Abstract
The rapid onset of massive, systemic viral replication during primary HIV/SIV infection and the immune evasion capabilities of these viruses pose fundamental problems for vaccines that depend upon initial viral replication to stimulate effector T cell expansion and differentiation1–5. We hypothesized that vaccines designed to maintain differentiated “effector memory” T cell (TEM) responses5,6 at viral entry sites might improve efficacy by impairing viral replication at its earliest stage2, and have therefore developed SIV protein-encoding vectors based on rhesus cytomegalovirus (RhCMV), the prototypical inducer of life-long TEM responses7–9. RhCMV vectors expressing SIV Gag, Rev/Nef/Tat, and Env persistently infected rhesus macaques (RM), regardless of pre-existing RhCMV immunity, and primed and maintained robust SIV-specific, CD4+ and CD8+ TEM responses (characterized by coordinate TNF, IFN-γ and MIP-1β expression, cytotoxic degranulation, and accumulation at extra-lymphoid sites) in the absence of neutralizing antibodies. Compared to control RM, these vaccinated RM showed increased resistance to acquisition of progressive SIVmac239 infection upon repeated, limiting dose, intra-rectal challenge, including four animals that controlled rectal mucosal infection without progressive systemic dissemination. These data suggest a new paradigm for AIDS vaccine development: that vaccines capable of generating and maintaining HIV-specific TEM might decrease the incidence of HIV acquisition after sexual exposure.
According to current concepts of T cell-directed HIV/AIDS vaccines3,4,10, a broadly targeted, high frequency, HIV-specific CD8+ memory T cell response would restrict acute phase HIV replication and decrease the subsequent chronic-phase HIV replication set-point. CD8+ T cell vaccines are not expected to provide “sterilizing” anti-HIV immunity and thereby prevent infection. Rather, by lowering viral load set points, these vaccines would ameliorate the AIDS epidemic by substantially reducing both the rate of disease progression in infected individuals, and the likelihood that such individuals would transmit the infection to others. Although experiments in monkey models have supported these principles11–14, the recent STEP phase IIb clinical trial using Adenovirus5 (Ad5) vectors, widely viewed as a test of the CD8+ T cell vaccine concept, was a clear failure, causing many to question the ability of T cell responses to contribute to an effective HIV/AIDS vaccine3,10.
In assessing the lack of efficacy seen in the STEP trial, it is instructive to consider whether the characteristics of the T cell responses elicited by current vaccine strategies are well matched to the goal of lentiviral containment. One important consideration is the influence of differentiation state on the efficacy of vaccine-generated memory T cells. Most current T cell vaccine strategies employ non-persistent vectors that produce antigen for a limited period, and as the antigen provided by these vectors wanes, the memory T cell responses they elicited become increasingly secondary lymphoid tissue-based or “central memory” T cell (TCM) in character5. TCM have limited immediate effector function, and require antigen-induced expansion, differentiation and migration to produce peak effector responses at viral replication sites5,6. TCM-derived effector responses are accelerated over primary responses, but still appear to develop too slowly to prevent the initial systemic dissemination and extensive early replication of SIV in NHP models11,12. In contrast, agents or vectors that provide a controlled, persistent level of antigen maintain functionally differentiated TEM in extra-lymphoid sites5,15,16. Given that TEM are the predominant type of T cell in mucosal effector sites8,17, and that mucosal HIV transmission is associated with small “seed populations” of limited genetic diversity18, we hypothesized that a vaccine capable of generating and maintaining a potent HIV-specific TEM-type response might improve efficacy by initiating adaptive anti-viral immunity at the outset of mucosal infection (the initial 24–48 hrs), when the virus is likely most susceptible to immune control.
