Abstract
Purpose
Ventilator-induced lung injury (VILI) is a recognized complication of mechanical ventilation. Although the specific mechanism by which mechanical ventilation causes lung injury remains an active area of study, both alveolar overdistension and cyclical airway collapse and recruitment have been suggested as contributing causes. We hypothesized that mechanical ventilation in the absence of PEEP causes VILI to be more severe and regionally variable as compared with PEEP = 8 cmH2O.
Materials and Methods
To test this hypothesis, anesthetized, supine rabbits were mechanically ventilated with an end inspiratory pressure of 28-cm H2O and either 0- or 8-cmH2O PEEP over 4-hours. Regional lung injury was determined by histological scoring.
Results
In the absence of PEEP, lung injury was regionally variable and greatest in the dorsal-caudal lung. This regional injury heterogeneity was abolished by the addition of PEEP = 8 cm H2O.
Conclusions
These results suggest that VILI is regionally heterogeneous and spatially correlates with regions in which cyclical airway collapse and recruitment is most likely to occur.
Keywords: mechanical ventilation, ARDS, animal model, ventilator-induced lung injury, PEEP
INTRODUCTION
Lung injury and edema are well-documented consequences of mechanical ventilation with high distending pressures in multiple experimental models (1–3); however, maintaining end-expiratory lung volume at some level above functional residual capacity (FRC) with positive end-expiratory pressure (PEEP) can attenuate ventilator-induced lung injury (VILI) in animal models (1–6). In contrast, with inadequate or absent PEEP, significant lung injury occurs with lower tidal volumes and distending pressures in a surfactant-depleted, isolated lung model (6). The protective effect of PEEP has been attributed primarily to prevention of repeated airway collapse and expansion (RACE) (6, 7), and to a lesser extent limitation of tidal excursion, and reduced cardiac output (8).
Much of the supporting evidence for the RACE hypothesis comes from the influential studies of Gattinoni and colleagues who used CT scans to measure gas content of lung tissue in patients with ARDS to show that the dependent, dorsal-caudal lung regions can be “recruited” with the application of PEEP or prone positioning (9, 10). However, the correlation between gray scale and alveolar size only holds true if a uniform amount of water in each alveolus is present. Otherwise, any gradients seen might reflect differences in alveolar water content rather than alveolar size/expansion (11). An additional line of evidence supporting the RACE hypothesis is that spatial analysis of lung injury distribution shows a dorsal-caudal bias, which is the lung region where RACE is predicted to occur (4, 12, 13).
Although improved outcomes in ARDS patients ventilated with PEEP set above the lower inflection point of the inspiratory pressure-volume curve has been observed (14), a recent multi-center trial failed to show a survival advantage with a high-PEEP ventilation strategy (15). The RACE hypothesis has been called into question recently, favoring the tidal movement of fluid and/or foam in the airways as an explanation for the mechanical behavior of the injured lung during mechanical ventilation (11). In experiments employing markers to measure regional lung parenchymal movement in an oleic acid-induced lung injury model, collapse of dependent lung units at FRC, increased vertical gradient of regional lung volumes at FRC, and cyclical collapse and reopening of dependent alveoli were not observed (16). Additionally, a recent report of saline lavage-induced lung injury found that high tidal volume/low PEEP ventilation resulted in lung injury in the non-dependent lung regions in supine rats, suggesting atelectasis in the dependent lung zones shifts stretch-induced injury to the non-dependent lung and argues against repetitive collapse and expansion as a cause of VILI (17). The current technological limitations of available imaging modalities preclude accurate real-time imagining of all but the most peripheral alveoli, therefore whether alveoli open and close during mechanical ventilation remains uncertain. However, it has been argued that alveoli are more likely to flood than collapse when subjected to the stresses of mechanical ventilation (11, 18).
The primary goal of this study was to test the hypothesis that lung injury due to large tidal volume ventilation is attenuated and its spatial distribution altered with the application of PEEP. We postulated that lung injury, unlike that reported in a recent study (17), would be predominant in the dorsal-caudal lung where RACE is most likely to occur and that the application of PEEP would not only reduce the severity of lung injury, which we recognize is a well establish finding, but that the distribution of lung injury would be altered. That is to say, lung injury would be more homogeneously distributed compared with what occurs in the absence of PEEP. We are unaware of any previous studies that have examined the effects of PEEP on the regional distribution of VILI.
