, Cary, NC), and P < .05 (two-sided) was considered statistically significant.
RESULTS
Demographic and clinical characteristics of this cohort of prostate cancer survivors are listed in Table 1. Median age was 62 years, and median PSA level was 6.0 ng/mL in this predominantly white population. More than half the cohort had good risk disease, whereas approximately one third was intermediate risk, in part reflecting the original cohort entry criteria. Neither age (P = .38) nor race (P = .69) was associated with risk of presenting with aggressive disease.
Table 1.
Patient Demographics and Clinical Characteristics at Diagnosis of Men With Prostate Cancer (N = 489)
| Characteristic | No. of Patients | % |
|---|---|---|
| Age at diagnosis, years | ||
| Median | 62 | |
| Range | 43-86 | |
| Interquartile range | 57-68 | |
| PSA at diagnosis, ng/mL | ||
| Median | 6.0 | |
| Range | 0.7-575.8 | |
| Interquartile range | 4.7-8.3 | |
| Plasma selenium, μg/L | ||
| Median | 121.4 | |
| Range | 64.2-221.1 | |
| Interquartile range | 110.4-135.1 | |
| Race | ||
| White | 468 | 95.7 |
| Other | 18 | 3.6 |
| Unknown | 3 | 0.6 |
| T stage at diagnosis | ||
| T1c | 259 | 53 |
| T2 | 21 | 4.3 |
| T2a | 94 | 19.2 |
| T2b | 9 | 1.8 |
| T3 | 2 | 0.4 |
| T3a | 1 | 0.2 |
| Tx | 103 | 21.1 |
| Biopsy Gleason sum | ||
| 6 or less | 312 | 63.8 |
| 7* | 153 | 31.3 |
| 8 or more | 24 | 4.9 |
| PSA at diagnosis, ng/mL | ||
| ≤ 10 | 405 | 82.8 |
| 10-20 | 52 | 10.6 |
| > 20 | 27 | 5.5 |
| Unknown | 5 | 1.0 |
| % of biopsy core positive | ||
| ≤ 33 | 230 | 47 |
| 33-50 | 59 | 12.1 |
| > 50 | 109 | 22.3 |
| Unknown | 91 | 18.6 |
| Disease risk categories† | ||
| High | 46 | 9.4 |
| Intermediate | 167 | 34.2 |
| Low | 276 | 56.4 |
Abbreviation: PSA, prostate-specific antigen.
Gleason sum 3 + 4, n = 94; Gleason sum 4 + 3, n = 45; and Gleason sum components unknown, n = 14.
D'Amico risk categories are as follows: low risk (≤ T2a, PSA ≤ 10 ng/mL, and Gleason sum ≤ 6), intermediate risk (T2b, PSA = 10-20 ng/mL, or Gleason sum = 7), and high risk (> T2b, PSA > 20 ng/mL, or Gleason sum > 7).
