Abstract
The International Union Against Tuberculosis and Lung Disease/World Health Organization Global Project on Anti-Tuberculosis Drug Resistance Surveillance recently released the fourth global survey, which documents the highest burden of multidrug-resistant tuberculosis (TB) yet reported. The best estimate of the number of new cases of multidrug-resistant disease occurring in 2006 is close to half a million and the recent recognition of extensively drug-resistant TB underscores the need for expanded surveillance, especially in areas in which TB control programs have been compromised by an escalating burden of TB and HIV. We review current methods used for drug resistance surveillance and describe methodologic obstacles for estimating the true extent of the problem, particularly in settings where HIV/TB coinfection is common or where a substantial portion of TB cases are treated in the private sector. We highlight practical challenges to the validity of surveillance studies and discuss how additional investment in laboratory capacity, diagnostic technologies, and sentinel site surveillance can improve our ability to estimate of the burden of drug-resistant TB.
Keywords: public health surveillance, drug resistance, epidemiology, bias (epidemiology), HIV
Recent recognition of the widespread distribution of highly drug resistant Mycobacterium tuberculosis has heightened concern that the emergence of drug resistance may compromise the effectiveness of tuberculosis (TB) control programs (1–3). In response to the emergence of multidrug-resistant (MDR; resistance to at least isoniazid and rifampin) and extensively drug-resistant TB (XDR; MDR with additional resistance to at least one fluorquinolone and one injectable TB antibiotic), the World Health Organization (WHO) and Stop TB partnership has revised the Global Plan to Stop TB, 2006–2015 (4). One of the key priorities outlined in the Global MDR TB and XDR TB Response Plan 2007–2008 is increasing investment in the accurate assessment of the magnitude and trend of drug-resistant disease (5).
Current estimates of the burden of drug-resistant TB reflect data amassed by the WHO and the International Union Against Tuberculosis and Lung Disease Global Project on Drug Resistance Surveillance (6). The most recent report provides data on anti-TB drug resistance in 81 countries studied between 2002 and 2007 (7). Approximately half of these data are from high-resource countries that perform routine diagnostic drug susceptibility testing (DST) on all patients with incident TB; the other half are from samples of incident TB cases in areas in which DST is not routinely performed. Despite the tremendous value of these surveillance data, the true global burden of MDR and XDR TB is not entirely clear; large gaps remain in some of the most affected areas, such as countries of the former Soviet Union, other parts of Asia, and much of Africa where the HIV burden is highest. Furthermore, because few national reference laboratories routinely test for susceptibility to second-line drugs, only a minority of countries can adequately measure the burden of XDR TB. In response to recent events, the WHO and the global TB community have called for additional surveillance studies to assess the true distribution and burden of MDR and XDR TB (1).
The surveillance of drug-resistant TB is complicated by the need to culture the organism to conduct DST. In many locations, the clinical diagnosis of TB relies on the demonstration of acid-fast bacilli by sputum smear microscopy. Unlike smear microscopy, culture of M. tuberculosis requires specialized laboratories equipped to minimize biosafety risk. DST to first-line drugs requires additional expertise beyond culture, whereas testing for second-line drug resistance remains a highly specialized activity that is reliably conducted at relatively few national and supranational reference laboratories. In high-resource settings, culture and DST are the standard of care and are conducted on all patients, and thus drug-resistance data from these countries represent all notified cases. In resource-constrained settings in which culture and DST are not routinely performed, surveillance for drug-resistant TB requires systematic studies that randomly sample patients with TB and which supplement standard diagnosis by microscopy with culture and DST to first- and sometimes second-line drugs.
Given the limited capacity of many national labs to conduct routine DST, it is essential to design and carry out studies that capture the data necessary to measure the burden of disease but which can also be practically implemented. Here we consider the role of surveillance in assessing the burden of drug-resistant TB, review the design of surveys in high-burden/low-resource settings in which sampling must be done, describe methodologic challenges and potential sources of bias associated with these studies, and discuss interim surveillance guidelines and recommendations recently issued by the WHO.
WHAT ARE THE OBJECTIVES FOR SURVEILLANCE OF DRUG-RESISTANT TB?
