Abstract
Purpose
Intensive postoperative surveillance is associated with improved survival and recommended for patients with late stage (stage IIB and III) colon cancer. We hypothesized that stage I and IIA colon cancer patients would experience similar benefits.
Patients and Methods
Secondary analysis of data from the Clinical Outcomes of Surgical Therapy trial was performed by analyzing results according to TNM stage; early (stage I and IIA, 537 patients) and late (stage IIB and III, 254 patients) stage disease. Five-year recurrence rates were higher in patients with late (35.7%) versus early stage disease (9.5%). Early and late stage salvage rates, recurrence patterns and methods of first detection were compared by χ2 test.
Results
Salvage rates for early- and late-stage disease patients with recurrence were the same (35.9% v 37%; P = .9, respectively). Median survival after second surgery after recurrence was 51.2 and 35.8 months for early- and late-stage patients, respectively. Single sites of first recurrence did not significantly differ between early and late stage, but multiple sites of recurrence occurred less often in early-stage patients (3.6% v 28.6%, for early v late, respectively; P < .001). Methods of first detection of recurrence were not significantly different: carcinoembryonic antigen (29.1% v 37.4%), computed tomography scan (23.6% v 26.4%), chest x-ray (7.3% v 12.1%), and colonoscopy (12.7% v 8.8%), for early versus late stage disease, respectively.
Conclusion
Patients with early-stage colon cancer have similar sites of recurrence, and receive similar benefit from postrecurrence therapy as late-stage patients; implementation of surveillance guidelines for early-stage patients is appropriate.
INTRODUCTION
Until recently, the potential clinical benefits of intensive follow-up for patients with resected colon cancer has been assumed, but not rigorously investigated or defined. A 2007 Cochrane comprehensive analysis confirmed the benefits of intensive follow-up for patients with resected colorectal cancer; leading to the recommendation of intensive follow-up as a new standard.1 The Cochrane review found, as expected, that rates of recurrence were not affected by follow-up intensity and yet, postrecurrence survival rates consistently favored patients who were followed more, rather than less intensively (odds ratio [OR], 0.73; 95% CI, 0.59 to 0.91). It is presumed that the improved survival for patients managed with intensive follow-up were due to earlier detection and a higher frequency of secondary curative intent surgery (28% v 12%; OR, 2.41; 95% CI, 1.63 to 3.54). Regardless of attribution, a new standard has been advised.
The Cochrane report presents the strongest analytic evidence to date that intensive postoperative surveillance is life-saving and appropriate for colon cancer patients. It stopped short, however, of providing guidance for how practitioners can implement this new standard. In the Cochrane review, the available data were not considered adequate to recommend specific methods of follow-up. The Cochrane report invites a series of logical next questions including which tests should be performed, in whom, and how often. Should risk factors, such as stage of disease, for example, enter into the decision on how to observe patients? Several published surveillance guidelines provide advice on how to implement this new standard,2,3,7 yet it is clear that there are major gaps in the knowledge on the particulars of best practices, including how best to treat postsurgical patients with early-stage disease. To address this specific question we conducted a study using the prospectively collected data from the Clinical Outcomes of Surgical Therapy (COST) Group laparoscopic colon trial, testing the hypothesis that early-stage patients with recurrence experience the same benefits from intensified follow-up as late-stage patients.
PATIENTS AND METHODS
The COST trial was a multi-institutional, National Cancer Institute–sponsored, phase III randomized prospective trial comparing laparoscopic-assisted colectomy with open colectomy for curable colon cancer patients. Surgeons were required to demonstrate experience with at least 20 laparoscopic colonic surgical patients and submit video demonstrating proper oncologic techniques before becoming credentialed and eligible to enter patients in the study. Laparoscopic-assisted colectomies and open colectomies were performed and monitored according to protocol guidelines and there was no significant difference in recurrence patterns between the two procedures.
Protocol specified follow-up included (Table 1): physical examination, including checking for tumor recurrence at wound sites; carcinoembryonic antigen (CEA) testing every 3 months for the first year and then every 6 months for 5 years; chest radiography every 6 months for 2 years and then annually; and colon evaluation, including colonoscopy or colon radiography, annually for the first year and then every 3 years if the colon was free of neoplasms. Computed tomography (CT) scans of the abdomen were at the discretion of the treating physician for symptoms, physical findings, or increased CEA values. Recurrence was defined as either radiologic or pathologic evidence of tumor in the follow-up period.