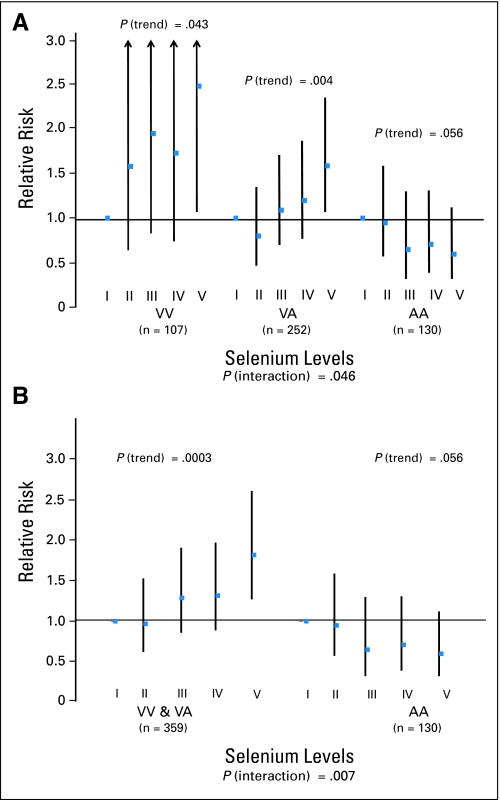
Median selenium level was similar among men with different SOD2 genotypes (approximately 121 ug/L), and SOD2 alone was not associated with risk of presenting with aggressive prostate cancer (Table 2). Higher plasma selenium level was slightly associated with increased risk of having aggressive prostate cancer at diagnosis (Table 3). This effect was statistically significantly modified by SOD2 genotype. Among men with the AA genotype, those in the highest versus lowest quintile of selenium had a borderline statistically significant 40% lower risk of aggressive prostate cancer (P for trend = .06). In contrast, among men with a V allele, those with high versus low plasma selenium had an increased risk of presenting with aggressive prostate cancer (RR = 2.48; 95% CI, 1.07 to 5.71; P for trend = .04 for VV; and RR = 1.59; 95% CI, 1.07 to 2.35; P for trend = .004 for VA; Fig 1 and Table 3). Overall, there was a statistically significant interaction between SOD2 and selenium and risk of aggressive prostate cancer (P = .007; Table 3 and Fig 1). This interaction remained evident in a cross-stratified analysis of all patients using a single reference group (ie, lowest quintile of selenium and having a V allele; Table 3). In this model, the association between selenium level and aggressive disease was clearly dependent on SOD2, with those in the highest versus lowest quintile of selenium having either no association or almost a two-fold increase in risk, depending on SOD2 variant (Table 3).
Table 2.
Distribution of SOD2 Genotypes and Association With Aggressive Disease and Plasma Selenium Levels Among 489 Men With Prostate Cancer
| Genotype | Patients |
Aggressive Disease |
Selenium Level (μg/L) |
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | Relative Risk | 95% CI | P* | Median | Range | P† | |
| VV | 107 | 21.9 | 1.00 | Reference | .346 | 120.9 | 85.4-221.1 | .376 |
| VA | 252 | 51.5 | 1.21 | 0.92 to 1.59 | 121.5 | 64.2-211.6 | ||
| AA | 130 | 26.6 | 1.10 | 0.81 to 1.51 | 121.7 | 84.2-193.5 | ||
χ2 test.
Kruskal-Wallis test.
Table 3.
RR for Aggressive Prostate Cancer According to Quintiles of Plasma Selenium for Men With Prostate Cancer and Stratified by SOD2 Genotype
| Genotype | No. of Patients | Quintile Levels of Plasma Selenium |
P* (trend) | P† (interaction) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I (low) |
II |
III |
IV |
V (high) |
|||||||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||||
| All patients | 489 | 1.00 | 0.99 | 0.70 to 1.40 | 1.08 | 0.77 to 1.52 | 1.11 | 0.80 to 1.55 | 1.35 | 0.99 to 1.84 | .042 | ||
| SOD2 genotype grouped separately | |||||||||||||
| VV | 107 | 1.00 | 1.58 | 0.64 to 3.85 | 1.95 | 0.83 to 4.58 | 1.73 | 0.74 to 4.06 | 2.48 | 1.07 to 5.71 | .043 | .046 | |
| VA | 252 | 1.00 | 0.80 | 0.47 to 1.35 | 1.09 | 0.70 to 1.71 | 1.20 | 0.77 to 1.87 | 1.59 | 1.07 to 2.35 | .004 | ||
| AA | 130 | 1.00 | 0.95 | 0.57 to 1.59 | 0.65 | 0.32 to 1.30 | 0.71 | 0.39 to 1.31 | 0.60 | 0.32 to 1.12 | .056 | ||
| SOD2 genotype with VV and VA groups combined | |||||||||||||
| VV/VA | 359 | 1.00 | 0.98 | 0.62 to 1.53 | 1.29 | 0.87 to 1.92 | 1.32 | 0.89 to 1.97 | 1.82 | 1.27 to 2.61 | .0003 | .007 | |
| AA | 130 | 1.00 | 0.95 | 0.57 to 1.59 | 0.65 | 0.32 to 1.30 | 0.71 | 0.39 to 1.31 | 0.60 | 0.32 to 1.12 | .056 | ||
| SOD2 genotype with VV and VA groups combined and using a common reference | |||||||||||||
| VV/VA | 359 | 1.00 | 0.98 | 0.62 to 1.53 | 1.29 | 0.87 to 1.92 | 1.32 | 0.89 to 1.97 | 1.82 | 1.27 to 2.61 | — | .007 | |
| AA | 130 | 1.56 | 0.97 to 2.53 | 1.49 | 0.93 to 2.39 | 1.01 | 0.52 to 1.98 | 1.11 | 0.62 to 1.98 | 0.93 | 0.51 to 1.69 | ||
Abbreviations: RR, relative risk.