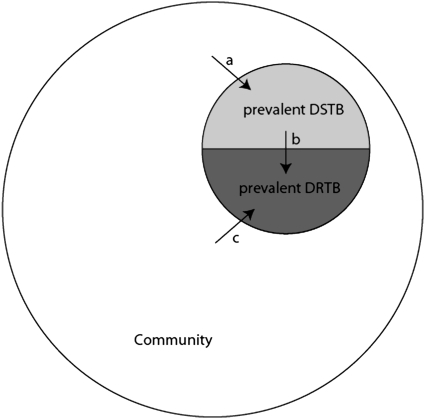
Although surveillance studies share the common goal of measuring the burden of drug-resistant TB, these studies may differ both in the specific objectives they are designed to accomplish and in the public health policy implications of their results. First, surveillance studies may quantify the incidence (rate of occurrence) of drug resistance among individuals initially infected with drug-sensitive TB who acquire resistance as a result of ineffective therapy (Figure 1, arrow b). If such a study detected a high incidence of acquired resistance, this result might indicate deficiencies in the treatment of individual patients and would suggest that improvements in availability, delivery, and adherence to standard treatment regimens would reduce resistance in this setting.
Figure 1.
Mechanisms of emergence of drug-resistant tuberculosis (DRTB) in a community. Incidence of drug-sensitive TB (DSTB) contributes to the pool of prevalent DSTB (arrow a). Incident DRTB occurs either through acquired drug resistance (arrow b) or through transmitted/primary resistance (arrow c). For simplicity, this figure groups all drug-resistant strains together; in reality, the pool of resistant strains is heterogeneous and strains that are resistant to some drugs can acquire additional resistance to others.
Second, surveillance studies may measure the transmission of drug-resistant TB by identifying individuals who are initially infected with drug-resistant strains of M. tuberculosis (Figure 1, arrow c). In this case, a high incidence of resistance among previously untreated individuals may indicate the failure of infection control, and would suggest that improvements in the detection, diagnosis, treatment, and isolation of individuals who are infectious with drug-resistant strains would reduce the transmission of these strains.
Third, surveillance studies may assess the prevalence of drug resistance (proportion of the population affected), a measure that reflects both the incidence of resistant TB (whether it occurs through acquisition or transmission) and the duration of disease. Often, the proportion of prevalent TB that is resistant is reported (Figure 1, lightly shaded semicircle/[lightly + darkly shaded semicircles]). The number of prevalent cases of drug-resistant TB represents the current resource demand for treatment with second-line antibiotics. Surveillance for chronic noninfectious diseases usually involves measuring the incidence of disease, rather than the prevalence, because a change in prevalence can reflect a change in either (or both) the occurrence and duration of the illness. For example, an increase in the prevalence of a terminal disease may not indicate a worrisome increase in occurrence but rather reflect an increase in the duration of the disease brought about by an intervention that improves prognosis but does not result in cure. In the case of an infectious disease like TB, however, an individual with resistant disease contributes to the disease burden both as a member of the pool of prevalent disease and as a source of transmission. A high prevalence of drug resistance could indicate an elevated incidence, a prolonged duration of disease, or a combination of both. In this case, improvements could be achieved by either reducing the inflow of patients to this disease state (through the improvement of treatment for individual patients with drug-sensitive disease if acquired drug resistance is common or by infection control if transmitted resistance is occurring) or by increasing the outflow of patients by improving the detection, diagnosis, and treatment of those with drug-resistant disease.
Ideally, a surveillance study would clarify the contribution of each mechanism (acquisition and transmission) to the emergence of resistance as well as the prevalence of resistant disease to help harness the resources to respond to the current need. In reality, there are practical challenges that limit our ability to achieve these objectives.
WHAT DO EXISTING SURVEILLANCE APPROACHES MEASURE?
Most drug-resistance surveillance studies measure resistance at the time individuals are diagnosed with TB. Where adequate laboratory capacity is available, all detected TB cases are included and surveillance is continuous. Where routine DST is not available, a random sample of patients with newly diagnosed TB is selected for testing. Assuming that this sampling is random, the proportion of newly diagnosed patients whose M. tuberculosis isolates is resistant provides an estimate of the burden of resistance among incident symptomatic sputum smear–positive patients who present to a TB diagnostic center during the survey period. Although this proportion is sometimes referred to as the prevalence of resistance among newly diagnosed cases, this terminology can be confusing because these sampling methods actually only estimate the proportion of isolates from incident sputum smear–positive cases that is resistant.