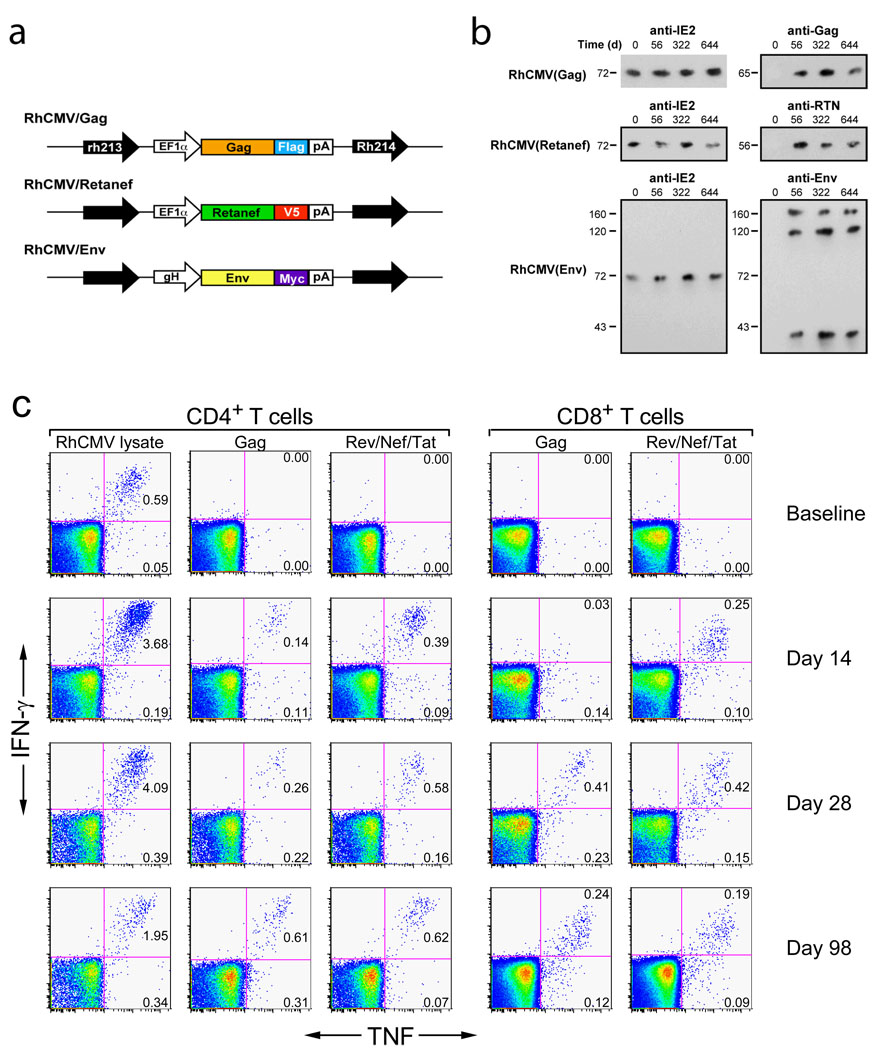
Primate cytomegaloviruses (CMV) comprise a family of closely related, but species-specific, β-herpes viruses that are ubiquitous, persistent, and for competent immune systems, largely benign19–21. CMV infections are associated with life-long, high frequency, and highly TEM-biased CD4+ and CD8+ T cell responses that protect against disease development, but do not eliminate the CMV infection or prevent CMV super-infection7–9,20,22–25. We therefore explored the possibility that RhCMV could be exploited as a persistent vaccine vector for generation of durable SIV-specific, TEM-biased CD4+ and CD8+ T cell responses in RM, and thereby allow assessment of the ability of these responses to prevent or completely contain SIV infection during repeated, limiting-dose, intra-rectal challenge. RhCMV/SIV vectors encoding SIV Gag, Rev/Nef/Tat and Env (Fig. 1a) were constructed, and shown to express high levels of these SIV antigens during lytic infection in vitro, and to possess wildtype RhCMV growth kinetics (Supplemental Figs. S1–S3). Administration of RhCMV/SIV vectors to either RhCMV-naïve or RhCMV+ RM resulted in clinically benign primary infection and super-infection, respectively. Virologic analysis showed the appearance of RhCMV/SIV vectors in urine and saliva by day 56 post-inoculation (Fig. 1b), but little to no measurable viremia. The persistence of these infections and the genetic stability of the three RhCMV/SIV vectors in vivo were demonstrated by the ability to co-culture SIV antigen-expressing RhCMV from urine and saliva co-cultures for up to 664 days after inoculation (Fig. 1b). Significantly, despite the presence of potentially competing RhCMV-derived T cell epitopes, and in RhCMV+ RM the pre-existent, high-frequency T cell responses to these epitopes, all RM inoculated with these RhCMV/SIV vectors developed both CD4+ and CD8+ T cell responses to the recombinant SIV protein(s) (Fig. 1c). These responses have remained detectable for >3 years after vector administration (see below and data not shown), suggesting that the RhCMV-vectored, SIV-specific responses persist indefinitely, similar to native RhCMV-specific T cell responses. In contrast to these robust SIV-specific T cell responses, RhCMV/SIV vectors elicited only low titre anti-SIV antibodies (≤1:10) that were unable to neutralize either SIVmac239 or a highly neutralization sensitive, tissue culture-adapted variant of SIVmac251 (data not shown).
Figure 1. RhCMV vectors engineered to express SIV proteins can re-infect RhCMV+ RM and initiate a de novo SIV-specific CD4+ and CD8+ T cell response.
(a) Schematic of the SIV protein expression cassettes inserted into the RhCMV genome in the intergenic region between rh213 and Rh214 to create the RhCMV/Gag, RhCMV/Retanef and RhCMV/Env vectors. [See also Supplementary Figs. S1–S3] (b) Western blot analysis of telomerized rhesus fibroblasts co-cultured for 4 weeks with virus pelleted from urine collected at the designated intervals from initially RhCMV+ RM following their inoculation with RhCMV/Gag, RhCMV/Retanef and RhCMV/Env. (c) Flow cytometric intracellular cytokine analysis (FCICA) of peripheral blood T cells responding to wildtype RhCMV lysate, SIV Gag or Rev/Nef/Tat overlapping 15mer peptides in a typical, initially RhCMV+ RM following inoculation with the RhCMV/Gag and RhCMV/Retanef vectors. The values in the upper and lower right quadrants of the flow cytometric profiles indicate the net% (minus background) of the total CD4+ or CD8+ T cell population responding to the designated antigen with production of both TNF and IFN-γ or TNF alone, respectively.
