Abstract
Purpose
To investigate the feasibility of intensity-modulated radiation therapy (IMRT) with or without chemotherapy, and to assess toxicities, failure patterns, and survivals in patients with nasopharyngeal carcinoma (NPC).
Patients and Methods
Radiation consisted of 70 Gy given to the planning target volumes of primary tumor plus any N+ disease and 59.4 Gy given to subclinical disease, delivered over 33 treatment days. Patients with stage T2b or greater or with N+ disease also received concurrent cisplatin (100 mg/m2) on days 1, 22, and 43 followed by adjuvant cisplatin (80 mg/m2) on day 1; fluorouracil (1,000 mg/m2/d) on days 1 through 4 administered every 4 weeks for three cycles. Tumor, clinical status, and acute/late toxicities were assessed. The primary objective was to test the transportability of IMRT to a multi-institutional setting.
Results
Between February 2003 and November 2005, 68 patients with stages I through IVB NPC (of which 93.8% were WHO types 2 and 3) were enrolled. Prescribed IMRT (target delineation) was given to 83.8%, whereas 64.9% received chemotherapy per protocol. The estimated 2-year local progression-free (PF), regional PF, locoregional PF, and distant metastasis–free rates were 92.6%, 90.8%, 89.3%, and 84.7%, respectively. The estimated 2-year PF and overall survivals were 72.7% and 80.2%, respectively. Acute grade 4 mucositis occurred in 4.4%, and the worst late grade 3 toxicities were as follows: esophagus, 4.7%; mucous membranes, 3.1%; and xerostomia, 3.1%. The rate of grade 2 xerostomia at 1 year from start of IMRT was 13.5%. Only two patients complained of grade 3 xerostomia, and none had grade 4 xerostomia.
Conclusion
It was feasible to transport IMRT with or without chemotherapy in the treatment of NPC to a multi-institutional setting with 90% LRPF rate reproducing excellent reports from single institutions. Minimal grade 3 and lack of grade 4 xerostomia were encouraging.
INTRODUCTION
Although rare among whites, nasopharyngeal carcinoma (NPC) is rather common among Asians.1 Standard treatment for NPC is radiotherapy (RT) for early-stage lesions or chemoradiotherapy for more advanced lesions.2,3 Historical local control (LC) rates for patients who undergo conventional RT range from 64% to 95% for T1-2 tumors but decrease to 44% to 68% in T3-4 lesions.4–9 Modern series, including recent phase II/III trials have shown an improvement in LC (up to 95%), which is likely attributed to major advances in imaging, radiation techniques, and the incorporation of chemotherapy into standard management.2,10–13
Retrospective studies that used RT alone for NPC suggest a correlation between LC and the dose delivered to the tumor.8,14 One study showed that LC was significantly improved when greater than 67 Gy were delivered to the tumor. In another retrospective series, improved LC was also attributed to improvements in technical accuracy. Because the nasopharynx is surrounded by normal critical structures, accuracy in dose delivery is essential in any dose-escalation studies.
Intensity-modulated RT (IMRT) modulates the radiation beams so that a high dose can be delivered to the tumor while the dose to the normal tissues is reduced.15 Investigators compared dosimetric plans of IMRT with conventional techniques for NPC and concluded that IMRT provided improved tumor coverage and spared normal tissues.16,17 Lee et al18 reported that the clinical experience of IMRT for NPC showed considerable recovery of salivary function with time. The authors also reported an excellent local progression-free rate of 97%. Two recent, phase III trials also showed improvements in salivary function in patients treated with IMRT versus conventional RT for early-stage NPC.19,20
Although IMRT for NPC is promising in terms of improvement of salivary function from different single institutions,18–23 the data still need to be validated in a multi-institutional setting. Therefore, the Radiation Therapy Oncology Group (RTOG) conducted this multicenter, phase II trial to test the feasibility of IMRT with or without chemotherapy for all stages of NPC. Preliminary results are reported in this article. This trial accrued patients before the publication of the two phase III trials that established improvement in salivary function by IMRT.
PATIENTS AND METHODS
Study Objectives/Patient Eligibility
Patients with previously untreated stages I to IVB NPC, with an Eastern Cooperative Oncology Group performance status of 0 or 1, who met criteria for blood counts and other tests (ie, WBC ≥ 4,000/μL; platelets ≥ 100,000/μL; serum creatinine ≤ 1.6 mg/dL) were eligible. Patients younger than 18 years old or those with a prior (ie, within 5 years) or synchronous malignancy, other than nonmelanoma skin cancer, were excluded. Pretreatment evaluations consisted of history and physical, dental, nutritional, audiogram, and laboratory studies. Magnetic resonance imaging (MRI) of the nasopharynx/neck was required unless there was a contraindication (ie, pacemaker); a computed tomography (CT) scan of the nasopharynx/neck was required if there was a contraindication to MRI. Additional tests to evaluate the extent of disease included chest x-ray, alkaline phosphatase, liver function tests, lactate dehydrogenase, and—when indicated—liver or bone scans. Positron emission tomography (PET) was optional. The disease was staged according to the 1997 American Joint Committee on Cancer Staging. All patients signed written informed consents, and the study was approved by participating centers' institutional review boards.