Table 1.
Surveillance Recommendations
| Parameter | COST Protocol | ASCO (2005)3 | NCCN (2008)7 |
|---|---|---|---|
| History and physical exam | Every 3 months for 1 year then every 6 months to 5 years | Every 3 to 6 months for 3 years; then every 6 months to 5 years | Every 3 to 6 months for 2 years then every 6 months to 5 years |
| CEA | Every 3 months for 1 year then every 6 months to 5 years | Every 3 months for 3 years* | Every 3 to 6 months for 2 years then every 6 months to 5 years† |
| Chest screening | CXR every 6 months for 2 years then every 1 year to 5 years | CT chest every 1 year for 3 years‡ | CT chest every 1 year for 3 years§ |
| Colonoscopy | Annual exam if positive for neoplasm; exam every 3 years if negative | At 3 years and if results are normal, then every 5 years | At 1 year, 3 years, and 5 years if negative‖ |
| CT abdomen | At discretion of physician for symptoms, signs, or increased h CEA | CT every 1 year for 3 years‡ | CT abdomen/pelvis every 1 year for 3 years§ |
Abbreviations: COST, Clinical Outcomes of Surgical Therapy; ASCO, American Society of Clinical Oncology; NCCN, National Comprehensive Cancer Network; CEA, carcinoembryonic antigen; CXR, chest x-ray; CT, computed tomography.
If the patient is a candidate for surgery or systemic therapy.
If patient is a potential candidate for further intervention.
For patients who are at a higher risk of recurrence and who could be candidates for curative intent surgery.
For patients at high risk for recurrence; CT scan may be useful for patients at high risk for recurrence (eg, lymphatic or venous invasion by tumor, or poorly differentiated tumors).
Colonoscopy in 1 year except if no preoperative colonoscopy due to obstructing lesion, colonoscopy in 3-6 months; if abnormal, repeat in 1; if no advanced adenoma (villous polyp, polyp > 1 cm, or high grade dysplasia), repeat in 3 years, then every 5 years.
Five-year follow-up data is available on 90% of living patients. The primary end point of the COST study was tumor recurrence; data on postrecurrence treatment was not collected. Our study is a secondary analysis of the data from the COST trial, analyzing the results according to stage; early-stage (stage I and IIA) and late-stage (stage IIB and III) colon cancer. Recurrence rates, median time to recurrence, overall 5-year survival, patterns of first recurrence, and methods of first detection of recurrence and confirmation of recurrences were compared between the two stage-based groups. Locoregional recurrences included anastomotic recurrences; intra-abdominal recurrences include metastasis on pelvic organs, peritoneum, abdominal wall, and any organ in the abdomen except liver.
A cutoff value of 5 units was used for CEA monitoring. Recurrence was considered as first detected by CEA if the recurrence was first suspected based on a high CEA value and further battery of investigations documented the recurrence. Recurrence was considered as first detected by chest x-ray when a suspicious nodule was first detected on chest x-ray (CXR) and further investigations confirmed recurrence. Recurrences which were first detected by colonoscopy with no elevation in CEA or no evidence on CT scan were considered as first detected by colonoscopy. Patients with no abnormality of CEA, chest x-ray, or colonoscopy whose recurrence was detected by CT scan were considered as first detected by CT scan. We included anastomotic recurrence and metachronous cancer as a single group and considered it as positive for colonoscopy during surveillance. Further analysis of the recurrence group with special emphasis on the methods of detection in each group was also performed. The study protocol was institutional review board approved and Health Insurance Portability and Accountability Act compliant.
Statistical Analysis
Analyses from this retrospective review of data from the COST trial are mainly descriptive. The separation of early- versus late-stage disease (stage I and IIA v stage IIB and III) was prospectively specified and quality controlled by a designated pathologist at the institutional level, and a single trial pathologist for all enrolled patients. Sites of recurrence and method of detection were compared between early- versus late-stage disease using a χ2 test. Duration of survival was estimated using the Kaplan-Meier method and comparisons of time-to-event end points were compared via the log-rank test.