METHODS
Animals Preparation
The University of Washington Animal Care Committee, in accordance with National Institutes of Health guidelines, approved all methods. New Zealand white rabbits (either sex, 2.4 to 2.8 kg) were sedated with intramuscular ketamine (30 mg/kg) and xylazine (7.5 mg/kg) to allow placement of a 20ga catheter in each marginal ear vein. A surgical plane of anesthesia was then maintained with a continuous intravenous infusion of ketamine (0.05 mg/kg/hr) and xylazine (0.003 mg/kg/hr) for the remainder of the protocol. A 3.5 mm endotracheal tube was inserted orally to allow positive pressure mechanical ventilation. Arterial catheters were inserted for blood gas sampling and arterial pressure measurement. Pancuronium bromide (0.15 to 0.2 mg/kg) was administered intravenously, after adequate anesthesia established, to suppress spontaneous respiratory efforts. A 30-min stabilization period followed the completion of surgical preparation, after which baseline data were collected. During this period, animals were ventilated in pressure control mode (Servo 900C; Semens-Elema, Stockholm, Sweden) with a 50% inspiratory time and no inspiratory pause. Tidal volume was set at 10 – 12 cc/kg, PEEP=5 cm H2O and a respiratory rate to achieve PaCO2 = 35 – 45 mmHg during the stabilization period.
Physiologic Measurements
Data were recorded using Powerlab data acquisition system (AD-Instruments Castle Hill, New South Wales, Australia). Arterial blood pressure, heart rate, arterial blood gases (Radiometer ABL 5, Copenhagen, Denmark) and ventilatory parameters were measured for each experimental condition after a 20-minute stabilization period.
An in-line spirometer (KORR RSS 100; Medical Technologies Research Spirometry System, Salt Lake City, UT) was used to measure airway pressures and tidal volume (VT). Plateau pressure was measured as the pressure achieved at the end of a 5-second end-inspiratory hold maneuver.
Ventilator-induced Lung Injury Protocol
Animals were prospectively randomized to either, 1) PEEP= 0 cm H2O or 2) PEEP = 8 cm H2O. All animals were then ventilated in pressure control mode (inspiratory time 0.5) for 4 hours with a total end-inspiratory pressure (inspiratory pressure + PEEP) of 28 cm H2O (i.e. for PEEP = 0 cm H2O group, pressure control set at 28 cm H2O; for PEEP = 8 cm H2O group, pressure control set at 20 cm H2O). This would guarantee an equal magnitude of end-inspiratory distending pressure between the two groups. Respiratory rate was kept at 32 breaths per minute to insure an equal number of potential injurious insults in every animal. Inspired oxygen fraction (FIO2) was kept at 0.5 and CO2 (F ICO2 = 4–5%) was added to the inspired gas mixture to maintain eucapnia (PaCO2 = 30–45). Lactated ringers solution was infused at 10 cc/kg/hour and 10 cc/kg boluses as needed to support blood pressure. Physiological data were collected at baseline, 15 min after initiation of injury protocol, and hourly thereafter.