Cochran-Armitage test for trend.
From the generalized linear model where selenium was coded as quintile levels.
Fig 1.
Relative risk and 95% CI for aggressive prostate cancer according to quintiles of plasma selenium level (A) by each SOD2 genotype and (B) for AA and the combined group of VV or VA SOD2 genotype.
Results reflected in Table 3 were similar when age and race were included in the multivariate models (P for interaction = .02), when we adjusted for year of diagnosis (P for interaction = .004), when we examined clinical Gleason sum as a single outcome variable (Gleason ≥ v < 7; P for interaction = .04); and when we used logistic regression models to compute prevalence odds ratios as estimates of the relative risk (P for interaction = .007; estimates consistent and stronger; data not shown).
DISCUSSION
We observed strong support for the hypothesis that plasma selenium levels and SOD2 genotype interact to influence risk of presenting with aggressive prostate cancer at diagnosis in men with localized or locally advanced prostate cancer. Higher selenium levels alone were also slightly positively associated with worse prognostic risk at diagnosis, which largely reflected an association with higher Gleason sum. There was no association for SOD2 alone and prostate cancer aggressiveness in this population.
The median selenium level in this population (121.4 ng/mL) was comparable to that in several other studies, where the range of median (or mean) circulating selenium levels among healthy control or placebo groups was 108 to 141 ng/mL.1,5,7,8,14,23,25 The trend toward a slightly higher risk of aggressive disease for higher levels of selenium in this case population is unique and somewhat in contrast to several studies that reported decreased prostate cancer risk with higher selenium levels.1,2,4,5,7–10,14,17 However, some data indicate that the association between selenium and cancer risk may not be entirely linear and that higher doses could have null or adverse effects.
In SELECT, men receiving selenium supplementation had a median selenium level that was substantially higher (252 μg/L) than that of men in the current study (121 μg/L; range, 64 to 221 μg/L), and the trial reported null results for selenium supplementation versus placebo and incident (mainly early-stage) prostate cancer risk and a nonsignificant elevation in diabetes risk.25 In the large National Institutes of Health–American Association of Retired Persons Diet and Health Study, more than daily usage of multivitamins was associated with an elevated risk of incident advanced and fatal prostate cancer, in particular among men who also took individual supplements of selenium, β-carotene, or zinc or who had a family history of prostate cancer.35 Waters et al19 conducted a randomized controlled feeding study of selenium supplementation versus placebo in male dogs, the only other species besides humans to experience spontaneous prostate cancer. Dogs receiving selenium had significantly less DNA damage and more apoptosis in their peripheral-blood lymphocytes and prostate cells,19 and there was a U-shaped dose-response curve between toenail measures of selenium and extent of DNA damage in prostate tissue.36 In the Nutritional Prevention of Cancer Trial, selenium supplementation reduced total and prostate cancer incidence; however, this effect was limited to those with lower baseline circulating selenium levels (hazard ratio = 0.14; 95% CI, 0.03 to 0.61 for selenium levels < 106.4 ng/mL). Among men in the highest tertile (> 123.2 ng/mL), selenium supplementation was unassociated with prostate cancer risk, although in the positive direction (hazard ratio = 1.14; 95% CI, 0.51 to 2.59).13 Also, among those randomly assigned to selenium, being in the highest versus lowest tertile of circulating selenium at baseline was associated with an 88% increased risk of total cancer (P = .01).23 Together, these data suggest that obtaining a certain level of selenium may confer some protection against prostate cancer; however, the relationship may not be linear, and caution is warranted before assuming that obtaining increasingly higher levels confers increased benefit.