Although surveillance studies of incident cases have the great advantage of being readily incorporated into the workflow of busy clinics, the information obtained has several limitations. Because resistance among prevalent cases is not determined, these studies cannot directly be used to estimate the numbers of existing drug-resistant TB cases and the demand for second-line antibiotics. Because standard first-line drug regimens are less likely to cure those with resistant TB, the average duration of infectiousness of individuals with drug-resistant disease may be longer than that of individuals with drug-sensitive disease. Thus, the proportion of patients with prevalent drug-resistant disease is likely to be greater than the proportion of incident cases that is resistant.
The data from studies of incident cases are typically stratified by the patients' previous TB treatment histories; the occurrence of resistance in individuals without prior TB treatment is used to estimate the proportion of transmitted disease that is resistant, whereas resistance in previously treated cases has been considered a proxy for the acquisition of resistance during treatment (6). Although resistance among cases who have never been treated should accurately measure transmitted resistance among new untreated cases, it can underestimate transmission of resistance among all incident cases because previously treated patients may be at higher risk for transmitted drug-resistant TB than individuals without previous treatment. This is because individuals with prior disease represent a subpopulation that is especially vulnerable to TB disease once infected and also because they may more likely come into contact with patients with drug-resistant TB in hospitals or clinic settings (8, 9). Drug resistance among previously treated cases has been used as proxy for acquired resistance; however, resistance among this group reflects a combination of acquisition, reinfection, and primary infection with a drug-resistant strain with subsequent treatment failure.
Because incidence-based studies enumerate patients at the time of TB diagnosis, those who acquire resistance during treatment will not be counted unless they discontinue therapy and re-present as “previously treated” incident cases. The extent of this potential source of bias depends on the relative frequency of resistance generated through transmission (which is represented in the study) compared with resistance generated through suboptimal treatment (which is included only if and when failed cases are re-registered as incident cases).
In summary, surveillance based on incident disease does not quantify the burden of resistance among prevalent cases, may misclassify the mechanism of emergence among individual cases of drug-resistant TB, may underestimate transmitted resistance if resistance among never-treated cases is used as a proxy, and may underestimate resistance among incident cases when individuals who acquired resistance during therapy are not re-registered as previously treated cases.
CHALLENGES TO QUANTIFYING THE BURDEN OF DRUG RESISTANCE
Although incidence-based surveillance methods may underestimate the proportion of incident drug-resistant TB cases due to transmission, estimation of the proportion of incident drug-resistant TB cases due to acquired resistance is even more difficult. Because resistance among retreatment cases includes cases due to both acquisition and transmission, it is not an optimal proxy for acquired resistance. In principle, direct measurement of the incidence of acquired drug resistance would require a study in which patients with confirmed drug-sensitive disease were prospectively followed with repeat cultures and DST to identify newly occurring resistance. To avoid misclassifying reinfection with a resistant strain as an occurrence of acquired resistance, molecular fingerprinting of strains would be required. Clearly, this study design is more complex and expensive than current surveillance strategies and in most high-burden settings this approach would not be a feasible routine method of surveillance.
Direct estimation of the prevalence of drug-resistant TB in a community requires population-based sampling, which is both logistically challenging and expensive. In some cases, investigators may be interested in the prevalence of drug-resistant TB among a high-risk group (e.g., hospital inpatients) or among patients with TB currently receiving treatment; in these circumstances, determining the prevalence of resistance among a defined subset of the population is relatively straightforward. Estimates of the prevalence of resistance can also be indirectly obtained if the incidence and average duration of drug-resistant disease are known.
To obtain valid estimates of either the incidence or prevalence of drug resistance from a subset of cases in a community, the probability that a case is included in the sample must be independent of the drug-resistance status of that case. In other words, the sample must be randomly chosen from all cases with respect to drug resistance. In most communities in which sampling is required to estimate the burden of resistance, there are obstacles to obtaining a random sample of eligible cases. Here we focus on several potential sources of bias that are present in many communities in which drug-resistant TB is circulating and in which the measurement of the burden of drug resistance is important.
HIV Coinfection
In settings that perform culture and DST as the standard of diagnosis, drug resistance can be measured among both smear-positive and smear-negative cases. However, surveys are often restricted to individuals who present with smear-positive TB (10). Because individuals with HIV/TB coinfection are less likely to have smear-positive TB (11–14), HIV-infected cases may be underrepresented in these samples. A survey sample that preferentially excludes those with HIV/TB coinfection will yield biased estimates of the proportion resistant among all incident cases if resistance is associated with HIV status. Although some studies have found no association between HIV and drug resistance among patients with TB (15–19), others have demonstrated a higher risk of resistance among the coinfected patients (20, 21). Thus, in areas where a high proportion of patients with TB also are infected with HIV, it is possible that drug resistance is underestimated.