RESULTS
Eight hundred seventy-two colon cancer patients from 48 institutions were included in the trial. No significant differences in the recurrence rate, pattern of recurrence, disease-free survival, or overall survival were present between the laparoscopic and open colectomy groups. There were 537 patients with early-stage (stage I and IIA) and 254 patients with late-stage (stage IIB and III) colon cancer (81 patients were either stage IV at primary surgery or benign pathology and are excluded from all subsequent analyses).
Recurrence was noted in a total of 146 patients of which 55 had early-stage colon cancer, and 91 had late-stage disease. The overall 5-year survival rate for early-stage disease was 82.9% (95% CI, 79.7% to 86.2%) and 64.4% (95% CI, 58.7% to 70.7%) for late stage (P < .0001). The cumulative incidence of recurrence at 2 and 5 years was 6.0% (95% CI, 4.0% to 8.0%) and 9.5% (95% CI, 7.0% to 12.0%) for early-stage, and 23.7% (95% CI, 18.7% to 29.3%) and 35.7% (95% CI, 29.9% to 42.1%) for late-stage patients (both P < .0001). For those that recurred, median time to first recurrence was 1.8 years in patients with early, and 1.4 years in patients with late-stage disease.
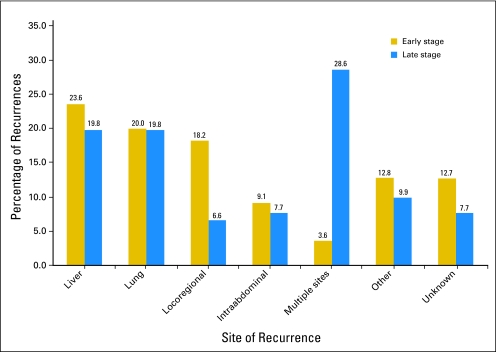
There was no significant difference between patients with early- and late-stage disease for single sites of first recurrence. Sites of first recurrence for early- and late-stage were: liver (23.6% v 19.8%), lung (20.0% v 19.8%), locoregional (18.2% v 6.6%), intra-abdominal recurrences (9.1% v 7.7%), other (12.8% v 9.9%), and unknown (12.7% v 7.7%; Fig 1). Patients with early-stage disease were significantly less likely to experience multiple sites of first recurrence (3.6% for early stage v 28.6% for late stage; P < .001).
Fig 1.
Plot comparing the patterns of first recurrence in early- and late-stage colon cancer.
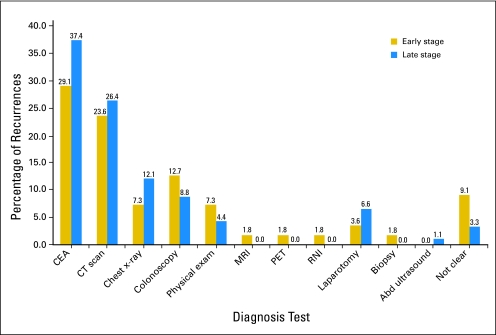
The methods of first detection of recurrence showed no significant difference between patients with early- and late-stage disease (P = .60). The detection methods for initial recurrence for early- and late-stage recurrence were with CEA (29.1% v 37.4%), CT scan (23.6% v 26.4%), chest x-ray (7.3% v 12.1%), and colonoscopy (12.7% v 8.8%). Physical examination, exploratory laparotomy, magnetic resonance imaging, positron emission tomography, and radionuclide imaging detected a higher proportion of patients with recurrences in the early than late stage group (16.4% v 11.0%), but this difference was not significant (Fig 2).
Fig 2.
Plot comparing the methods of detection of first recurrence in early- and late-stage colon cancer.
Confirmation of recurrence in early and late stage was accomplished primarily by biopsy/fine needle aspiration cytology (34.5% v 29.7%) and CT scan (20.0% v 34.1%). Other confirmatory methods for early- and late-stage disease were colonoscopy (3.6% v 1.1%), positron emission tomography scan (1.8% v 4.4%), laparotomy (1.8% v 4.4%), magnetic resonance imaging (1.8% v 2.2%), and abdominal ultrasound (0% v 2.2%).