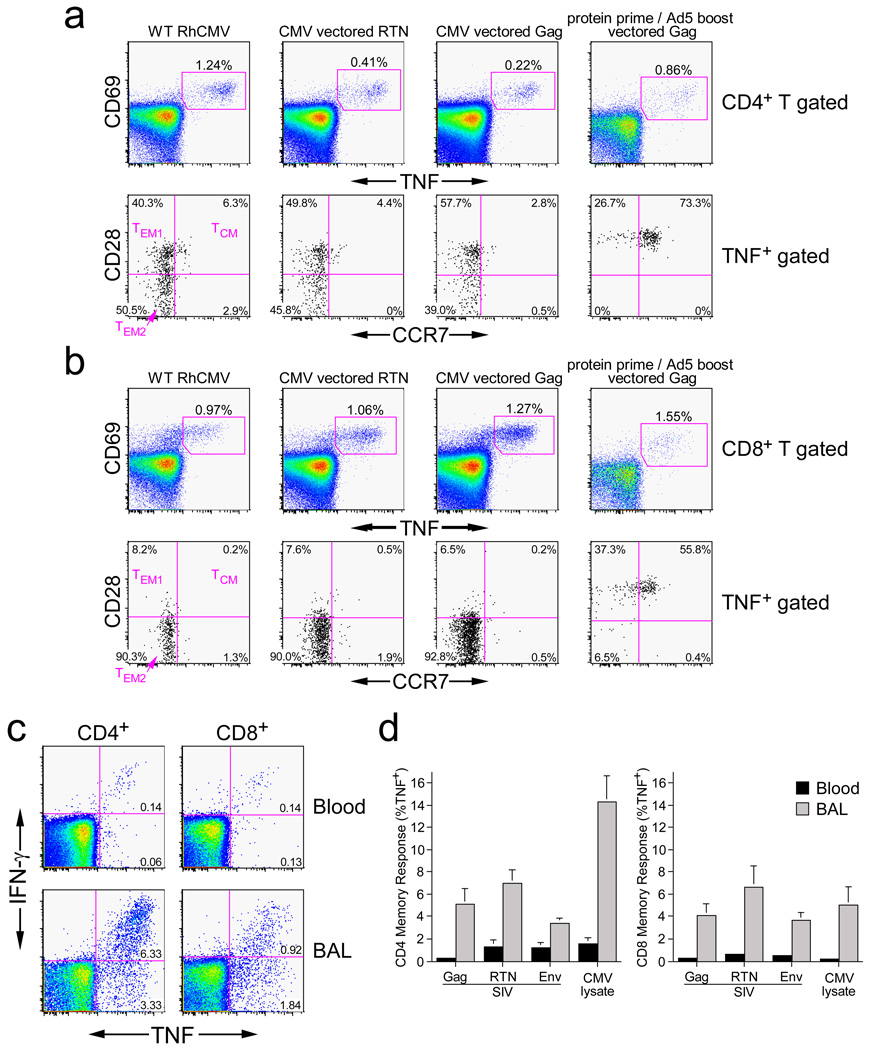
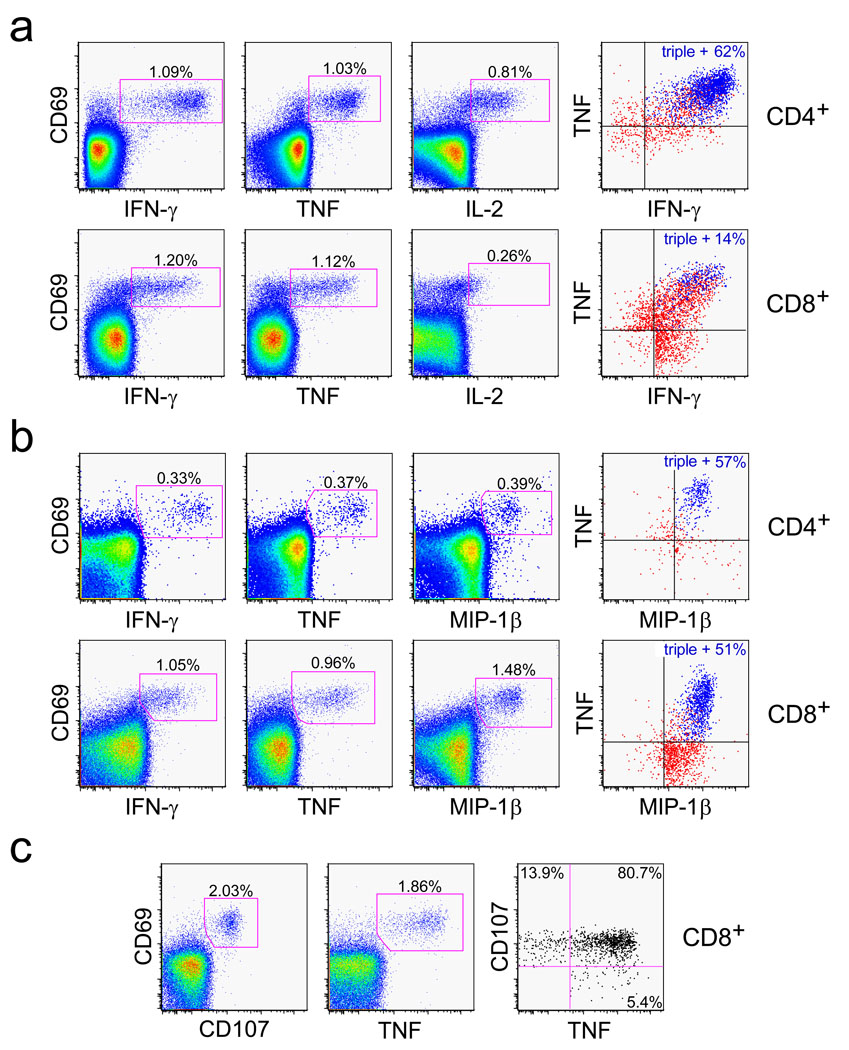
We next studied the differentiation and functional characteristics of established SIV-specific memory T cells induced by RhCMV/SIV-vector vaccination. The markers CD28 and CCR7 define the TCM to TEM differentiation axis among RM T cells, with TCM, transitional TEM1, and fully differentiated TEM2 displaying a CD28+/CCR7+, CD28+/CCR7−, and CD28−/CCR7− phenotype, respectively8,17. Both RhCMV-specific and RhCMV-vectored, SIV-specific T cells in peripheral blood displayed a nearly exclusive (>90%) TEM phenotype, with CD8+ T cells being almost entirely TEM2 and CD4+ T cells being ~ equally split between TEM1 and TEM2. In sharp contrast, a protein prime-Ad5 boost vaccine induced a predominantly TCM phenotype among SIV-specific, CD4+ and CD8+ T cells (p <.0001; Fig. 2a; Supplemental Fig. S4). In keeping with TEM localization in extra-lymphoid sites8,17, both RhCMV-specific and RhCMV-vectored, SIV-specific T cells were highly enriched among bronchoalveolar lavage (BAL) lymphocytes (Figs. 2b,c). Finally, RhCMV-specific and RhCMV-vectored, SIV-specific T cells showed essentially identical functional potential (Figs. 3a,b; Supplemental Fig. S5). Specifically, CD4+ T cells were predominantly polyfunctional, capable of simultaneous production of TNF, IFN-γ, IL-2 and MIP-1β, the latter being a CCR5-binding chemokine capable of blocking HIV/SIV infection26. CD8+ T cells were also polyfunctional for TNF, IFN-γ and MIP-1β, but consistent with a highly polarized TEM2 phenotype, lacked significant IL-2 production. The polarized TEM2 phenotype of the RhCMV-vectored, SIV-specific CD8+ T cells would predict cytotoxic potential6, and accordingly, these cells efficiently extruded cytotoxic granules (externalized CD107) upon antigen recognition (Fig. 3c).
Figure 2. RhCMV-vectored, SIV-specific T cell responses persist with a polarized TEM phenotype and maintain high representation at extra-lymphoid effector sites.