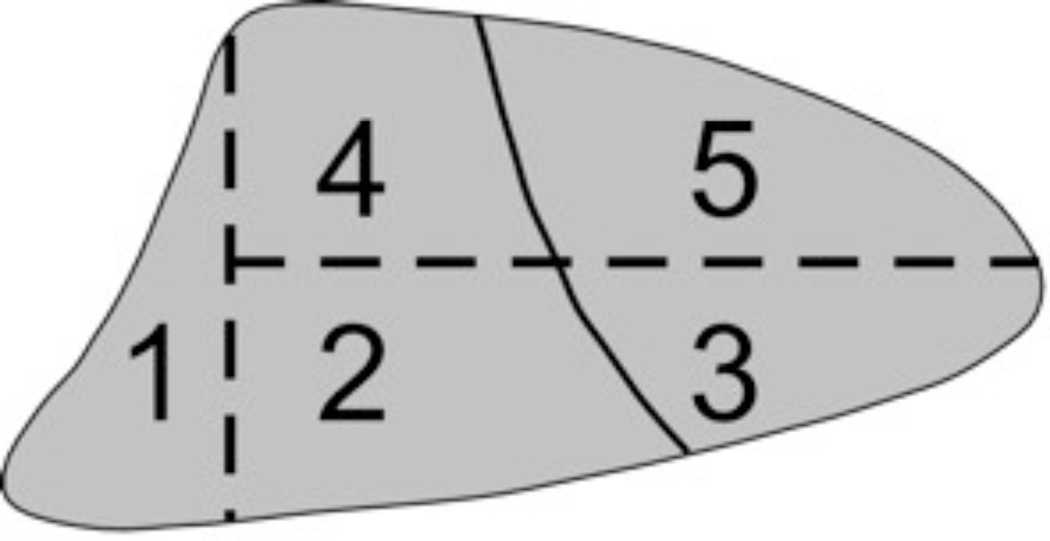
At the conclusion of each experiment, animals were exsanguinated and the heart and lungs removed en bloc. The lungs were fixed with intratracheal 10% buffered formalin at 20 cmH2O pressure overnight and subsequently divided into 5 regions with a #11 blade scalpel (Figure 1). Each region was then sectioned, stained with hematoxylin and eosin, and scored by a pathologist blinded to experimental conditions. Samples were assigned an injury score in each of 4 categories (interstitial edema, alveolar edema, neutrophil infiltration, and hemorrhage) based on severity (0 = not present, 4 = severe and present throughout) as previously described (5). Regional composite lung injury scores were calculated by summing the category scores within each lung region. Whole lung injury scores were calculated by summing the regional composite lung scores within each animal.
Figure 1.
Lung Divisions: Each lung was divided into five regions. The first region included all lung pieces in all ventral-dorsal sections that were adjacent to the diaphragm at any point. The remaining lung was divided into four regions as indicated based on bisecting transverse and coronal planes.
Data Analysis and Statistics
All data are presented as mean ± SD, and all statistical analyses were done using JMP software (SAS, Cary, NC). Paired t-tests were used to evaluate change in physiological data between the end of the experiment and baseline. Comparisons between the different groups were made by unpaired t-tests. Whole lung injury scores consisting of the sum of regional composite injury scores were compared between the PEEP = 0 cm H2O and PEEP = 8 cm H2O groups. For each animal, the standard deviation of regional composite lung injury scores was used to assess spatial heterogeneity of lung injury. For purposes of statistical testing, the logarithms of the standard deviations were used to achieve a more normal distribution of values prior to comparison by t-test. Comparisons between PEEP = 0 cm H2O and PEEP = 8 cm H2O animals were made using t-tests. The statistical methods were evaluated and approved by a statistical consultant.
RESULTS
Ventilator-induced lung injury
Five animals were studied in each group (PEEP = 0 Vs. PEEP = 8, total n = 10). None of the measured physiological parameters were statistically different between the PEEP = 0 cm H2O and PEEP = 8 cm H2O groups at baseline (Table 1). Over the 4 hr ventilation period, animals in both groups received similar total amounts of i.v. fluids (p = 0.7).
Table 1.
Baseline Data for the Ventilator Induced Lung Injury (prior to randomization)
| PEEP = 0 | PEEP = 8 | |
|---|---|---|
| Weight (kg) | 3.0±0.2 | 2.9±0.2 |
| Tidal Volume (ml) | 25±1.8 | 24±1.4 |
| Respiratory Rate (min−1) | 34.3±9.9 | 34.0±18.9 |
| Mean PAW (cmH2O) | 11.3±0.9 | 10.9±0.4 |
| PEEP (cmH2O) | 5.2±0.6 | 5.3±0.2 |
| Compliance (ml•cmH2O−1) | 2.6±0.4 | 2.4±0.2 |
| Heart Rate (min−1) | 168±16 | 171±11 |
| MAP (mmHg) | 71.8±9 | 72.5±10 |
| Arterial pH | 7.43±0.04 | 7.44±0.06 |
| Arterial PO2 (torr) | 281.8±6.8 | 285.0±11.2 |
| Arterial PCO2 (torr) | 38.8±3.4 | 39.5±4.7 |
At the end of the 4 hr period, there were no statistically significant differences in any hemodynamic parameters between the PEEP = 8 cmH2O and PEEP = 0 cmH2O groups (Table 2).