Alternatively, in areas where HIV/TB coinfection is common, the total burden of drug-resistant TB may be overstated by standard surveys of incident TB cases. HIV/TB-coinfected individuals may have a shorter period of infectiousness either because they have a more rapid onset of symptoms and receive earlier effective therapy or because they experience an increased rate of mortality. If this is the case, and drug-resistant TB is more common among those with HIV coinfection, the proportion of prevalent disease that is resistant may be less than the proportion of incident disease that is resistant because the average duration of drug-resistant TB will be shorter than that of drug-sensitive TB. The relationship between the duration of infectiousness of HIV-related TB may differ between settings; Corbett and colleagues found a substantially reduced duration of infectiousness for these individuals among South African gold miners (22) and among workers in Zimbabwe (23), whereas Wood and coworkers report a slightly longer duration of TB for those with concurrent HIV living in a crowded South African setting (24).
The speed of emergence of resistance in areas affected by HIV may also affect the performance of surveillance studies. In low-HIV-prevalence settings, models predict that TB epidemics progress slowly (25); as such, the WHO recommends that periodic drug surveillance surveys take place at 3- to 5-year intervals. However, the dynamics of TB epidemics in areas of high HIV prevalence are likely to be altered by the rapid progression to TB in HIV-seropositive individuals, which could greatly accelerate the spread of drug resistance within these populations, making intermittent surveillance a less sensitive approach for detecting its emergence.
Diagnosis and Treatment of TB Cases in the Private Sector
Most drug-resistant TB surveys are conducted in the public sector where the national TB programs mandate treatment regimens in accordance with internationally accepted guidelines. However, in many countries, a substantial portion of TB treatment occurs in the private sector where TB treatment often does not conform to recommended care, either because patients are given suboptimal drug regimens, because they are not treated for an adequate period of time, or because they are not followed up if they discontinue care before the end of their treatment course (26, 27). Because these are often the settings in which resistance emerges, we expect that burden of drug-resistant TB may be greater within the portion of the population treated by private practitioners, precisely the portion that is usually excluded from surveys for resistance. Thus, it is possible that current surveys underestimate the actual burden of drug-resistant TB in some settings by excluding those most likely to be affected, either because they are smear-negative patients with HIV in high-HIV-prevalence areas or because they are diagnosed and treated within the private sector.
Geographic Heterogeneity
In those areas where not all TB cases receive DST, the use of sampling can lead to misestimation of the burden of TB if the distribution of resistant cases is not homogeneous. The uneven distribution of drug-resistant TB may reflect variation in treatment practices within a country with poorly functioning areas generating more resistant strains. Heterogeneity may also emerge if factors facilitating the spread of drug-resistant TB are not uniformly distributed. If transmission of drug-resistant TB is occurring within specific health care settings in which infection control practices are not optimal, then microepidemics could rapidly occur in the communities around these facilities. This occurred during an outbreak of MDR TB in New York during the early 1990s (28) and is a contributing factor to the spread of XDR TB in KwaZulu-Natal (2). Finally, M. tuberculosis is phenotypically diverse, and an association of drug resistance with specific strain families, most notably the Beijing/W genotype, has been reported (29). If the distribution of these strains is not uniform (30), we may also observe an uneven distribution of drug resistance. In principle, the existence of heterogeneity in the distribution of resistant cases can result in either over- or underestimation of the burden of resistance depending on whether pockets of resistance are included or excluded from the survey sample.
CONCLUSIONS
The recent emergence of XDR TB emphasizes the need for scaling up surveillance of drug-resistant TB, especially in areas in which TB control programs have been compromised by an escalating burden of TB and HIV. Current approaches to surveillance include testing of all diagnosed cases in areas where routine DST is available and incidence-based surveys in areas where routine DST is not conducted. Although incidence-based studies remain the most feasible approach to surveillance in areas where resources are constrained, they may underestimate the true burden of drug resistance because of methodologic problems inherent in their design. Factors such as the emergence of HIV, the role of the private sector in diagnosis and treatment of TB, and the presence of geographic heterogeneity also challenge the validity of these studies. Although incidence-based surveys should be continued as a standard form of evaluation, additional studies are necessary to improve our ability to gauge the true magnitude of this public health threat.