The effect of selenium status on prostate cancer may also depend on how it interacts with components of the oxidative stress pathway, as evidenced by the current study. The observed 40% decreased risk of aggressive prostate cancer associated with higher selenium among men with the AA variant (P for trend = .06) was consistent and expands on our previous findings in the PHS. However, an important difference was that in the current study, men with a V allele had an elevated risk of aggressive disease with higher selenium levels, and there was no such trend in the PHS. Differences in study design and patient population may explain this apparent inconsistency. In particular, PHS focused on prediagnostic selenium levels from years before diagnosis, in contrast to the present study, which examined levels at or shortly after diagnosis. Also, we contrasted cases only, whereas the PHS compared men with and without prostate cancer diagnoses to examine risk of developing total or advanced prostate cancer. Furthermore, in the PHS, the significant inverse relation was observed only for risk of developing advanced-stage, not localized, prostate cancer; and in the current study, we examined only patients that had stage T3 disease or less. Despite these differences, the findings converge on demonstrating an interaction of selenium with SOD2 genotype in prostate cancer.
Several other studies have reported on possible effect modification by SOD2 on the relationship between dietary factors and prostate cancer incidence.37–40 In the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, having SOD2 AA genotype was associated with an increased risk of developing prostate cancer, in particular among those with lower vitamin E intake who were also smokers, although there was no effect modification by selenium level.38 In the Carotene and Retinol Efficacy Trial, SOD2 alone was not associated with prostate cancer risk, but among VV carriers, never versus ever use of vitamin supplements or greater iron intake was associated with double the risk of incident prostate cancer.37,40 In contrast, SOD2 AA genotype (v VV or VA) was associated with more than 2.5-fold elevation in risk of high-grade prostate tumors in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study, whereas there was no evidence for an interaction by vitamin E or β-carotene.39 A few other studies have also reported a higher frequency of A alleles among prostate cancer patients versus controls or healthy adults.41,42 Further evidence that SOD2 genotype modifies diet or lifestyle associations with carcinogenesis comes from studies of breast,43–48 bladder,49 and skin50 cancer.
The observed interaction between SOD2 and selenium levels may reflect the dual roles of selenium as a pro- and antioxidant, depending on dose, form, and function of other enzymes.51,52 The AA variant of SOD2 may be more effective at transporting the enzyme through the mitochondrial membrane, thereby increasing breakdown of superoxide radicals into hydrogen peroxide.30 If there is sufficient selenium, then (selenium-dependent) GPX can next act to efficiently break down hydrogen peroxide into water. However, if there is not sufficient selenium, the GPX reaction is halted, and a buildup of hydrogen peroxide may occur, leading to toxicity, oxidation, propensity for DNA damage, and increased cancer risk. This may be one way in which lower versus higher selenium levels and the AA variant could be associated with an increased risk of aggressive prostate cancer.26
The direct relation of higher selenium with more aggressive disease among carriers of the V allele was unexpected and has not previously been reported. One explanation might be that in men with established cancer, antioxidants may promote cancer cell survival through an antiapoptotic mechanism by neutralizing the higher levels of reactive oxygen species found in cancer cells. Alternatively, given the cross-sectional nature of our study, it could be that prostate tumors that develop despite high selenium status tend to have higher Gleason grade. However, the interaction and different associations observed based on SOD2 argues against this reverse causality explanation. One could speculate that if there is chronic lower normal SOD2 activity and high superoxide anion production as a result of high concentrations of selenite, then there may be an imbalance toward oxidative stress that cannot be fully compensated by GPX or catalase actions alone.51 Finally, this finding may be a result of chance and warrants further confirmation in humans.