The primary objective was to test the feasibility of delivering IMRT in a multi-institutional setting. Other objectives were to determine rates of late xerostomia, locoregional (LR) control, distant metastasis (DM), progression-free survival (PFS), and overall survival (OS) and to determine compliance to combined modality.
Treatment
Computerized optimization was used with fusion of MRI and/or PET with treatment planning CT images, when possible, to accurately delineate the gross tumor volume (GTV), which included the primary disease and nodes greater than 1 cm in diameter or nodes with necrotic centers. The clinical target volume (CTV) denoted the subclinical regions at risk for involvement. Different CTVs were defined, as follows: CTV70 = GTV + 5-mm margin; CTV59.4 = CTV70 + 5 mm margin plus areas at risk for microscopic involvement, including the entire nasopharynx, retropharyngeal nodal regions, skull base, clivus, pterygoid fossae, parapharyngeal space, sphenoid sinus, the posterior third of the nasal cavity/maxillary sinuses that includes the pterygopalatine fossae, and levels I through V nodal regions. To account for organ motion/daily treatment set-up uncertainties, a planning target volume (PTV) was added (ie, additional margin of 3 to 5 mm) to each of the above CTVs. In areas where the GTV or the CTV was adjacent to critical normal structures (ie, brainstem) the margin could be reduced to 1 mm.
RT was delivered by using a simultaneous-integrated IMRT boost technique. PTV70 received 70 Gy in 2.12 Gy/fraction, and PTV59.4 received 59.4 Gy in 1.8 Gy/fraction, over 33 days. The lower neck could be included in the IMRT fields by using proper contours of CTVs (1.8 Gy/fraction) and by keeping the dose to the larynx to as low as possible without compromising target coverage. Alternatively, split-field IMRT technique was used, in which the low neck was treated with conventional anterior-posterior or anterior-posterior/posterior-anterior fields and received a total of 50.4 Gy. However, all involved nodes received a total dose of 70 Gy.
Patients with stage T2b or greater and/or N+ disease also received concurrent cisplatin (CDDP; 100 mg/m2) on days 1, 22, and 43 followed by adjuvant CDDP (80 mg/m2) on day 1; fluorouracil (FU; 1,000 mg/m2/d) on days 1 through 4 every 4 weeks for three cycles, as per Intergroup 0099. Guidelines for dose modifications were specified in the protocol.
Follow-Up/Statistical Considerations
Patients underwent weekly examinations during treatment. Follow-up evaluations occurred every 3 months during the first 2 years; every 6 months during years 3 to 5; then annually. In addition to tumor/clinical status, acute/late (ie, occurring > 90 days after start of RT) normal tissue effects were graded. Systemic/acute RT adverse effects were scored by using the National Cancer Institute Common Toxicity Criteria, version 2.0, whereas late RT effects were scored according to the RTOG/European Organisation for Research and Treatment of Cancer criteria. Whole salivary flows, both unstimulated and stimulated, also were collected at pretreatment and at 3, 6, and 12 months after treatment. No validated instruments were used in this protocol.
All treatment plans were reviewed by the Image-Guided Therapy Center, Washington University (St Louis, MO). All target volumes were evaluated by study chairs. Patients who were scored as having no or minor variations were considered compliant; those who had major variations or were not evaluable were considered noncompliant. Noncompliance could result from inaccurate target volume delineation (eg, not contouring of neck level V, 93% of the isodose surface covering less than 95% of the target volumes, greater than 5% of the PTV70 receiving greater than 115%, and/or greater than 60% of each parotid glands receiving greater than 30 Gy). An important aspect of this study was the determination that the locoregional progression-free (LRPF) rate—in which failure was defined as local and/or regional progression, death as a result of study cancer without documented site of failure, or death as a result of unknown causes—was not compromised by the use of IMRT. The sample size was estimated on the basis of this end point. A rate of 80% was targeted, and 65% was considered unacceptable. By using Fleming's one-sample, multiple-testing procedure with type I and type II errors both set at 0.10, 57 evaluable patients were required.24 The sample size was adjusted by 10% to allow for ineligible patients and loss to follow-up, so the total sample size was 64 patients. If 15 of the first 57 evaluable patients failed, the LRPF rate would be considered unacceptable. For the feasibility end point, a compliance rate of 90% was targeted, and 75% was considered unacceptable. If 10 or more of the first 57 patients were noncompliant, the treatment was deemed nontransportable to a multi-institutional setting.