Table 2.
Physiological Response to Ventilation Protocol PEEP = 0 versus 8 cm H2O (Total inspiratory pressure = 28 cm H2O in both groups)
| Tidal Volume | Time = 0 | Time = 4 hours | ||
|---|---|---|---|---|
| PEEP | PEEP = 0 | PEEP = 8 | PEEP = 0 | PEEP = 8 |
| Tidal Volume (ml) | 72±9.2 | 28±3.3† | 71±13.5 | 36±3.4*† |
| Respiratory Rate (min−1) | 32 | 32 | 32 | 32 |
| Mean PAW (cmH2O) | 13.3±0.7 | 16.9±0.2† | 14.4±1.6 | 16.7±0.6 |
| Compliance (ml•cmH2O−1) | 3.3±0.4 | 1.7±0.2† | 2.8±0.5 | 2.3±0.3 |
| Heart Rate (min−1) | 188±27 | 198±30 | 197±32 | 185±50 |
| MAP (mmHg) | 65±9 | 59±11 | 60±3 | 58±5 |
| Arterial pH | 7.45±0.06 | 7.45±0.05 | 7.36±0.03 | 7.38±0.03 |
| Arterial PO2 (torr) | 309±7 | 296±10 | 133±88*† | 305±8 |
| Arterial PCO2 (torr) | 39±3 | 40±4 | 45±3 | 39±1 |
p<0.05 comparing different time points within the same group
p<0.05 comparing different groups (PEEP = 0 vs. PEEP = 8 cm H2O) levels at the same time point. T-tests used for statistical comparisons. MAP- mean arterial pressure, RVP – right ventricular pressure, PAW – airway pressure
The application of PEEP in one group and not the other precludes any meaningful direct comparisons of total thoracic compliance between these groups. However, static compliance showed a marked decline over the 4-hours of mechanical ventilation in the PEEP = 0 cm H2O group (−0.29±0.19 ml•cmH2O−1) compared with an improved static compliance when PEEP = 8 cm H2O was applied (0.53±0.16 ml•cmH2O−1; p < 0.0001). , Arterial oxygenation, which was similar at baseline between the two groups (309±6 vs. 296±10 torr, p = 0.11), was markedly lower in the PEEP = 0 cm H2O animals after 4 hrs (133±88 vs. 305±8 torr, p = 0.006). This physiological evidence of more severe lung injury in the PEEP = 0 group was associated with gross differences in the appearances of the lungs at the end of the experiment (Fig 2) and worse total histological lung injury score (11.6±6.9 vs. 2.7±2.2, p=0.02, Figure 3A). Additionally, lung injury scores were more variable across the five lung regions in the PEEP = 0 cm H2O animals (standard deviation of 1.40±0.40 vs. 0.45±0.34, p = 0.003, Figure 3B), indicating greater spatial heterogeneity of lung injury. In the absence of PEEP, the highest regional lung injury scores were seen in dorsal-caudal lung regions (Figure 4).
Figure 2.