This study has several limitations that must be considered. It was cross-sectional, and we cannot entirely rule out the possibility that the slight positive association for selenium and risk of aggressive disease was a result of patients with worse clinical features taking selenium supplements out of concern about their diagnosis. However, most blood was donated shortly after diagnosis, and the strong interaction with SOD2 genotype argues against this. Other limitations include our inability to adjust for potential confounding factors, such as body size or diet, and lack of racial diversity.
In conclusion, this cross-sectional analysis of 489 men with prostate cancer provides evidence that selenium levels interact with SOD2 genotype to influence the risk of presenting with aggressive prostate cancer at diagnosis. Among the approximate 25% of men with the AA genotype, having greater selenium levels may protect against aggressive disease. However, for the 75% men who carry a V allele, higher selenium levels might increase the likelihood of having worse tumor characteristics. Together, these data underscore the importance of genotype for understanding the potential benefits and dangers of selenium on prostate cancer and may, in part, explain apparently conflicting results from previous studies. Complete interpretation of results from SELECT may depend on assessment of SOD2 genotype in trial participants.
Acknowledgment
We sincerely thank Opeyemi Talabi and Carolyn Evan for their assistance in study management and data collection and all the participants without whom none of this work would be possible.
Footnotes
Supported by National Cancer Institute/National Institutes of Health Grant No. R01 CA106947; Prostate Cancer Specialized Programs of Research Excellence (SPORE) Grants No. P50CA90381 to Dana-Farber Harvard Cancer Center and P50CA89520 to University of California, San Francisco from the National Institutes of Health/National Cancer Institute; American Association for Cancer Research and the California Department of Public Health Early Career Development Award; Prostate Cancer Foundation; and The Arthur and Linda Gelb Center.
Presented in part at the American Society of Clinical Oncology Genitourinary Cancer Symposium, February 14-16, 2008, San Francisco, CA; and at the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL.
The funding agencies provided financial support for this work but were not directly involved in the design of the study, collection of data, analysis, interpretation, decisions regarding publication, or writing of the article.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: June M. Chan, William K. Oh, Meir J. Stampfer, Miyako Abe, Philip W. Kantoff
Financial support: June M. Chan, Meir J. Stampfer, Philip W. Kantoff
Administrative support: June M. Chan, William K. Oh, Philip W. Kantoff
Provision of study materials or patients: William K. Oh, Irena B. King, Miyako Abe, Philip W. Kantoff
Collection and assembly of data: William K. Oh, Wanling Xie, Meredith M. Regan, Irena B. King, Miyako Abe
Data analysis and interpretation: June M. Chan, William K. Oh, Wanling Xie, Meredith M. Regan, Meir J. Stampfer, Irena B. King, Miyako Abe, Philip W. Kantoff
Manuscript writing: June M. Chan, William K. Oh, Wanling Xie, Meredith M. Regan, Meir J. Stampfer, Irena B. King, Miyako Abe, Philip W. Kantoff
Final approval of manuscript: June M. Chan, William K. Oh, Wanling Xie, Meredith M. Regan, Meir J. Stampfer, Irena B. King, Miyako Abe, Philip W. Kantoff
REFERENCES