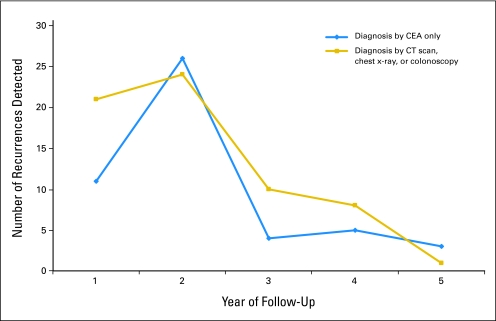
Subset analysis of the detection methods by each follow-up year was performed to estimate the importance of individual tests. CEA was the single most important diagnostic test to detect early recurrences and the maximum number of recurrences. This was particularly noticeable in the second year of follow-up where 26 recurrences were detected by CEA alone compared to 24 recurrences by combined CT scan, CXR, and colonoscopy during second year (Fig 3).
Fig 3.
Plot comparing detection of recurrence by carcinoembryonic antigen (CEA) alone with combined computed tomography (CT) scan, chest x-ray, and colonoscopy.
In the 146 patients who developed recurrence, 59 underwent a second surgery for cancer, 52 of which were considered curative intent, constituting 7% of the 791 primary patients and 36% of the recurrences. Fifty-five early-stage colon cancer patients experienced a recurrence and of these, 20 patients (36%) went on to a second surgery for recurrence involving the liver (n = 5), lung (n = 4); anastomosis (n = 7), and locoregional sites (n = 4). The median survival of the 20 patients with recurrence and early-stage colon cancer group who went on to second surgery was 51.2 months compared with 8.8 months for the 35 patients who had recurrence but did not undergo second surgery (P = .0002).
Ninety-one patients of the late-stage colon cancer group experienced recurrence and of these, 32 underwent second surgery (35.2%). Of these 32 patients, second surgery was for resection of recurrence involving the liver (n = 8), lung (n = 6), anastomosis (n = 8), and locoregional (n = 10). The median survival of the 32 late-stage colon cancer patients undergoing surgery for recurrence was 35.8 months compared with 11.3 months for the 59 patients who did not undergo second surgery (P < .0001).
Among patients who had curative intent surgery, subsequent analysis was performed focusing on the particular detection methods that diagnosed their recurrence. Only those whose recurrence was detected by colonoscopy had sufficient patients to compare survival between the early- and late-stage groups. For those in this group, median survival from salvage surgery was 51.0 months in the early-stage group versus 47.4 months in the late-stage group (P = .64).
DISCUSSION
Our analysis of the COST trial database confirms what was reported by the Cochrane review; that is, roughly one third of patients who experience recurrences after primary colon cancer resection can be treated with secondary curative-intent surgery when followed intensively after primary surgery. The 36% rate of secondary surgery we report from the COST trial is quite similar to the 28% rate of secondary surgery reported in the Cochrane review, and the published rates of between 35% to 47% for multiple series.4–6 Our report also confirmed that patients undergoing secondary surgery experience median survival of between 35.8 and 51.2 months, which is not dissimilar to median survivals in the literature of between 39.2 and 84.8 months.4 To our knowledge, this is the first prospective study to test and confirm the hypothesis that patients with early-stage disease experience the same benefits as those with late-stage disease after curative intent secondary resection. Indeed, our study showed that patients with recurrent disease who had early stage disease at the time of their original diagnosis can expect secondary surgery rates of 37%, and a median survival of 51.2 months, both of which are comparable to what is seen with late stage patients: 35.9% and 35.8 months, respectively. Having established benefits in early- and late-stage patients, we next investigated the database to address best methods of detection and compared our findings with recommended standards from national guidelines.