(a,b) Combined FCICA and surface phenotype analysis of CD4+ (a) and CD8+ (b) peripheral blood T cells responding to wildtype RhCMV lysate, SIV Gag or Rev/Nef/Tat overlapping 15mer peptides. The figure compares the CD28 vs. CCR7 phenotype of RhCMV and SIV antigen-responsive CD4+ or CD8+ T cells (CD69+; TNF+) in a representative, initially RhCMV+ RM that was inoculated 595 days and 330 days earlier with RhCMV/Retanef) and RhCMV/Gag, respectively (left and middle columns), and also compares the SIV Gag response of this RM to a representative RM that received a Gag protein prime and Ad5(Gag) boost (105 days after the boost; right column). [See also Supplementary Fig. S4] (c) FCICA of the response of peripheral blood vs. BAL T cells (CD4+ or CD8+) to SIV Rev/Nef/Tat overlapping 15mer peptides in an RM that received the RhCMV/Retanef vector 192 and 94 days earlier. The values in the profiles indicate the net% (minus background) of the total CD4+ or CD8+ T cell population responding to the Rev/Nef/Tat peptides with the designated cytokines. (d) Comparison of the mean frequencies (±SEM) of RhCMV-vectored SIV Gag-, Rev/nef/tat-, and Env-specific T cell responses in the peripheral blood memory compartment vs. the BAL (memory) T cell compartment in a group of 6 RM that are 192 days post initial inoculation and 94 days post a second inoculation with the RhCMV(Gag), RhCMV/Retanef and RhCMV/Env vectors.
Figure 3. RhCMV-vectored SIV-specific T cell responses maintain potent effector function.
Representative FCICA of peripheral blood CD4+ and/or CD8+ T cells responding to SIV Gag or Rev/Nef/Tat overlapping peptides from RM that were inoculated with the corresponding RhCMV vector greater than 500 days earlier. The figures show coordinate analysis of a, TNF vs. IFN-γ vs. IL-2; b, TNF vs. IFN-γ vs. MIP-1β, and c, TNF vs. CD107 externalization (indicating degranulation of cytoplasmic cytotoxic granules). The left and middle profiles are gated on the overall CD4+ or CD8+ T cell populations with the percentage of the designated responding populations (CD69+, cytokine+ or CD107+) shown in each profile. The right profiles are Boolean gated on total responding T cells (those making any of the designated cytokines or expressing CD107, alone or in combination), with either the percentage of triple producers (a and b, colored blue), or the percentage of responding cells showing CD107 and TNF reactivity alone or in combination (c) designated in the figure [see also Supplementary Figs. S4 and S5].