- 1.Peters U, Foster CB, Chatterjee N, et al. Serum selenium and risk of prostate cancer: A nested case-control study. Am J Clin Nutr. 2007;85:209–217. doi: 10.1093/ajcn/85.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkman M, Reulen RC, Kellen E, et al. Are men with low selenium levels at increased risk of prostate cancer? Eur J Cancer. 2006;42:2463–2471. doi: 10.1016/j.ejca.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Etminan M, FitzGerald JM, Gleave M, et al. Intake of selenium in the prevention of prostate cancer: A systematic review and meta-analysis. Cancer Causes Control. 2005;16:1125–1131. doi: 10.1007/s10552-005-0334-2. [DOI] [PubMed] [Google Scholar]
- 4.Allen NE, Morris JS, Ngwenyama RA, et al. A case-control study of selenium in nails and prostate cancer risk in British men. Br J Cancer. 2004;90:1392–1396. doi: 10.1038/sj.bjc.6601701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks JD, Metter EJ, Chan DW, et al. Plasma selenium level before diagnosis and the risk of prostate cancer development. J Urol. 2001;166:2034–2038. [PubMed] [Google Scholar]
- 6.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial—Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 7.Vogt TM, Ziegler RG, Graubard BI, et al. Serum selenium and risk of prostate cancer in U.S. blacks and whites. Int J Cancer. 2003;103:664–670. doi: 10.1002/ijc.10866. [DOI] [PubMed] [Google Scholar]
- 8.Nomura AM, Lee J, Stemmermann GN, et al. Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:883–887. [PubMed] [Google Scholar]
- 9.Yoshizawa K, Willett WC, Morris SJ, et al. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J Natl Cancer Inst. 1998;90:1219–1224. doi: 10.1093/jnci/90.16.1219. [DOI] [PubMed] [Google Scholar]
- 10.van den Brandt PA, Zeegers MP, Bode P, et al. Toenail selenium levels and the subsequent risk of prostate cancer: A prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2003;12:866–871. [PubMed] [Google Scholar]
- 11.Combs GF., Jr Status of selenium in prostate cancer prevention. Br J Cancer. 2004;91:195–199. doi: 10.1038/sj.bjc.6601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson MA, Porterfield BW, Jacobs ET, et al. Selenium and prostate cancer prevention. Semin Urol Oncol. 1999;17:91–96. [PubMed] [Google Scholar]
- 13.Duffield-Lillico AJ, Dalkin BL, Reid ME, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Stampfer MJ, Giovannucci EL, et al. A prospective study of plasma selenium levels and prostate cancer risk. J Natl Cancer Inst. 2004;96:696–703. doi: 10.1093/jnci/djh125. [DOI] [PubMed] [Google Scholar]
- 15.Combs GF, Clark LC, Turnbull BW. Reduction of cancer risk with an oral supplement of selenium. Biomed Environ Sci. 1997;10:227–234. [PubMed] [Google Scholar]
- 16.Combs GF, Jr, Clark LC, Turnbull BW. Reduction of cancer mortality and incidence by selenium supplementation. Med Klin. 1997;92(suppl 3):42–45. doi: 10.1007/BF03041964. [DOI] [PubMed] [Google Scholar]
- 17.Helzlsouer KJ, Huang HY, Alberg AJ, et al. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000;92:2018–2023. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 18.Corcoran NM, Najdovska M, Costello AJ. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J Urol. 2004;171:907–910. doi: 10.1097/01.ju.0000092859.16817.8e. [DOI] [PubMed] [Google Scholar]
- 19.Waters DJ, Shen S, Cooley DM, et al. Effects of dietary selenium supplementation on DNA damage and apoptosis in canine prostate. J Natl Cancer Inst. 2003;95:237–241. doi: 10.1093/jnci/95.3.237. [DOI] [PubMed] [Google Scholar]
- 20.Webber MM, Perez-Ripoll EA, James GT. Inhibitory effects of selenium on the growth of DU-145 human prostate carcinoma cells in vitro. Biochem Biophys Res Commun. 1985;130:603–609. doi: 10.1016/0006-291x(85)90459-0. [DOI] [PubMed] [Google Scholar]