Current guidelines from the American Society of Colon and Rectal Surgeons (ASCRS)2 recommend at a minimum that colon cancer patients resected for cure should have CEA levels drawn every 4 months for 2 years and a colonoscopy performed at 1 year after surgery. Maximum recommendations from American Society of Clinical Oncology (ASCO),3 and National Comprehensive Cancer Network (NCCN)7 advise that CEA levels should be drawn every 3 months for 2 years and then twice yearly until 5 years after surgery. Further the ASCO and NCCN guidelines advise that patients should undergo an annual CT of the chest and abdomen with a colonoscopy at 1 or 3 years postoperative. The COST postoperative surveillance protocol, developed in 1993 for the purpose of rigorous local relapse detection, included, more frequent testing than advised by any of the current guidelines. The high frequency of postoperative testing, coupled with the excellent rate of long-term follow-up, made the COST database a unique resource from which we could investigate sites of first recurrence and methods of first detection to study the issue of optimal postoperative surveillance methods. The COST trial data, in fact, supports the frequent use of CEA and colonoscopy and suggests greater emphasis on lung imaging with less certainty on the value of abdomen and pelvis CT.
CEA blood levels were required as part of the COST trial study protocol to be drawn every 3 months for the first year and then twice a year for 4 subsequent years, with colonoscopy required year 1 and year 4 after resection. In our study, CEA elevation was the single most common test identifying recurrence, and CEA was equally effective in detecting recurrence in early- and late-stage disease. The COST data supports the value of CEA as a monitoring tool, and consistent with national guidelines we would encourage the 3-month testing interval for at least 1 year, if not for 2 years, and agree with twice yearly values until year 5 of follow-up. Similarly the COST trial data supports the nearly universal recommendations for colonoscopy to be performed 1 year postoperative.2,7 One third of the patients who went on to curative secondary surgery in the COST trial were diagnosed with colonoscopy within the first 2 years with the same benefits for early- and late-stage disease patients.
In regard to the role of CT of the chest and abdomen, this type of imaging was not advised as part of the ASCRS guidelines2 but was advised in both the ASCO3 and NCCN7guidelines. Based on our data, the emphasis on lung imaging is appropriate, but the role of CT of the abdomen is less certain. In the COST trial, a twice yearly chest x-ray was required in the first 2 years and an annual chest x-ray required for the subsequent 3 years. It is noteworthy that with this approach 23.6% of the recurrences occurred in the lungs. This rate exceeds the approximate 10% rate typically reported in the literature. Given that patients with isolated lung metastases often experience excellent 5-year survival rates (27% to 41%),8–10 we would recommend that patients undergo some manner of chest imaging as part of their postoperative surveillance approach. Whether annual CT of the chest is superior to a twice yearly CXR cannot be resolved at this juncture. In addition, we cannot conclude with confidence whether the CT of the abdomen provided benefit beyond CEA serum testing. Our uncertainty regarding the role of CT imaging in postoperative surveillance highlights a shortcoming of our study.
The COST trial provided excellent long-term data pertinent to a surgical population with stage I to III disease, treated with curative intent and observed using a specific practice-friendly protocol. It provided robust data on sites of first recurrence and comparisons between early- and late-stage patients. This database was not, however, designed to specifically test best practices for postoperative surveillance. We acknowledge the limitations of the database in particular with regards to results specific to best methods of detection and frequency of testing. The variable use of CT imaging for example prevents us from drawing conclusions regarding the value of CT beyond CEA. We also readily acknowledge that this report cannot address the larger question of whether intensive follow-up in patients with early-stage disease is as cost effective as other accepted medical practices.
We have demonstrated that patients with early-stage disease who develop a recurrence benefit to at least the same extent as their late-stage counterparts from intensive follow-up. We found that early-stage patients were less likely to experience recurrences involving multiple sites and demonstrated a trend toward longer median survival after second surgery. The societal and economic questions remain as to whether the frequency of events in patients with early-stage disease balances the increased effort and expense associated with implementation of such a follow-up regimen. Based on our data, one secondary resection would be possible for each, approximately 27 patients with early-stage disease under surveillance; this compares with approximately one per every eight patients with later-stage disease. There can be little doubt that finding recurrences early brings benefit for patients of either stage. Patients with isolated hepatic metastasis can expect 36% to 58% 5-year survival11–14 and those with isolated lung metastasis a 27% to 41%8–10 5-year survival. Chemotherapy can prolong life by approximately 1 to 2 years15 and improve quality of life.16,17 Only with additional economic studies will we be positioned to judge the broader question of cost to benefit ratio of early detection.