Another end point was the rate of grade 2 or greater xerostomia 1 year (± 3 months) after the start of IMRT. A rate of 30% was targeted, and 55% (eg, if 27 of 57 patients experienced this toxicity) was considered unacceptable. Other secondary end points were acute/late effects; DM-free rates, in which failure was DM; PFS, in which failure was local, regional, or distant progression or death as a result of any cause; overall survival (OS), in which failure was death as a result of any cause. All percentages reported for the sites of first failures were based on all 68 patients.
OS and PFS were estimated by using the Kaplan-Meier method,25and local, regional, LRPF, and DM-free rates were calculated by using the method of cumulative incidence26 to account for the competing risk of death without failure. All time-to-failure end points were calculated from the date of registration to the date of failure/last follow-up for patients who did not experience failure. Patients who developed DM were still observed for LR failure.
RESULTS
Patient Characteristics/Treatment Compliance
From February 2003 to November 2005, 68 patients were accrued from 17 centers in North America, and nine centers accrued greater than two patients who were eligible for analysis. Table 1 lists the pretreatment patient demographic and clinical tumor characteristics. Fifty-seven patients (83.8%) had stages IIB to IVB disease that required chemotherapy.
Table 1.
Distribution of Patient Demographic and Clinical Tumor Characteristics
| Characteristic | Patients |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 48.5 | |
| Range | 18-73 | |
| Sex | ||
| Male | 51 | 75.0 |
| Female | 17 | 25.0 |
| Ethnicity | ||
| White | 37 | 54.4 |
| Asian | 23 | 33.8 |
| Black | 5 | 7.4 |
| Native Hawaiian or other Pacific Islander | 1 | 1.5 |
| Unknown | 2 | 2.9 |
| Zubrod performance scale | ||
| 0 | 47 | 69.1 |
| 1 | 21 | 30.9 |
| Primary site | ||
| Nasopharynx NOS | 35 | 51.5 |
| Posterior superior wall | 24 | 35.3 |
| Lateral wall | 9 | 13.2 |
| T stage | ||
| 1 | 17 | 25.0 |
| 2a | 12 | 17.6 |
| 2b | 16 | 23.5 |
| 3 | 10 | 14.7 |
| 4 | 13 | 19.1 |
| N stage | ||
| 0 | 18 | 26.5 |
| 1 | 21 | 30.9 |
| 2 | 21 | 30.9 |
| 3a | 2 | 2.9 |
| 3b | 6 | 8.8 |
| AJCC stage grouping | ||
| I | 9 | 13.2 |
| IIA | 2 | 2.9 |
| IIB | 17 | 25.0 |
| III | 21 | 30.9 |
| IVA | 11 | 16.2 |
| IVB | 8 | 11.8 |
| WHO histology | ||
| I | 6 | 8.8 |
| II or IIA | 24 | 35.3 |
| III or IIB | 37 | 54.4 |
| Unknown | 1 | 1.5 |
Abbreviations: NOS, not otherwise specified; AJCC, American Joint Committee on Cancer.
Only one patient was deemed not evaluable for assessment of target delineation, because digital radiation planning data was not received. Major target variation was noted in 10 patients overall and in nine of the first 57 patients. Reasons were incorrect contouring of CTV59.4; missing the posterior third of the maxillary sinus; or incomplete coverage of the level-V nodal region. The estimated compliance rate was 83.8% (95% CI, 72.9 to 91.6). A total of 89.7% of the patients received 70 Gy.
Chemotherapy was given to all 57 patients who had stages IIB to IVB disease. Thirty-seven patients (64.9%) were scored by the medical oncology chair per protocol, 19 (33.3%) of whom had deviations, and one (1.8%) of whom was not evaluable. Fifty patients (87.7%) received all three concurrent CDDP cycles, whereas an additional six patients (10.5%) received two concurrent cycles. Only 26 patients (45.6%) received all three cycles of adjuvant CDDP/FU. Overall, only 25 patients (43.9%) received all cycles of concurrent and adjuvant chemotherapy. Only 13 patients (22.8%) received all cycles with at least 80% of the planned dose.