- 21.Venkateswaran V, Fleshner NE, Sugar LM, et al. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004;64:5891–5896. doi: 10.1158/0008-5472.CAN-04-0690. [DOI] [PubMed] [Google Scholar]
- 22.Karunasinghe N, Ryan J, Tuckey J, et al. DNA stability and serum selenium levels in a high-risk group for prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:391–397. [PubMed] [Google Scholar]
- 23.Duffield-Lillico AJ, Reid ME, Turnbull BW, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: A summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 24.Klein EA, Thompson IM, Lippman SM, et al. SELECT: The next prostate cancer prevention trial—Selenium and Vitamin E Cancer Prevention Trial. J Urol. 2001;166:1311–1315. doi: 10.1016/s0022-5347(05)65759-x. [DOI] [PubMed] [Google Scholar]
- 25.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2008;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Kantoff PW, Giovannucci E, et al. Manganese superoxide dismutase polymorphism, prediagnostic antioxidant status, and risk of clinical significant prostate cancer. Cancer Res. 2005;65:2498–2504. doi: 10.1158/0008-5472.CAN-04-3535. [DOI] [PubMed] [Google Scholar]
- 27.Sutton A, Khoury H, Prip-Buus C, et al. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003;13:145–157. doi: 10.1097/01.fpc.0000054067.64000.8f. [DOI] [PubMed] [Google Scholar]
- 28.Oh WK, Hayes J, Evan C, et al. Development of an integrated prostate cancer research information system. Clin Genitourin Cancer. 2006;5:61–66. doi: 10.3816/CGC.2006.n.019. [DOI] [PubMed] [Google Scholar]
- 29.Rosenblum JS, Gilula NB, Lerner RA. On signal sequence polymorphisms and diseases of distribution. Proc Natl Acad Sci U S A. 1996;93:4471–4473. doi: 10.1073/pnas.93.9.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimoda-Matsubayashi S, Matsumine H, Kobayashi T, et al. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene: A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson's disease. Biochem Biophys Res Commun. 1996;226:561–565. doi: 10.1006/bbrc.1996.1394. [DOI] [PubMed] [Google Scholar]
- 31.Satia JA, King IB, Morris JS, et al. Toenail and plasma levels as biomarkers of selenium exposure. Ann Epidemiol. 2006;16:53–58. doi: 10.1016/j.annepidem.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Ericson SP, McHalsky ML, Rabinow BE, et al. Sampling and analysis techniques for monitoring serum for trace elements. Clin Chem. 1986;32:1350–1356. [PubMed] [Google Scholar]
- 33.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 34.Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med 65: 2008;481:501–506. doi: 10.1136/oem.2007.034777. [DOI] [PubMed] [Google Scholar]
- 35.Lawson KA, Wright ME, Subar A, et al. Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. J Natl Cancer Inst. 2007;99:754–764. doi: 10.1093/jnci/djk177. [DOI] [PubMed] [Google Scholar]
- 36.Waters DJ, Shen S, Glickman LT, et al. Prostate cancer risk and DNA damage: Translational significance of selenium supplementation in a canine model. Carcinogenesis. 2005;26:1256–1262. doi: 10.1093/carcin/bgi077. [DOI] [PubMed] [Google Scholar]
- 37.Choi JY, Neuhouser ML, Barnett M, et al. Iron intake, oxidative stress-related genes (MnSOD and MPO), and prostate cancer risk in CARET cohort. Carcinogenesis. 2008;29:964–970. doi: 10.1093/carcin/bgn056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang D, Lee KM, Park SK, et al. Functional variant of manganese superoxide dismutase (SOD2 V16A) polymorphism is associated with prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer study. Cancer Epidemiol Biomarkers Prev. 2007;16:1581–1586. doi: 10.1158/1055-9965.EPI-07-0160. [DOI] [PubMed] [Google Scholar]