The allocation of additional resources for accurate assessment of the burden of resistance will be an essential step in a global response to MDR and XDR TB. Fundamental to this process will be the development of global laboratory capacity to scale up routine culture and DST according to the Global Plan to Stop TB (5) and the universal provision of first- and second-line treatment for TB, which will encourage patients and caregivers to improve efforts to find and diagnose patients with TB. To improve the estimation of the burden of drug-resistant TB, the WHO has published interim recommendations for the surveillance of drug resistance in TB (9). These recommendations reflect the consensus of the XDR TB Global Task Force meeting held in October 2006 and address the challenges of drug-resistant TB surveillance. The recommendations first emphasize the need to improve routine recording and reporting of notification data and to incorporate results of DST to first- and second-line drugs when and where these data are available. Second, in areas where routine testing is not yet available, they stress the need to extend incidence-based surveys by increasing sample sizes, expanding access to second-line drug testing, ascertaining HIV status of TB cases, and recruiting incident cases over longer periods of time; these efforts will help to improve validity and precision of the resultant estimates of resistance. Third, they call for conducting specific surveys among high-risk populations, such as treatment failures, hard-to-reach populations, and migrant workers, to improve our understanding of the distribution of resistance and help guide the allocation of resources for the control of resistance. Fourth, they suggest the incorporation of rapid diagnostic methods for assessing resistance to expand the scope of surveillance. Additional molecular tests that can help assess the relative contribution of acquired and transmitted drug resistance can also help to determine which interventions may be most effective within particular settings.
In addition to these recommendations, we suggest the establishment of global sentinel surveillance sites to assess all suspect TB cases for resistance over an extended period of time. These sentinel sites should be selected so that each site contributes distinctive information about how resistance emerges (and is potentially controlled) within particular types of epidemic settings. For example, sentinel sites located in areas of high HIV prevalence would permit the study of the timing and mechanisms of resistance emergence in areas where a large proportion of the population is at high risk of rapid TB progression. Sentinel sites in locations where a substantial proportion of patients are treated in the private sector would provide data to assess possible differences in resistance among patients presenting for treatment—and potentially also to identify differential risks of resistance acquisition—in the two treatment settings. In territories where worrisome numbers of MDR and XDR TB cases have been identified, sentinel sites would allow for accurate tracking of the epidemics of highly resistant disease. Detailed surveillance data from such sentinel sites would help to clarify the role of important modifiers on the dynamics of resistance and, more importantly, this information would be instrumental in informing the design of effective interventions against the further emergence of drug-resistant TB within these settings.
T.C. is supported by a National Institutes of Health grant (5K08AI055985–05). The funding source had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
A.W., M.Z., and M.M. were members of the XDR TB Global Task Force; A.W. and M.Z. were authors of the recent IUATLD/WHO Anti-tuberculosis Drug Resistance in the World Report No. 4.
Originally Published in Press as DOI: 10.1164/rccm.200801-175PP on March 27, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.World Health Organization. Anti-tuberculosis Drug Resistance in the World Report No. 4. Geneva, Switzerland: World Health Organization; 2008. Publication No. WHO/HTM/TB/2008.394.
- 2.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006;368:1575–1580. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep 2006;55:301–305. [PubMed] [Google Scholar]
- 4.World Health Organization. Global Plan to Stop TB, 2006–2015. Geneva, Switzerland: World Health Organization; 2006. Publication No. WHO/HTM/STB/2006.35.
- 5.World Health Organization. The Global MDR-TB and XDR-TB Response Plan 2007–2008. Geneva, Switzerland: World Health Organization; 2007. Publication No. WHO/HTM/STB/2007.2007.387.
- 6.World Health Organization. Guidelines for surveillance of drug resistance in tuberculosis. Geneva, Switzerland: World Health Organization; 2003. Publication No. WHO/TB/2003.320.
- 7.World Health Organization. Report of the meeting of the WHO task force on XDR-TB, October 9–10. Geneva, Switzerland: World Health Organization; 2007. Publication No. WHO/HTM/TB/2007.375.