Failure Pattern
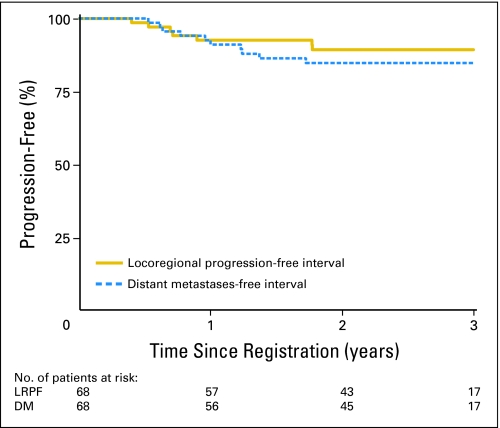
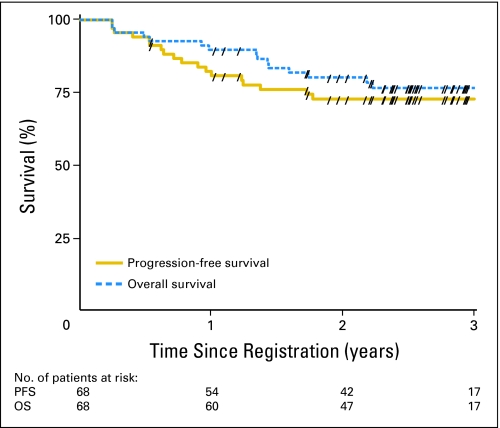
Median follow-up for surviving patients was 2.6 years (range, 0.5 to 4.6 years). There were seven patients who experienced LR failure: one was local only, two were regional only, three were local and regional, and one was death as a result of study cancer without documented site of failure. All seven failures occurred in the first 57 evaluable patients, and all had T2b-4 and/or N+ and WHO disease types 2 to 3. Only one patient had WHO type 1 disease. Some regional failures were attributed to incorrect contouring of level V. Almost all patients were treated with a single IMRT technique. Ten patients developed DM; sites of failure were liver (n = 3), bone (n = 2), lung (n = 2), liver and lung (n = 1), epidural space of spine (n = 1), and trachea (n = 1). Eight of these 10 patients received all three cycles of both concurrent and adjuvant chemotherapies, but only three had at least 80% of planned dose. First failure sites were as follows: distant (n = 10; 14.7%), locoregional (n = 2; 2.9%), local (n = 1; 1.5%), regional (n = 1; 1.5%), death with no evidence of progression (n = 3; 4.4%), and death as a result of study cancer without documented site of failure (n = 1; 1.5%). This last patient failed treatment (locally, regionally, and distantly) and died. The cause of death was not clearly documented and, hence, was scored as LR failure. Two patients developed second primaries (one each in the nasal cavity and prostate). At the time of publication, 48 patients were alive without any documented progression. Fifteen patients died; the cause of death was the study cancer in 12 of these patients; treatment complications were the cause in three patients. Table 2 lists the 2-year estimated rates and 95% CIs for all time-to-event end points. Figures 1 and 2 show the Kaplan-Meier curves for LRPF and DM-free and for PFS and OS rates, respectively. At the time of publication, the median had not been reached for any time-to-event end point.
Table 2.
2-Year Estimates of Time-to-Event End Points
| End Point | Patient Group |
|||
|---|---|---|---|---|
| All |
Stages IIB to IVB |
|||
| 2-Year Estimate | 95% CI | 2-Year Estimate | 95% CI | |
| Local progression-free interval | 92.6 | 86.3 to 98.9 | 91.2 | 83.8 to 98.6 |
| Regional progression-free interval | 90.8 | 83.6 to 97.9 | 89.2 | 81.0 to 97.5 |
| Locoregional progression-free interval | 89.3 | 81.7 to 96.9 | 87.5 | 78.7 to 96.3 |
| Distant metastases–free interval | 84.7 | 75.9 to 93.5 | 82.1 | 72.0 to 92.3 |
| Progression-free survival | 72.7 | 61.9 to 83.5 | 68.0 | 55.7 to 80.2 |
| Overall survival | 80.2 | 70.5 to 89.8 | 76.7 | 65.6 to 87.8 |
Fig 1.
Locoregional progression-free (LRPF) and distant metastasis (DM) -free rates in patients with nasopharyngeal cancer treated with intensity-modulated radiation therapy with or without chemotherapy.
Fig 2.
Progression-free survival (PFS) and overall survival (OS) in patients with nasopharyngeal cancer treated with intensity-modulated radiation therapy with or without chemotherapy. Forward slashes indicate censored patients.
Acute/Late Toxicity
Three patients (4.4%) died as a result of complications of protocol treatment (dysphagia/esophagitis 83 days after start of IMRT; febrile neutropenia at 80 days; pneumonitis at 74 days). In addition, eight patients (11.8%) experienced grade 4 acute adverse effects, and 42 patients (61.8%) experienced grade 3 acute adverse effects. The most common acute, grade 4 adverse effects were leukopenia (n = 4), anorexia (n = 3), radiation mucositis (n = 3), hyponatremia (n = 2), and neutropenia (n = 2). Acute grades 2, 3, and 4 mucositis/stomatitis toxicities were 29.4%, 36.8%, and 4.4%, respectively. Table 3 summarizes the chemotherapy and acute IMRT toxicities.
Table 3.