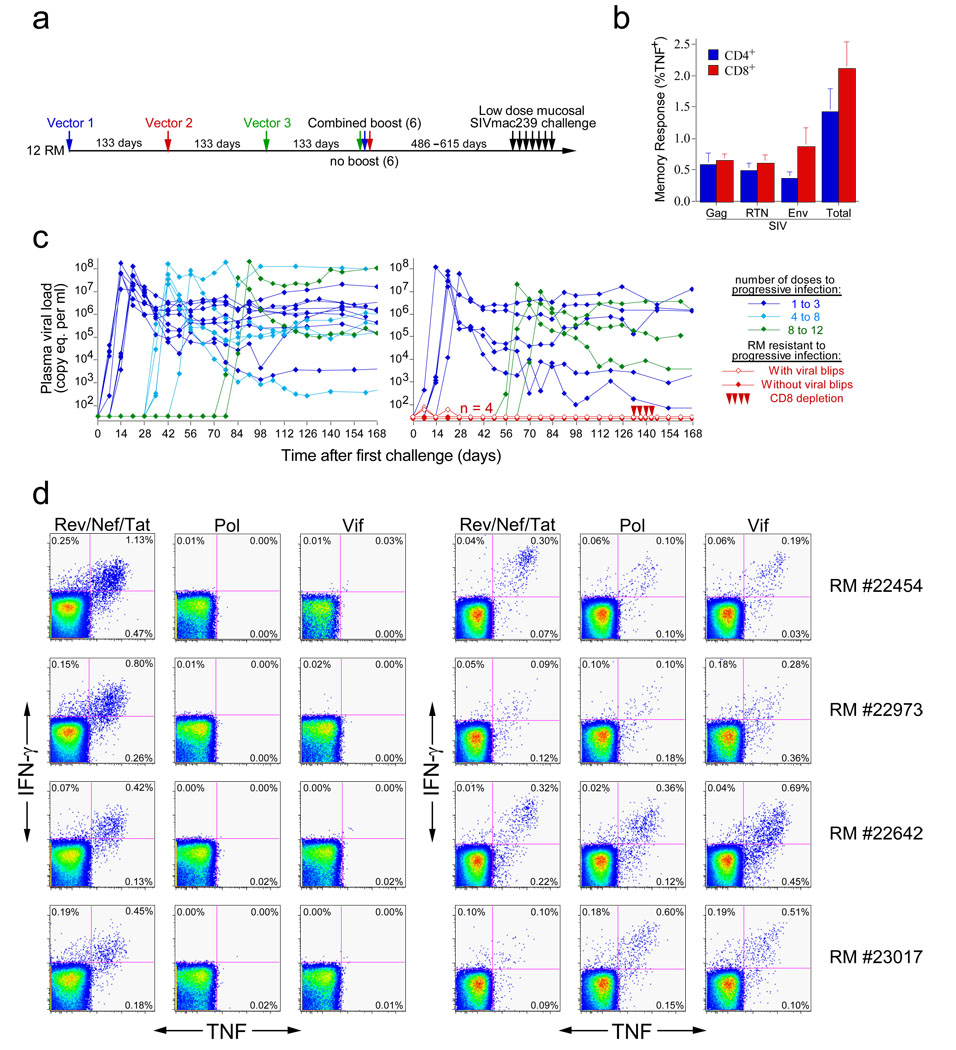
These results indicate that the RhCMV/SIV vectors elicit and maintain SIV-specific T cells that are highly skewed towards TEM differentiation. To test whether this TEM-skewed anti-SIV T cell response would protect against SIV infection, we assembled a cohort of 12 long-term RhCMV/SIV vector-vaccinated RM (which were >486 days from their last RhCMV/SIV vaccination) and 16 wildtype RhCMV+, negative control RM for repeated, limiting dose intra-rectal challenge with the highly pathogenic SIVmac239 (Fig. 4a). At the time of challenge, the CMV/SIV vector-vaccinated RM had approximately equivalent CD4+ and CD8+ responses to Gag, Rev/Nef/Tat and Env, totaling, on average, 1.5% and 2.0% of blood memory T cells for CD4+ and CD8+ responses, respectively (Fig. 4b). All 16 control RM manifested progressive infection over the course of challenge with 50% of these RM becoming infected with the first or second dose of virus, and the last control RM infected with the 12th dose (Fig. 4c). In contrast, the median number of doses to achieve progressive, systemic infection was 8 for the vaccinated group. Most notably, 4 of the 12 vaccinees failed to show progressive infection (p = 0.024). Two of these 4 RM showed transient plasma virus levels of 60–80 SIV RNA copy eq/ml after the first challenge, whereas the other 2 RM underwent 13 SIVmac239 challenges without measurable plasma virus. All 4 RM, however, appeared to be SIVmac239-infected based on their development of reproducible de novo CD8+ T cell responses to two SIV proteins, Pol and Vif, that were not included in the vaccine (Fig. 4d). Such new SIV-specific T cell responses did not develop in naïve RM repeatedly challenged with equivalent doses of AT2-inactivated SIVmac239, or in 3 unvaccinated control RM that were not overtly infected in the 6 initial challenges with competent SIVmac239 (Supplemental Fig. S6). These controls argue against the possibility that the de novo CD8+ T cell responses to Pol and Vif in the four protected RM were related to cross-presentation of SIV proteins in the challenge virus preparation in the absence of infection, and in agreement with previous work27, indicate that the typical outcome of repeated, limiting dose, intra-rectal SIV challenge of unvaccinated RM is either no infection or overt progressive infection, with no immune sensitization in the absence of overt infection.
Figure 4. RM inoculated with RhCMV vectors expressing Gag, Rev/Nef/tat, and Env are protected from progressive SIVmac239 infection following repeated, limiting dose intra-rectal challenge.