- 39.Woodson K, Tangrea JA, Lehman TA, et al. Manganese superoxide dismutase (MnSOD) polymorphism, alpha-tocopherol supplementation and prostate cancer risk in the alpha-tocopherol, beta-carotene cancer prevention study (Finland) Cancer Causes Control. 2003;14:513–518. doi: 10.1023/a:1024840823328. [DOI] [PubMed] [Google Scholar]
- 40.Choi JY, Neuhouser ML, Barnett M, et al. Polymorphisms in oxidative stress-related genes are not associated with prostate cancer risk in heavy smokers. Cancer Epidemiol Biomarkers Prev. 2007;16:1115–1120. doi: 10.1158/1055-9965.EPI-07-0040. [DOI] [PubMed] [Google Scholar]
- 41.Taufer M, Peres A, de Andrade VM, et al. Is the Val16Ala manganese superoxide dismutase polymorphism associated with the aging process? J Gerontol A Biol Sci Med Sci. 2005;60:432–438. doi: 10.1093/gerona/60.4.432. [DOI] [PubMed] [Google Scholar]
- 42.Ergen HA, Narter F, Timirci O, et al. Effects of manganase superoxide dismutase Ala-9Val polymorphism on prostate cancer: A case-control study. Anticancer Res. 2007;27:1227–1230. [PubMed] [Google Scholar]
- 43.Slanger TE, Chang-Claude J, Wang-Gohrke S. Manganese superoxide dismutase Ala-9Val polymorphism, environmental modifiers, and risk of breast cancer in a German population. Cancer Causes Control. 2006;17:1025–1031. doi: 10.1007/s10552-006-0043-5. [DOI] [PubMed] [Google Scholar]
- 44.Silva SN, Cabral MN, Bezerra de Castro G, et al. Breast cancer risk and polymorphisms in genes involved in metabolism of estrogens (CYP17, HSD17beta1, COMT and MnSOD): Possible protective role of MnSOD gene polymorphism Val/Ala and Ala/Ala in women that never breast fed. Oncol Rep. 2006;16:781–788. [PubMed] [Google Scholar]
- 45.Cai Q, Shu XO, Wen W, et al. Genetic polymorphism in the manganese superoxide dismutase gene, antioxidant intake, and breast cancer risk: Results from the Shanghai Breast Cancer Study. Breast Cancer Res. 2004;6:R647–R655. doi: 10.1186/bcr929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ambrosone CB, Freudenheim JL, Thompson PA, et al. Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res. 1999;59:602–606. [PubMed] [Google Scholar]
- 47.Tamimi RM, Hankinson SE, Spiegelman D, et al. Manganese superoxide dismutase polymorphism, plasma antioxidants, cigarette smoking, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:989–996. [PubMed] [Google Scholar]
- 48.Millikan RC, Player J, de Cotret AR, et al. Manganese superoxide dismutase Ala-9Val polymorphism and risk of breast cancer in a population-based case-control study of African Americans and whites. Breast Cancer Res. 2004;6:R264–R274. doi: 10.1186/bcr786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hung RJ, Boffetta P, Brennan P, et al. Genetic polymorphisms of MPO, COMT, MnSOD, NQO1, interactions with environmental exposures and bladder cancer risk. Carcinogenesis. 2004;25:973–978. doi: 10.1093/carcin/bgh080. [DOI] [PubMed] [Google Scholar]
- 50.Han J, Colditz GA, Hunter DJ. Manganese superoxide dismutase polymorphism and risk of skin cancer (United States) Cancer Causes Control. 2007;18:79–89. doi: 10.1007/s10552-006-0079-6. [DOI] [PubMed] [Google Scholar]
- 51.Husbeck B, Nonn L, Peehl DM, et al. Tumor-selective killing by selenite in patient-matched pairs of normal and malignant prostate cells. Prostate. 2006;66:218–225. doi: 10.1002/pros.20337. [DOI] [PubMed] [Google Scholar]
- 52.Zhong W, Yan T, Webber MM, et al. Alteration of cellular phenotype and responses to oxidative stress by manganese superoxide dismutase and a superoxide dismutase mimic in RWPE-2 human prostate adenocarcinoma cells. Antioxid Redox Signal. 2004;6:513–522. doi: 10.1089/152308604773934279. [DOI] [PubMed] [Google Scholar]