Type and Frequency of Acute Adverse Effects Observed in 68 Patients
| Adverse Effect | No. of Patients by Toxicity Grade |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Allergy/immunology | 3 | 0 | 0 | 0 | 0 |
| Auditory/hearing | 7 | 20 | 10 | 0 | 0 |
| Blood/bone marrow | 5 | 14 | 31 | 4 | 0 |
| Cardiovascular | |||||
| Arrhythmia | 3 | 2 | 0 | 0 | 0 |
| General | 6 | 3 | 5 | 0 | 0 |
| Constitutional symptom | 17 | 24 | 16 | 1 | 0 |
| Dermatologic/skin | 16 | 30 | 9 | 0 | 0 |
| Endocrine | 1 | 6 | 1 | 0 | 0 |
| Gastrointestinal | 3 | 19 | 41 | 4 | 1 |
| Hemorrhage | 13 | 0 | 0 | 0 | 0 |
| Hepatic | 21 | 9 | 1 | 1 | 0 |
| Infection febrile neutropenia | 4 | 5 | 5 | 1 | 1 |
| Lymphatic | 2 | 1 | 0 | 0 | 0 |
| Metabolic/laboratory | 16 | 9 | 11 | 3 | 0 |
| Musculoskeletal | 1 | 2 | 2 | 0 | 0 |
| Neurology | 21 | 6 | 3 | 1 | 0 |
| Ocular/visual | 2 | 1 | 1 | 0 | 0 |
| Pain | 7 | 21 | 10 | 1 | 0 |
| Pending | 3 | 0 | 0 | 0 | 0 |
| Pulmonary | 10 | 7 | 0 | 1 | 1 |
| Renal/genitourinary | 12 | 7 | 2 | 0 | 0 |
| Sexual/reproductive function | 1 | 1 | 3 | 0 | 0 |
| Worst nonhematologic | |||||
| No. of patients | 3 | 13 | 43 | 6 | 3 |
| % of total patients | 4.4 | 19.1 | 63.2 | 8.8 | 4.4 |
| Worst overall | |||||
| No. of patients | 3 | 12 | 42 | 8 | 3 |
| % of total patients | 4.4 | 17.6 | 61.8 | 11.8 | 4.4 |
Table 4 lists the type and frequency of late IMRT adverse effects. Overall, 13 patients (20.3%) had grade 3 late toxicity, most commonly hearing impairment5 and dysphagia.3 Of the 48 patients alive without progression of disease, two (4.2%) were still dependent on percutaneous endoscopic gastrostomy feeding at their last follow-up visits. Prophylactic percutaneous endoscopic gastrostomy placement was not mandatory for this protocol.
Table 4.
Type and Frequency of Late Radiotherapy Adverse Effects Observed in 64 Patients
| Adverse Effect | No. of Patients by Toxicity Grade |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Skin* | 16 | 3 | 0 |
| Mucous membrane | 26 | 13 | 2 |
| Subcutaneous tissue* | 15 | 3 | 1 |
| Salivary gland | 29 | 19 | 2 |
| Esophagus | 14 | 9 | 3 |
| Larynx | 10 | 2 | 0 |
| Spinal cord | 3 | 0 | 0 |
| Brain | 2 | 0 | 0 |
| Bone† | 1 | 1 | 0 |
| Joint | 10 | 1 | 0 |
| Auditory/hearing | 14 | 4 | 5 |
| Other | 14 | 11 | 3 |
| Worst overall | |||
| No. of patients | 18 | 28 | 13 |
| % of total patients | 28.1 | 43.8 | 20.3 |
Within radiation therapy field.
Including osteonecrosis.
Xerostomia
The worst late, grade 2 and 3 xerostomia scores from start of treatment were 29.7% and 3.1%, respectively. The 1-year estimated rates of grades 1 and 2 xerostomia were 51.9% (95% CI, 37.6 to 66.0) and 13.5% (95% CI, 5.6 to 25.8), respectively. Seven patients overall and four of the first 57 had grade 2 xerostomia at 1 year. Salivary output in grams at pretreatment and at 3, 6, and 12 months from the end of IMRT were recorded. There was minimal difference between pretreatment and post-treatment objective mean stimulated and unstimulated salivary function at 12 months, which indicated recovery of salivary flow after treatment with IMRT.
DISCUSSION
The proximity of the nasopharynx to critical normal tissues, such as the brainstem/optic structures, makes it difficult to treat the tumor with two-dimensional techniques while the dose to surrounding organs is kept within an acceptable range.16,17,27 As a result, portions of the tumor were often underdosed with the goal of normal tissue protection. IMRT enables coverage of irregularly shaped tumor while it limits the dose to critical organs, and several single institutions have reported greater than 90% LR control rates.18,21,22,23 Lee et al18 reported a remarkable LC rate of 97%, despite around 50% of the patients having T3-4 disease. It is noteworthy that other factors, such as advances in imaging for better tumor delineation, the addition of chemotherapy, and better supportive care, also contribute to the significant improvements in LR control.
Building on the result from University of California, San Francisco, RTOG conducted this phase II trial to test whether the result of IMRT can be reproduced in a multi-institutional setting. Per statistical design, the treatment was considered transportable if less than 10 patients of 57 were scored as having major variations. Because of the protocol requirement for centralized review, it was possible to assess the quality of the target delineation and, when needed, to provide feedback to participating centers on target volume specifications. Consequently, there was a lower percentage of major deviations for the second/subsequent patients entered at a given institution (31% deviation for the first patient, which diminished to 12% with subsequent patients).