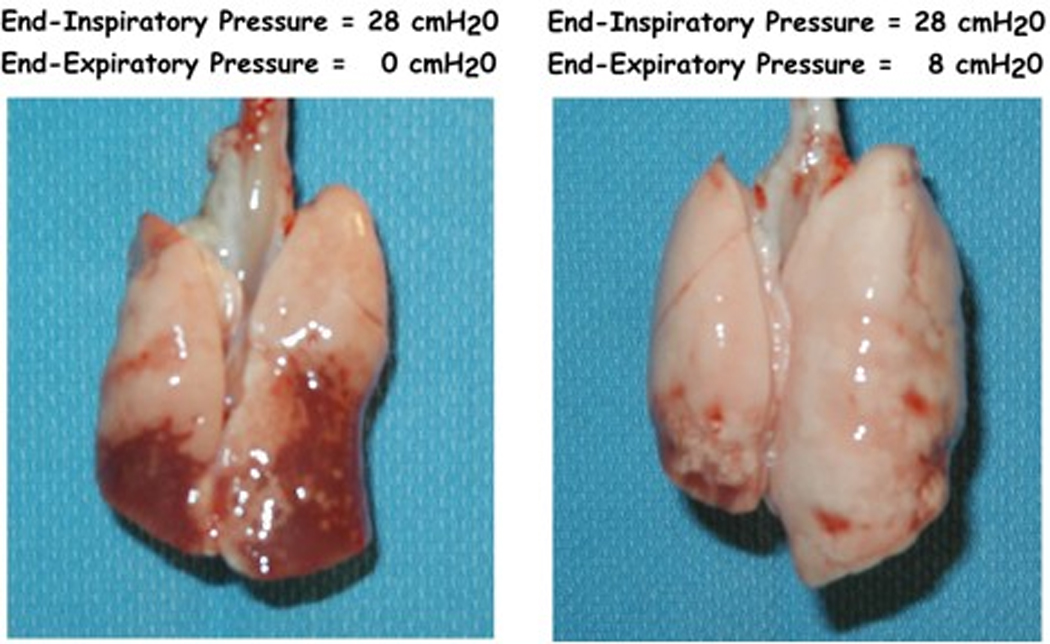
Gross appearance of lungs mechanically ventilated with to an end-inspiratory pressure of 28-cmH2O over for hours either in the absence (left) or presence (right) of PEEP = 8-cm H2O.
Figure 3.
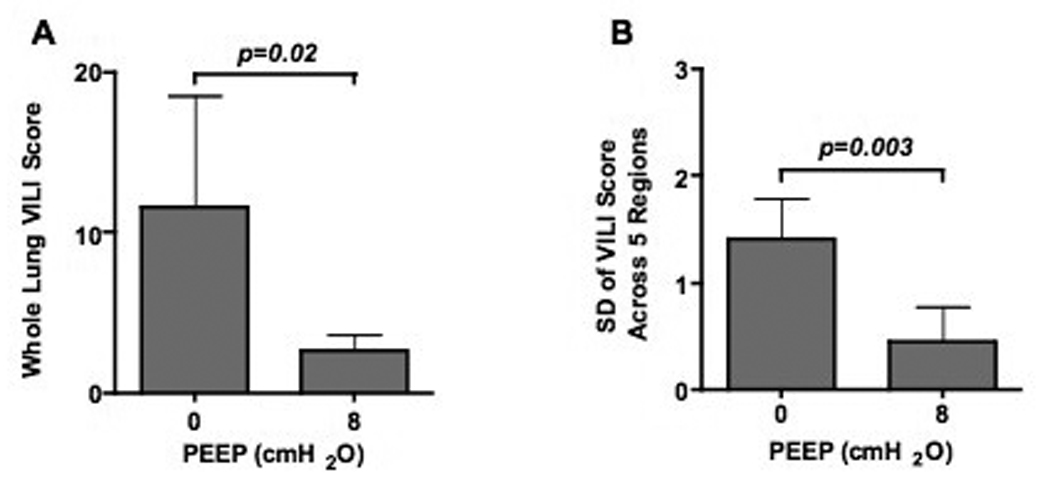
Total lung injury scores (A) and standard deviation of regional lung injury scores across the five lung divisions (B) for rabbits ventilated with 0- or 8-cmH2O PEEP.
Figure 4.