- 8.Verver S, Warren RM, Beyers N, Richardson M, van der Spuy G, Borgdorff MW, Enarson DA, Behr MA, van Helden PD. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med 2005;171:1430–1435. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Zhang Y, Shen X, Shen G, Gui X, Sun B, Mei J, DeRiemer K, Small PM, Gao Q. Transmission of drug-resistant tuberculosis among treated patients in Shanghai, China. J Infect Dis 2007;195:864–869. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Interim recommendations for the surveillance of drug resistance in tuberculosis. Geneva, Switzerland: World Health Organization; 2007. Publication No. WHO/HTM/TB/2007.385.
- 11.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 2003;163:1009–1021. [DOI] [PubMed] [Google Scholar]
- 12.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 1999;282:677–686. [DOI] [PubMed] [Google Scholar]
- 13.Raviglione MC, Harries AD, Msiska R, Wilkinson D, Nunn P. Tuberculosis and HIV: current status in Africa. AIDS 1997;11(Suppl B):S115–S123. [PubMed] [Google Scholar]
- 14.Shafer RW, Edlin BR. Tuberculosis in patients infected with human immunodeficiency virus: perspective on the past decade. Clin Infect Dis 1997;22:683–704. [DOI] [PubMed] [Google Scholar]
- 15.Kenyon TA, Mwasekaga MJ, Huebner R, Rumisha D, Binkin N, Maganu E. Low levels of drug resistance amidst rapidly increasing tuberculosis and human immunodeficiency virus co-epidemics in Botswana. Int J Tuberc Lung Dis 1999;3:4–11. [PubMed] [Google Scholar]
- 16.Warndorff DK, Yates M, Ngwira B, Chagaluka S, Jenkins PA, Drobniewski F, Pönnighaus JM, Glynn JR, Fine PE. Trends in antituberculosis drug resistance in Karonga District, Malawi, 1986–1998. Int J Tuberc Lung Dis 2000;4:752–757. [PubMed] [Google Scholar]
- 17.Chum HJ, O'Brien RJ, Chonde TM, Graf P, Rieder HL. An epidemiological study of tuberculosis and HIV infection in Tanzania, 1991–1993. AIDS 1996;10:299–309. [DOI] [PubMed] [Google Scholar]
- 18.Churchyard GJ, Corbett EL, Kleinschmidt I, Mulder D, De Cock KM. Drug-resistant tuberculosis in South African gold miners: incidence and associated factors. Int J Tuberc Lung Dis 2000;4:433–440. [PubMed] [Google Scholar]
- 19.Espinal MA, Laserson K, Camacho M, Fusheng Z, Kim SJ, Tlai RE, Smith I, Suarez P, Antunes ML, George AG, et al. Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int J Tuberc Lung Dis 2001;5:887–893. [PubMed] [Google Scholar]
- 20.Haar C, Cobelens F, Kalisvaart N, van der Have JJ, van Gerven PJHJ, van Soolingen D. Tuberculosis drug resistance and HIV infection, the Netherlands. Emerg Infect Dis 2007;13:776–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax 2006;61:158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbett EL, Charalambous S, Moloi VM, Fielding K, Grant AD, Dye C, De Cock KM, Hayes RJ, Williams B, Churchyard GJ. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med 2004;170:673–679. [DOI] [PubMed] [Google Scholar]
- 23.Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Fausett P, Hayes R, Churchyard G, Butterworth A, Mason P. Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med 2007;4:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker L. Undiagnosed tuberculosis in a community with high HIV-prevalence: implications for TB control. Am J Respir Crit Care Med 2007;175:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blower SM, McLean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, Moss AR. The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med 1995;1:815–821. [DOI] [PubMed] [Google Scholar]
- 26.Uplekar M, Pathania V, Raviglione M. Private practitioners and public health: weak links in tuberculosis control. Lancet 2001;358:912–916. [DOI] [PubMed] [Google Scholar]
- 27.Greaves F, Ouyang H, Pefole M, MacCarthy S, Cash RA. Compliance with DOTS diagnosis and treatment recommendations by private practitioners in Kerala, India. Int J Tuberc Lung Dis 2007;11:110–112. [PubMed] [Google Scholar]
- 28.Frieden TR, Sterling T, Pablos-Mendez A, Kilburn JO, Cauthen GM, Dooley SW. The emergence of drug-resistant tuberculosis in New York City. N Engl J Med 1993;328:521–526. [DOI] [PubMed] [Google Scholar]
- 29.European Concerted Action on New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg Infect Dis 2006;12:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, et al. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 2006;103:2869–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]