Although NPC can be treated effectively with non-IMRT planning techniques, many patients complain of permanent xerostomia as a result of the delivery of a near full dose of RT to the bilateral parotid glands.5,6,28,29 The degree of xerostomia is dependent largely on the dose and volume of salivary gland in the radiation field. Studies have shown that salivary flows are markedly reduced after 10 to 15 Gy of RT.30,31 High doses to most of the gland will result in permanent xerostomia, which compromises patient quality of life.32–34 The degree of xerostomia is largely dependent on the dose/volume of the salivary gland in the radiation field.30,31 IMRT can limit the dose delivered to these glands without compromising tumor coverage. Multiple clinical studies have shown a lesser degree of xerostomia after IMRT relative to the two-dimensional technique.18,21-23,35 Kam et al19 showed that IMRT had lower incidence of observer-rated xerostomia versus the two-dimensional arm (39.3% v 82.1%; P = .001) at 1 year after treatment for early-stage NPC. There was also higher stimulated parotid flow rate (P < .001) as well as a higher stimulated whole saliva flow rate (P = .001) in patients who received IMRT. Pow et al20 showed that IMRT was significantly better than conventional RT in terms of parotid sparing and improved quality of life for early-stage NPC. Although xerostomia and sticky saliva were problems reported in both groups at 2 months post-RT, there was consistent improvement over time with xerostomia-related symptoms at 12 months post-IMRT. This study also showed that only 14% of patients reported grade 2 xerostomia at 1 year from the start of IMRT and that 35% of the patients had no complaints of xerostomia at all.
One of our study end points focused on compliance to combined modality treatment. Our hypothesis was that a potential reduction in the radiation adverse effects on salivary flow by using IMRT would increase patient compliance to combined therapy. We found that 90% of the patients received the full 70 Gy and that 88% of the patients with stageT2b or greater and/or N+ disease received three cycles of concurrent cisplatin. This compares favorably to the compliance rates of 63% in the Intergroup 0099 trial,2 71% in the Singapore randomized trial,12 and 52% in the Hong Kong NPC-9901 trial.11 In addition, 98% of the patients received at least two cycles of CDDP compared with 86% in the Intergroup 0099 trial. Adjuvant chemotherapy compliance to three cycles of CDDP and FU was, however, slightly lower (46% v 55%, 57%, and 76%) in this RTOG study versus in the Intergroup 0099, Singapore, and Hong Kong NPC-9901 trials, respectively. The value of the adjuvant treatment is not clearly known, as at least one study did not include adjuvant chemotherapy also showed superb outcomes.36
Because RT, whether by using IMRT or non-IMRT techniques, with chemotherapy achieved superb LR control in patients who presented with locoregionally advanced NPC, the development of DM has become the main pattern of relapse (up to 30%) and cause of death.18,21,22 The 2-year DM rate of patients who had T2b or greater and/or N+ disease in this series was 18%. This resulted in 2-year PFS and OS of 68% and 77%, respectively. These numbers are similar to the combined therapy arm of Intergroup 0099 trial (3-year PFS and OS rates were 69% and 78%, respectively).2
Given that the predominant pattern of failure in locoregionally advanced NPC treated with IMRT and chemotherapy is DM, and given that patients with NPC who have elevated vascular endothelial growth factor have a higher likelihood of recurrence, DM, and decreased survival, we have embarked on testing the role of antiangiogenic agents to the treatment strategy in this study for this population.37–39 In a recently closed, phase II trial (RTOG 0615), bevacizumab (a monoclonal antibody directed against vascular endothelial growth factor) was added to the concurrent and adjuvant phases of therapy. The reason for combining bevacizumab with the concurrent phase of chemoradiotherapy is to enhance the effect of CDDP in potentially sterilizing distant micrometastases from the beginning of treatment, because compliance to the adjuvant phase has been generally low.
In summary, IMRT with or without chemotherapy produced excellent LPF, RPF, and LRPF rates for NPC. This is the first paper to demonstrate the transportability of IMRT from large institutions to multi-institutional setting. No excessive, unwarranted toxicities have been observed. High rates of compliance to concurrent chemotherapy were achieved, but compliance to adjuvant chemotherapy was poor. Late xerostomia associated with this regimen has decreased. On the basis of the excellent LR control and still-high rates of DM reported in this trial, along with other IMRT and none-IMRT studies, more effective systemic therapy is highly warranted to additionally improve the outcome for these patients.
Appendix
Table A1.