(a) Vaccination and challenge protocol for efficacy assessment of RhCMV vectors. RhCMV/Gag, RhCMV/Retanef, and RhCMV/Env vectors were given individually at 133 day intervals in the following orders: 1) Gag/Retanef/Env, 2) Retanef/Env/Gag, and 3) Env/Gag/Retanef for 4 RM each. Half of each group was subsequently provided with a combined boost of all 3 vectors. The long-term anti-SIV T cell responses did not differ between these sub-groups, and all 12 of these RM were therefore combined into one vaccinated group for challenge (in comparison to 16 unvaccinated, but RhCMV+, control RM). (b) Mean pre-challenge frequencies (± SEM) of RhCMV-vectored, SIV Gag-, Rev/Nef/Tat-, and Env-specific responses among blood CD4+ and CD8+ memory T cells of the 12 vaccinated RM. (c) Plasma viral loads of the control (left panel) and vaccinated (right panel) RM cohorts over the course of, and subsequent to, limiting dose intra-rectal SIVmac239 challenge. Four of 12 vaccinated RM resisted progressive infection: these protected RM were treated with the humanized anti-CD8 mAb cMT807 at days 133, 136, 140 and 143 post-initial challenge (10, 5, 5, and 5 mg/kg doses, respectively) and were profoundly depleted of CD8+ T cells from blood (< 2.5% of baseline absolute counts) for 14 to 21 days. (d) FCICA of peripheral blood CD8+ T cells from the 4 protected vaccinees examining the response of these cells to SIV proteins that were (Rev/Nef/Tat) or were not (Pol and Vif) expressed by the administered RhCMV vectors, before (right panel) and 133 days after (left panel) initiation of SIVmac239 intra-rectal challenge protocol.
At 19 weeks after the first challenge, the 4 protected RM were depleted of CD8+ cells by administration of mAb cM-T807, a treatment capable of transiently reversing CD8+ T cell-mediated control of SIV replication28. This treatment caused profound depletion of circulating CD8+ T cells, but did not result in measurable viremia (Fig. 4c). Moreover, cell-associated SIV DNA or RNA were not detected in isolated peripheral blood and lymph node CD4+ T cells from these 4 RM using sensitive PCR assays, both before and during maximal CD8+ cell depletion (data not shown). Thus, 4 of 12 RhCMV/SIV vector-vaccinated RM were infected with SIVmac239 via a mucosal route, but manifested no systemic evidence of infection months later, strongly suggesting that infection was effectively contained locally, before dissemination and establishment of typical systemic infection. This study did not directly compare the efficacy of RhCMV/SIV vectors to other vaccine regimens that induce “TCM-biased” responses, but it should be noted that this degree of protection was not achieved in a test of a potent DNA prime/Adenovirus boost vaccine regimen that used the same challenge virus and limiting dose, intra-rectal challenge procedure12. Our data also do not directly demonstrate the mechanism of this protection, but among the possibilities – T cell, antibody or innate immunity—the most likely candidate is certainly the robust, SIV-specific TEM responses observed in the vaccinees. SIV-specific antibody responses were weak with no neutralization activity, and both vaccinated and control monkeys were chronically infected with RhCMV and >486 days away from any overt RhCMV exposure, making it highly unlikely that innate immune activation contributed to protection (although it should be noted that the control RM were not experimentally RhCMV re-infected via vector administration, as were the vaccinated animals).
The presence of class I MHC alleles such as Mamu B*08 and B*17, associated previously with spontaneous, CD8+ T cell-mediated control of viral replication in the chronic phase of SIV infection4, did not correlate with the acute phase protective effects we observed (only 1/4 protected vs. 5/8 unprotected RM were B*17+ or B*08+), suggesting potential differences in the mechanism(s) mediating early/local vs. late/systemic protection. We speculate that in the 4 early-protected RM reported here, RhCMV vector-generated, SIV-specific TEM responses in rectal lamina propria responded to challenge by creating adverse local conditions for viral replication and spread, effectively lowering the basic reproductive ratio (R0) of the infection below that required for sustained infection (<1)2,29. In the 8 non-protected RM, the local TEM responses may have been insufficient to suppress R0<1, perhaps due to intrinsic genetic differences among these RM, or to stochastic variation in local TEM distribution and function. In future studies it will clearly be important to quantify the distribution and function of SIV-specific TEM in the rectal mucosa, so as to define precise correlates of local protection. Not surprisingly, given the extra-lymphoid localization and relatively low expansion potential of TEM, the RhCMV-vectored, SIV-specific TEM responses were unable to provide significant protection once progressive, systemic infection was initiated (Supplemental Fig. S7). However, it should also be noted that the vaccine generated SIV-specific, CD4+, CCR5+ TEM did not accelerate or enhance viral replication in these “break-through” infections.
Consistent with our original hypothesis, these results suggest that TEM-biased, T cell immunity, in the absence of neutralizing antibody, can prevent establishment of progressive systemic infection after mucosal challenge with a highly pathogenic SIV, presumably by interfering with viral replication at its earliest stages. CMV-based vectors offer one strategy to generate such a TEM component, but other strategies that provide persistent, low-level antigen expression could also be explored. It may also be possible to engineer HIV/AIDS vaccines that generate and maintain both TEM and TCM components, the latter serving as a second line of defense if the initial “TEM barrier” is breached. Such TEM or combination TEM/TCM vaccines will likely not provide absolute protection against all HIV exposure (against parenteral routes, for example). However, our data suggest, for the first time, that vaccine-generated T cell responses are able to do more than simply lower viral replication set points. Specifically, HIV/AIDS vaccines with a TEM component may have the ability to protect against the sexual transmission of HIV.