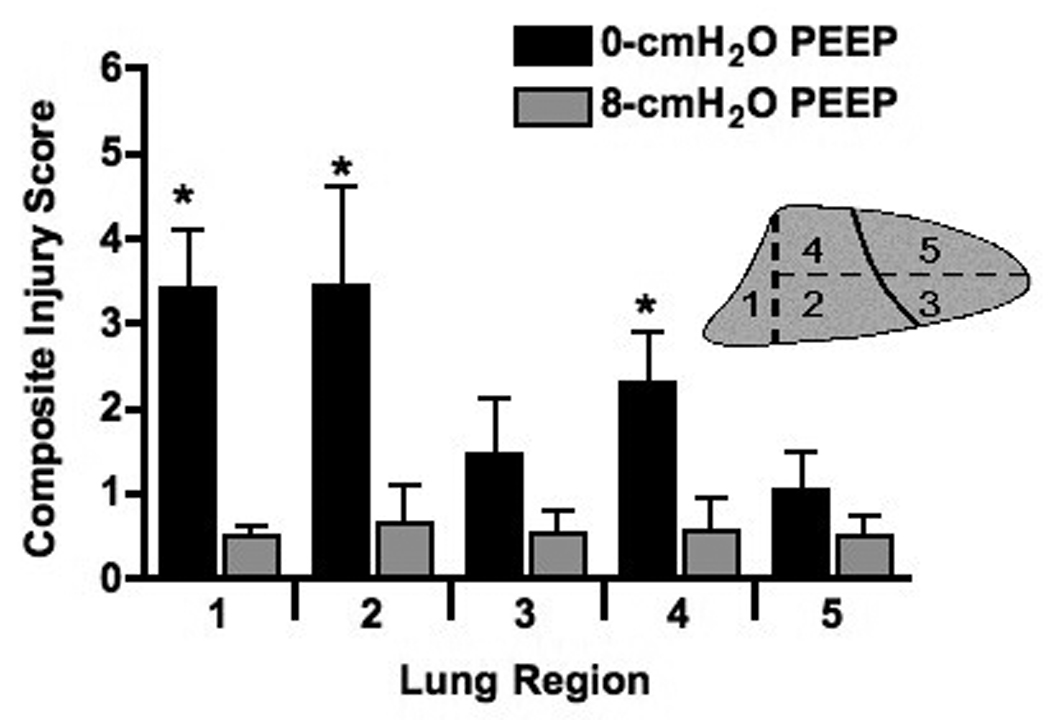
Lung injury scores separated by lung region for rabbits ventilated with 0- or 8-cmH2O PEEP. * indicates a significant (p≤0.05) difference between 0- and 8-cmH2O PEEP.
In summary, animals ventilated with high distending pressures in the absence of PEEP had greater evidence of lung injury than did animals ventilated with an identical end-inspiratory pressure but with the application of PEEP = 8 cm H2O. The difference in lung injury was evident by both physiological and histological measures. This was associated with a greater degree of spatial variability of injury in the PEEP = 0 cm H2O group with the most severe injury occurring in the dorsal-caudal lung regions. With the application of PEEP = 8 cm H2O, lung injury was reduced in severity and more homogeneous in its spatial distribution.
DISCUSSION
In this study, we hypothesized that ventilator-induced lung injury would be more regionally variable, with a dorsal-caudal predominance, in animals ventilated with high distending pressures in the supine posture in the absence of PEEP as compared with those ventilated with equally high distending pressures and PEEP = 8 cm H2O. The important findings of this study were that: 1) VILI was spatially heterogeneous and greatest in the dorsal-caudal lung in the absence of PEEP and 2) the application of PEEP decreased both the severity and spatial variability of VILI. Although demonstrating that PEEP attenuates VILI is not a novel finding, to our knowledge this is the first report to document the effect of PEEP on the spatial distribution of VILI in an animal model. Gattinonni (9, 19) and others have reported increased tissue density in the dorsal-caudal lung on computed tomographic images in patients with ALI/ARDS that were ameliorated (increased gas to tissue ratio) with the application of PEEP. However, the acute changes seen in these studies suggest that the changes in tissue density represent atelectasis and/or fluid redistribution rather than lung injury per se. In the current study we found changes in the distribution of histological injury with the application of PEEP in previously normal lungs.