Accrual Centers
| Accrual Center | Patients |
|
|---|---|---|
| No. | % | |
| M. D. Anderson Cancer Center | 14 | 20.6 |
| University of California, San Francisco | 10 | 14.7 |
| Radiological Associates of Sacramento | 7 | 10.3 |
| Washington University | 7 | 10.3 |
| Memorial Sloan-Kettering Cancer Center | 5 | 7.4 |
| University of Alabama, Birmingham | 5 | 7.4 |
| Tom Baker Cancer Centre | 4 | 5.9 |
| University of California, Davis | 4 | 5.9 |
| Cross Cancer Institute | 2 | 2.9 |
| Mayo Clinic | 2 | 2.9 |
| McGill University | 2 | 2.9 |
| Fox Chase Cancer Center | 1 | 1.5 |
| Gulf Coast Cancer Treatment Center | 1 | 1.5 |
| McKay-Dee Hospital | 1 | 1.5 |
| Medical College of Wisconsin | 1 | 1.5 |
| Thomas Jefferson University | 1 | 1.5 |
| Wilford Hall US Air Force Medical Center | 1 | 1.5 |
Footnotes
Supported by Grants No. U10 CA21661, U10 CA37422, U10 CA32115, and U24 CA81647 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Nancy Lee, Jonathan Harris, Adam S. Garden, K. Kian Ang
Provision of study materials or patients: Nancy Lee, Adam S. Garden, Walter Bosch, William H. Morrison, Jeanne Quivey, Wade Thorstad, Christopher Jones, K. Kian Ang
Collection and assembly of data: Nancy Lee, Jonathan Harris, William Straube
Data analysis and interpretation: Nancy Lee, Jonathan Harris, Adam S. Garden, Bonnie Glisson, K. Kian Ang
Manuscript writing: Nancy Lee, Jonathan Harris, Adam S. Garden, K. Kian Ang
Final approval of manuscript: Nancy Lee, Jonathan Harris, Adam S. Garden, Bonnie Glisson, Ping Xia, Walter Bosch, William H. Morrison, Jeanne Quivey, Wade Thorstad, Christopher Jones, K. Kian Ang
REFERENCES
- 1.Yu MC. Diet and nasopharyngeal carcinoma. FEMS Microbiol Immunol. 1990;2:235–242. doi: 10.1111/j.1574-6968.1990.tb03524.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 3.Langendijk JA, Leemans CR, Buter J, et al. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: A meta-analysis of the published literature. J Clin Oncol. 2004;22:4604–4612. doi: 10.1200/JCO.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 4.Chu AM, Flynn MB, Achino E, et al. Irradiation of nasopharyngeal carcinoma: Correlations with treatment factors and stage. Int J Radiat Oncol Biol Phys. 1984;10:2241–2249. doi: 10.1016/0360-3016(84)90229-3. [DOI] [PubMed] [Google Scholar]
- 5.Hoppe RT, Goffinet DR, Bagshaw MA. Carcinoma of the nasopharynx: Eighteen years' experience with megavoltage radiation therapy. Cancer. 1976;37:2605–2612. doi: 10.1002/1097-0142(197606)37:6<2605::aid-cncr2820370607>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Mesic JB, Fletcher GH, Goepfert H. Megavoltage irradiation of epithelial tumors of the nasopharynx. Int J Radiat Oncol Biol Phys. 1981;7:447–453. doi: 10.1016/0360-3016(81)90129-2. [DOI] [PubMed] [Google Scholar]
- 7.Bailet JW, Mark RJ, Abemayor E, et al. Nasopharyngeal carcinoma: Treatment results with primary radiation therapy. Laryngoscope. 1992;102:965–972. doi: 10.1288/00005537-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Vikram B, Mishra UB, Strong EW, et al. Patterns of failure in carcinoma of the nasopharynx: I. Failure at the primary site. Int J Radiat Oncol Biol Phys. 1985;11:1455–1459. doi: 10.1016/0360-3016(85)90332-3. [DOI] [PubMed] [Google Scholar]
- 9.Sanguineti G, Geara FB, Garden AS, et al. Carcinoma of the nasopharynx treated by radiotherapy alone: Determinants of local and regional control. Int J Radiat Oncol Biol Phys. 1997;37:985–996. doi: 10.1016/s0360-3016(97)00104-1. [DOI] [PubMed] [Google Scholar]
- 10.Lee AW, Tung SY, Chan AT, et al. Preliminary results of a randomized study (NPC-9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:142–151. doi: 10.1016/j.ijrobp.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 11.Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. 2005;23:6966–6975. doi: 10.1200/JCO.2004.00.7542. [DOI] [PubMed] [Google Scholar]
- 12.Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23:6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 13.Lee AW, Sze WM, Au JS, et al. Treatment results for nasopharyngeal carcinoma in the modern era: The Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 14.Marks JE, Bedwinek JM, Lee F. Dose-response analysis for nasopharyngeal carcinoma. Cancer. 1982;50:1042–1050. doi: 10.1002/1097-0142(19820915)50:6<1042::aid-cncr2820500604>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.Nutting C, Dearnaley DP, Webb S. Intensity-modulated radiation therapy: A clinical review. Br J Radiol. 2000;73:459–469. doi: 10.1259/bjr.73.869.10884741. [DOI] [PubMed] [Google Scholar]