METHODS
Animals
We used 49 purpose-bred juvenile and adult male RM (Macaca mulatta) of Indian genetic background in this study, including 12 RhCMV+ and 6 RhCMV− RM inoculated with RhCMV/SIV vectors, 9 RM that received a gag protein prime followed by Ad5(gag) boost vaccine protocol, and 22 RhCMV+ RM used as unvaccinated controls. All RM were free of cercopithicine herpesvirus 1, D type simian retrovirus, simian T-lymphotrophic virus type 1 and SIV infection at the start of the study, and were used with approval of the Oregon National Primate Research Center’s Animal Care and Use Committee, under the standards of the NIH Guide for the Care and Use of Laboratory Animals. We administered RhCMV/SIV vectors subcutaneously at a dose of 107 plaque-forming units (pfu). The gag protein prime/Ad5(gag) boost protocol consisted of 450µg SIVgag protein (Protein Sciences) emulsified in montanide ISA-51 carrier (SEPPIC), with or without 1 mg of poly I:C (Invitrogen), 3M-12 (3M Pharmaceuticals), or class C CpG (Pfizer) adjuvant, at weeks 0 and 10 followed by Ad5(gag) (1010 particles, from NIH Vaccine Research Center) at week 20. We performed SIVmac239 challenge by administering 300 focus-forming units (ffu) of virus weekly via the intra-rectal route for the first 8 challenges, and then, if necessary, 5 additional weekly challenges at 1000 ffu. We challenged all of the vaccinated and 12 of the control RM concurrently; the remaining 4 control RM were challenged as part of titration performed immediately prior to the primary challenge, using the same viral dose and challenge procedure. We followed plasma viral loads weekly, with challenge being discontinued the week following detection of >30 copy eq/ml of SIV gag RNA. For CD8+ cell depletion, we treated RM with 10, 5, 5, and 5 mg/kg of the humanized monoclonal antibody cM-T80730 on days 1, 3, 5 and 10, respectively. BAL cell collection was performed as previously described7.
Viruses and vectors
We prepared RhCMV constructs expressing SIV proteins (designated RhCMV/SIV viruses; Fig. 1a) using a bacterial artificial chromosome (BAC) containing the entire RhCMV strain 68-1 genome (pRhCMV/BAC-Cre), as described31,32 (see Supplementary Fig. S1). We confirmed the genomic integrity of the recombinant RhCMV/SIV BACs by restriction enzyme digestion and Southern analysis, and direct DNA sequence analysis of the SIV open reading frames (data not shown). We reconstituted RhCMV/SIV viruses by transfection of BAC DNA into RhCMV permissive rhesus fibroblasts, and confirmed expression of SIV antigens by western analysis of RhCMV/SIV-infected cell lysates (Supplementary Fig. S2). Multi-step growth analysis of RhCMV/SIV viruses31 showed growth kinetics comparable to wildtype RhCMV (Supplementary Fig. S3). The pathogenic SIV challenge stock (kindly provided by CJ Miller) was generated by expanding the SIVmac239 clone33 in RM peripheral blood mononuclear cells (PBMC), and was quantified using the sMAGI cell assay34 and by quantitative RT-PCR for SIV genomic RNA35. We prepared AT-2-inactivated SIVmac239 (lot #P4146) as described36, and normalized this stock to the infectious SIVmac239 stock by real-time RT-PCR quantification of SIV genome copy number.
Viral detection assays
We centrifuged filter-sterilized (0.4 µm) urine at 16,000 × g for 1 hr @ 4° C to concentrate virus for co-culture on RF. After extensive cytopathic effect or 42 days of co-culture, we prepared cell lysates and assessed RhCMV/SIV vector replication based on expression of SIV antigen-specific epitope tags by western immunoblot. We measured whole blood RhCMV DNA, plasma SIV RNA, and cell-associated SIV DNA and RNA by real-time PCR and RT-PCR assays24,35,37, with the cell-associated SIV quantification using immunobead-isolated CD4+ T cells, before and after 48 hrs stimulation with immobilized anti-CD3 and IL-2, and including RT-PCR analysis of supernatants of the stimulated cells.
Immunologic assays
We measured RhCMV- and SIV-specific CD4+ and CD8+ T cell responses by flow cytometric intracellular cytokine analysis (FCICA) of peripheral blood mononuclear cells and BAL cells, as described7,24,25,38 (see Supplementary Methods). Anti-SIV binding antibodies were measured by ELISA of purified SIVmac239 viral lysates39. Neutralizing Abs against SIVmac239 and tissue culture adapted SIVmac251 were measured in luciferase reporter gene assays using the TZM-bl and M7 Luc cell lines, respectively, for SIVmac239 and tissue culture adapted SIVmac25140.
Statistical analysis
We performed statistical analysis with SAS version 9.1 (Statistical Analysis System). We assessed the significance of differences in 1) the phenotype or functional properties of RhCMV-specific T cells vs. RhCMV-vectored, SIV-specific T cells vs. prime-boost-elicited SIV-specific T cells using mixed effects analysis, 2) the proportion of RM within the vaccinated vs. control groups that completely controlled SIV infection after repeated, limiting dose mucosal challenge using a two-sided Fischer’s exact test, and 3) the peak and mean plateau phase (day 42 to day 91) plasma viral loads of the control vs. vaccinated RM with progressive infection by using general linear models with log10 transformed data. In all analysis, we used a two-sided significance level (alpha) of 0.05, with correction made for multiple comparisons using the Bonferroni method.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases, the International AIDS Vaccine Initiative, the Bill & Melinda Gates Foundation-supported Collaboration for AIDS Vaccine Discovery, the National Center for Research Resources, and the National Cancer Institute. We thank J. Edgar, A. Keech, J. Ford, J. Cook, M. Rohankhedkar, T. Ha, A. Sylwester, and J. Dewane for technical assistance, P. Barry, G. Pavlakis, G. Franchini, R. Seder, J. Bess, K. Reimann, C. Miller, and Centocor for provision of crucial constructs or reagents, M. Mori and J. O’Malley for statistical assistance, and K. Frueh and S. Wong for helpful discussion and advice.