Footnotes
Supported by grants No. CA65157 (to the Mayo Clinic) and CA25224 (to the North Central Cancer Treatment Group).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Brent Christensen, Richard Whelan, Jace Hyder, Peter Marcello, Sergio Larach, David Lauter, Daniel J. Sargent, Heidi Nelson
Financial support: Heidi Nelson
Administrative support: Vassiliki L. Tsikitis, Heidi Nelson
Provision of study materials or patients: Brent Christensen, Richard Whelan, Jace Hyder, Peter Marcello, Sergio Larach, David Lauter, Daniel J. Sargent, Heidi Nelson
Collection and assembly of data: Kishore Malireddy, Erin A. Green, Heidi Nelson
Data analysis and interpretation: Vassiliki L. Tsikitis, Kishore Malireddy, Erin A. Green, Daniel J. Sargent, Heidi Nelson
Manuscript writing: Vassiliki L. Tsikitis, Kishore Malireddy, Erin A. Green, Daniel J. Sargent, Heidi Nelson
Final approval of manuscript: Vassiliki L. Tsikitis, Kishore Malireddy, Erin A. Green, Brent Christensen, Richard Whelan, Jace Hyder, Peter Marcello, Sergio Larach, David Lauter, Daniel J. Sargent, Heidi Nelson
REFERENCES
- 1.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD002200.pub2. CD002200. [DOI] [PubMed] [Google Scholar]
- 2.Anthony T, Simmang C, Hyman N, et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004;47:807–817. doi: 10.1007/s10350-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 3.Desch CE, Benson AB, III, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 4.Arriola E, Navarro M, Pares D, et al. Imaging techniques contribute to increased surgical rescue of relapse in the follow-up of colorectal cancer. Dis Colon Rectum. 2006;49:478–484. doi: 10.1007/s10350-005-0280-9. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Moranta F, Salo J, Arcusa A, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: A prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24:386–393. doi: 10.1200/JCO.2005.02.0826. [DOI] [PubMed] [Google Scholar]
- 6.Secco GB, Fardelli R, Gianquinto D, et al. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: A prospective, randomized and controlled trial. Eur J Surg Oncol. 2002;28:418–423. doi: 10.1053/ejso.2001.1250. [DOI] [PubMed] [Google Scholar]
- 7.NCCN colon cancer clinical practice guidelines in oncology. J Natl Compr Cancer Network. 2008;1:1–MS18. doi: 10.6004/jnccn.2003.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tepper JE, O'Connell M, Hollis D, et al. Analysis of surgical salvage after failure of primary therapy in rectal cancer: Results from Intergroup Study 0114. J Clin Oncol. 2003;21:3623–3628. doi: 10.1200/JCO.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Regnard JF, Grunenwald D, Spaggiari L, et al. Surgical treatment of hepatic and pulmonary metastases from colorectal cancers. Ann Thorac Surg. 1998;66:214–218. doi: 10.1016/s0003-4975(98)00269-0. [DOI] [PubMed] [Google Scholar]
- 10.Rena O, Casadio C, Viano F, et al. Pulmonary resection for metastases from colorectal cancer: Factors influencing prognosis: Twenty-year experience. Eur J Cardiothorac Surg. 2002;21:906–912. doi: 10.1016/s1010-7940(02)00088-x. [DOI] [PubMed] [Google Scholar]
- 11.Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 12.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Gramont A, Buyse M, Abrahantes JC, et al. Reintroduction of oxaliplatin is associated with improved survival in advanced colorectal cancer. J Clin Oncol. 2007;25:3224–3229. doi: 10.1200/JCO.2006.10.4380. [DOI] [PubMed] [Google Scholar]
- 16.Nordic Gastrointestinal Tumor Adjuvant Therapy Group. Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal cancer: A randomized trial. J Clin Oncol. 1992;10:904–911. doi: 10.1200/JCO.1992.10.6.904. [DOI] [PubMed] [Google Scholar]
- 17.Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: Interim results of a phase III trial. J Clin Oncol. 2003;21:2059–2069. doi: 10.1200/JCO.2003.11.126. [DOI] [PubMed] [Google Scholar]