Mechanical ventilation with large tidal volumes is known to produce VILI presumably due to alveolar over-distension. However, if this were the sole cause of VILI, one would expect the most severe injury to occur where alveoli are most distended at end-inhalation, i.e. non-dependent lung regions. In this study, both dorsal-ventral and cranial-caudal gradients of injury severity were observed with supine ventilation in the absence of PEEP, with the dorsal-caudal segments incurring the most severe injury. With the application of 8 cmH2O of PEEP however, no such gradient was observed, rather a less severe, more homogeneously distributed injury pattern was found, despite identical end inspiratory (i.e. distending) pressures. The application of PEEP has been shown to ameliorate VILI in a number of experimental models (1, 4, 6, 20–23). One potential explanation for the protective effects of PEEP is the prevention of tidal collapse and re-expansion of distal lung units. In the supine posture, lower alveolar volumes at FRC may predispose dependent lung regions to cyclical collapse. Muscedere et. al. (6) demonstrated epithelial injury in distal airways and alveoli, even with low tidal volumes (6cc/kg), when isolated, unperfused rat lungs were allowed to deflate to volumes below the lower inflection point of the pressure-volume curve during exhalation. Although compelling, these findings must be interpreted in the context of an ex vivo, unperfused model which will favor tidal airway collapse and minimize edema formation in comparison with an intact animal model (11).
Several additional factors must be considered in interpreting the results of this study. We used a fixed end-inspiratory pressure of 28 cmH2O to produce lung injury. This resulted in tidal volumes of ~24 cc/kg in the PEEP= 0 group. This is similar to tidal volumes used in rat (24) and mouse (25) models of VILI, but is clearly not physiologic and is far greater than that currently recommended for patients receiving mechanical ventilation for ALI/ARDS. However, patients with ARDS have heterogeneous lung injury, predominantly in the dorsal-caudal regions, with intervening areas of relatively normal lung (19, 26). The relatively normal regions, being more compliant, receive the majority of the tidal distension with each positive pressure breath. Therefore, in a severely injured lung, where, for example, only ~25% of lung regions or less remain “normal”, the tidal volume the non-injured regions receive with only a 6 cc/kg tidal breath will impact those regions like a ~ 24 cc/kg tidal volume. Since this model begins with normal lungs, the higher tidal volume is essential to mimic the mechanical forces seen in mechanical ventilation in ARDS.
The application of PEEP, while maintaining a constant end-inspiratory pressure (28 cmH2O), resulted in a significant reduction in tidal volume in PEEP = 8 cm H2O vs the PEEP = 0 cm H2O group. It could be argued that this reduction in tidal volume alone is sufficient to explain the attenuated lung injury observed in the PEEP = 8 group. This argument alone however, would not explain the change in the distribution of lung injury seen with the application of PEEP. Alternatively, if the net change in volume during a given breath were the primary injurious insult causing VILI in this model, one could argue that this would be most pronounced in the dorsal-caudal lung in the absence of PEEP (i.e. dorsal caudal lung would experience a greater net change in tidal expansion per breath than non-dependent regions) and that the application of significant PEEP could more evenly distribute the smaller change in volume of a given breath over the entire lung. We have previously reported preliminary data that PEEP produced a similar effect when tidal volumes were kept constant in PEEP and no PEEP groups (4). Unfortunately it is impossible to keep end-inspiratory pressure and tidal volume constant between PEEP and no PEEP groups without artificially altering the thoracic compliance (2). Given the clinical benefits seen with keeping airway pressures low, we felt a set end-inspiratory pressure protocol would more closely mirror clinically relevant ventilator settings.
Our findings conflict with a recent report by Tsuchida and colleagues of the distribution of lung injury in a saline lavage model (17). Injury in this model was most pronounced in non-dependent lung regions and was attributed to over-distension of areas distant from areas of dependent atelectasis. Our model differs in that we began with uninjured lungs that were not atelectasis prone. We also kept the FIO2 at a relatively modest 0.5 to avoid resorption atelectasis. Since all animals were ventilated to an identical end-inspiratory pressure, the end-inspiratory volumes were likely equivalent, which would argue against alveolar over-distension as the sole cause of VILI in this model. Finally, we (4) and others have previously reported a similar reduction in both total lung injury and regional variability of lung injury by ventilation in the prone posture, another method of reducing RACE. In this study, tidal volumes were set at 25 ml/kg throughout the entire experimental protocol for rabbits ventilated in both the prone and the supine postures. These results are similar to that of previous observations in both oleic acid injured (13) and normal dogs (12).