Footnotes
AUTHOR INFORMATION:
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors have no competing financial interests.
REFERENCES
- 1.Johnson WE, Desrosiers RC. Viral persistence: HIV's strategies of immune system evasion. Ann Rev Med. 2002;53:499–518. doi: 10.1146/annurev.med.53.082901.104053. [DOI] [PubMed] [Google Scholar]
- 2.Haase AT. Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol. 2005;5:783–792. doi: 10.1038/nri1706. [DOI] [PubMed] [Google Scholar]
- 3.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 4.Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol. 2008;8:619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11:S25–S32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Ann Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 7.Pitcher CJ, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 8.Picker LJ, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J Clin Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan KS, Kaur A. Flow cytometric detection of degranulation reveals phenotypic heterogeneity of degranulating CMV-specific CD8+ T lymphocytes in rhesus macaques. J Immunol Methods. 2007;325:20–34. doi: 10.1016/j.jim.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14:617–621. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horton H, et al. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J Virol. 2002;76:7187–7202. doi: 10.1128/JVI.76.14.7187-7202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson NA, et al. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol. 2006;80:5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Letvin NL, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2008;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauduin MC, et al. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J Exp Med. 2006;203:2661–2672. doi: 10.1084/jem.20060134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pipeling MR, et al. Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection. J Immunol. 2008;181:546–556. doi: 10.4049/jimmunol.181.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossman Z, Picker LJ. Pathogenic mechanisms in simian immunodeficiency virus infection. Curr Opin HIV AIDS. 2008;3:380–386. doi: 10.1097/COH.0b013e3282fbaae6. [DOI] [PubMed] [Google Scholar]
- 18.Keele BF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, editors. Fields Virology. 4th edition. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2675–2706. [Google Scholar]
- 20.Britt W. Manifestations of human cytomegalovirus infection: Proposed mechanisms of acute and chronic disease. In: Shenk T, Stinski MF, editors. Human Cytomegalovirus. Heidelberg: Springer-Verlag; 2008. pp. 417–470. [DOI] [PubMed] [Google Scholar]
- 21.Powers C, Fruh K. Rhesus CMV: an emerging animal model for human CMV. Med Microbiol Immunol. 2008;197:109–115. doi: 10.1007/s00430-007-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sylwester AW, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kern F, et al. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur J Immunol. 1999;29:2908–2915. doi: 10.1002/(SICI)1521-4141(199909)29:09<2908::AID-IMMU2908>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Price DA, et al. Induction and evolution of cytomegalovirus-specific CD4+ T cell clonotypes in rhesus macaques. J Immunol. 2008;180:269–280. doi: 10.4049/jimmunol.180.1.269. [DOI] [PubMed] [Google Scholar]
- 25.Casazza JP, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVico AL, Gallo RC. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol. 2004;2:401–413. doi: 10.1038/nrmicro878. [DOI] [PubMed] [Google Scholar]
- 27.Letvin NL, et al. No evidence for consistent virus-specific immunity in simian immunodeficiency virus-exposed, uninfected rhesus monkeys. J Virol. 2007;81:12368–12374. doi: 10.1128/JVI.00822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedrich TC, et al. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J Virol. 2007;81:3465–3476. doi: 10.1128/JVI.02392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petravic J, et al. Estimating the Impact of Vaccination in Acute SHIV/SIV Infection. J Virol. 2008;82:11589–11598. doi: 10.1128/JVI.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz JE, et al. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Pathol. 1999;154:1923–1932. doi: 10.1016/S0002-9440(10)65450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rue CA, et al. A cyclooxygenase-2 homologue encoded by rhesus cytomegalovirus is a determinant for endothelial cell tropism. J Virol. 2004;78:12529–12536. doi: 10.1128/JVI.78.22.12529-12536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang WL, Barry PA. Cloning of the full-length rhesus cytomegalovirus genome as an infectious and self-excisable bacterial artificial chromosome for analysis of viral pathogenesis. J Virol. 2003;77:5073–5083. doi: 10.1128/JVI.77.9.5073-5083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kestler H, et al. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [see comment] [DOI] [PubMed] [Google Scholar]
- 34.Chackerian B, Haigwood NL, Overbaugh J. Characterization of a CD4-expressing macaque cell line that can detect virus after a single replication cycle and can be infected by diverse simian immunodeficiency virus isolates. Virology. 1995;213:386–394. doi: 10.1006/viro.1995.0011. [DOI] [PubMed] [Google Scholar]
- 35.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 36.Rossio JL, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venneti S, et al. Longitudinal in vivo positron emission tomography imaging of infected and activated brain macrophages in a macaque model of human immunodeficiency virus encephalitis correlates with central and peripheral markers of encephalitis and areas of synaptic degeneration. Am J Pathol. 2008;172:1603–1616. doi: 10.2353/ajpath.2008.070967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker JM, Maecker HT, Maecker VC, Picker LJ. Multi-color flow cytometric analysis in SIV-infected rhesus macaques. In: Darzynkiewicz Z, Roederer M, Tanke H, editors. Cytometry, 4th Edition, Methods in Cell Biology Series. San Diego: Academic Press/Elsevier; 2004. pp. 535–557. [DOI] [PubMed] [Google Scholar]
- 39.Lu X, et al. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS. 1998;12:1–10. doi: 10.1097/00002030-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montefiori DC. Chapter 12, Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol. 2005 doi: 10.1002/0471142735.im1211s64. Unit 12 11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.