- 16.Xia P, Fu KK, Wong GW, et al. Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000;48:329–337. doi: 10.1016/s0360-3016(00)00585-x. [DOI] [PubMed] [Google Scholar]
- 17.Kam MK, Chau RM, Suen J, et al. Intensity-modulated radiotherapy in nasopharyngeal carcinoma: Dosimetric advantage over conventional plans and feasibility of dose escalation. Int J Radiat Oncol Biol Phys. 2003;56:145–157. doi: 10.1016/s0360-3016(03)00075-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: An update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 19.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 20.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Wolden SL, Chen WC, Pfister DG, et al. Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: Update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys. 2006;64:57–62. doi: 10.1016/j.ijrobp.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 22.Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: The Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60:1440–1450. doi: 10.1016/j.ijrobp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 23.Kwong DL, Pow EH, Sham JS, et al. Intensity-modulated radiotherapy for early-stage nasopharyngeal carcinoma: A prospective study on disease control and preservation of salivary function. Cancer. 2004;101:1584–1593. doi: 10.1002/cncr.20552. [DOI] [PubMed] [Google Scholar]
- 24.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 25.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Amer Stat Assoc. 1958;53:457. [Google Scholar]
- 26.Kalbfleisch JG, Sprott DA. Statistical analysis of data bearing on the number of particles required to form a plaque. J Hyg (Lond) 1974;73:27–34. doi: 10.1017/s0022172400023792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chau RM, Teo PM, Kam MK, et al. Dosimetric comparison between 2-dimensional radiation therapy and intensity modulated radiation therapy in treatment of advanced T-stage nasopharyngeal carcinoma: To treat less or more in the planning organ-at-risk volume of the brainstem and spinal cord. Med Dosim. 2007;32:263–270. doi: 10.1016/j.meddos.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: Overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23:261–270. doi: 10.1016/0360-3016(92)90740-9. [DOI] [PubMed] [Google Scholar]
- 29.Perez CA, Devineni VR, Marcial-Vega V, et al. Carcinoma of the nasopharynx: Factors affecting prognosis. Int J Radiat Oncol Biol Phys. 1992;23:271–280. doi: 10.1016/0360-3016(92)90741-y. [DOI] [PubMed] [Google Scholar]
- 30.Leslie MD, Dische S. The early changes in salivary gland function during and after radiotherapy given for head and neck cancer. Radiother Oncol. 1994;30:26–32. doi: 10.1016/0167-8140(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 31.Mira JG, Wescott WB, Starcke EN, et al. Some factors influencing salivary function when treating with radiotherapy. Int J Radiat Oncol Biol Phys. 1981;7:535–541. doi: 10.1016/0360-3016(81)90140-1. [DOI] [PubMed] [Google Scholar]
- 32.Cooper JS, Fu K, Marks J, et al. Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys. 1995;31:1141–1164. doi: 10.1016/0360-3016(94)00421-G. [DOI] [PubMed] [Google Scholar]
- 33.Shannon IL, Starcke EN, Wescott WB. Effect of radiotherapy on whole saliva flow. J Dent Res. 1977;56:693. doi: 10.1177/00220345770560062201. [DOI] [PubMed] [Google Scholar]
- 34.Balogh JM, Sutherland SE. Osteoradionecrosis of the mandible: A review. J Otolaryngol. 1989;18:245–250. [PubMed] [Google Scholar]
- 35.Eisbruch A, Ship JA, Martel MK, et al. Parotid gland sparing in patients undergoing bilateral head and neck irradiation: Techniques and early results. Int J Radiat Oncol Biol Phys. 1996;36:469–480. doi: 10.1016/s0360-3016(96)00264-7. [DOI] [PubMed] [Google Scholar]
- 36.Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: Positive effect on overall and progression-free survival. J Clin Oncol. 2003;21:631–637. doi: 10.1200/JCO.2003.06.158. [DOI] [PubMed] [Google Scholar]
- 37.Krishna SM, James S, Balaram P. Expression of VEGF as prognosticator in primary nasopharyngeal cancer and its relation to EBV status. Virus Res. 2006;115:85–90. doi: 10.1016/j.virusres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Qian CN, Zhang CQ, Guo X, et al. Elevation of serum vascular endothelial growth factor in male patients with metastatic nasopharyngeal carcinoma. Cancer. 2000;88:255–261. [PubMed] [Google Scholar]
- 39.Wakisaka N, Wen QH, Yoshizaki T, et al. Association of vascular endothelial growth factor expression with angiogenesis and lymph node metastasis in nasopharyngeal carcinoma. Laryngoscope. 1999;109:810–814. doi: 10.1097/00005537-199905000-00024. [DOI] [PubMed] [Google Scholar]