In conclusion, we have shown in a rabbit model of VILI that lung injury is greater and more spatially variable in the absence of PEEP. One potential mechanism for this finding is regional repeated airway collapse and expansion during tidal breathing. An alternative explanation of our findings is that the change in volume during a given breath is more important than the absolute magnitude of lung distension in the development of VILI. We speculate that RACE may occur in normal lungs in the supine posture in the absence of PEEP and that this may contribute to the development of lung injury.
Acknowledgements
The authors thank Wayne Lamm and Dowon An for assistance in performing these experiments.
Research Support: NIH Grants HL004479 and HL081297
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dreyfuss D, Basset G, Soler P, et al. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis. 1985;132(4):880–884. doi: 10.1164/arrd.1985.132.4.880. [DOI] [PubMed] [Google Scholar]
- 2.Dreyfuss D, Soler P, Basset G, et al. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137(5):1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 3.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110(5):556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair S, Souders J, Hlastala M. Severity and distribution of ventilator-induced lung injury is altered by PEEP, prone position, and respiratory frequency in normal rabbits. Am J Respir Crit Care Med. 1998;157(153):A107. [Google Scholar]
- 5.Sinclair SE, Kregenow DA, Lamm WJ, et al. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2002;166(3):403–408. doi: 10.1164/rccm.200112-117OC. [DOI] [PubMed] [Google Scholar]
- 6.Muscedere JG, Mullen JB, Gan K, et al. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149(5):1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 7.Russell J, Slutsky A, Lemaire F, et al. International Consensus Conference in Intensive Care Medicine: Ventilator-associated Lung Injury in ARDS. Am J Resp Crit Care Med. 1999;160:2118–2124. doi: 10.1164/ajrccm.160.6.ats16060. [DOI] [PubMed] [Google Scholar]
- 8.Luce JM. The cardiovascular effects of mechanical ventilation and positive end-expiratory pressure. JAMA. 1984;252(6):807–811. [PubMed] [Google Scholar]
- 9.Gattinoni L, Pesenti A, Bombino M, et al. Relationships between lung computed tomographic density, gas exchange, and PEEP in acute respiratory failure. Anesthesiology. 1988;69(6):824–832. doi: 10.1097/00000542-198812000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Langer M, Mascheroni D, Marcolin R, et al. The prone position in ARDS patients. A clinical study. Chest. 1988;94(1):103–107. doi: 10.1378/chest.94.1.103. [DOI] [PubMed] [Google Scholar]
- 11.Hubmayr RD. Perspective on lung injury and recruitment: a skeptical look at the opening and collapse story. Am J Respir Crit Care Med. 2002;165(12):1647–1653. doi: 10.1164/rccm.2001080-01CP. [DOI] [PubMed] [Google Scholar]
- 12.Broccard A, Shapiro RS, Schmitz LL, et al. Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Crit Care Med. 2000;28(2):295–303. doi: 10.1097/00003246-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Broccard AF, Shapiro RS, Schmitz LL, et al. Influence of prone position on the extent and distribution of lung injury in a high tidal volume oleic acid model of acute respiratory distress syndrome. Crit Care Med. 1997;25(1):16–27. doi: 10.1097/00003246-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 15.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 16.Martynowicz MA, Minor TA, Walters BJ, et al. Regional expansion of oleic acid-injured lungs. Am J Respir Crit Care Med. 1999;160(1):250–258. doi: 10.1164/ajrccm.160.1.9808101. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchida S, Engelberts D, Peltekova V, et al. Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med. 2006;174(3):279–289. doi: 10.1164/rccm.200506-1006OC. [DOI] [PubMed] [Google Scholar]
- 18.Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med. 1995;332(1):27–37. doi: 10.1056/NEJM199501053320106. [DOI] [PubMed] [Google Scholar]
- 19.Gattinoni L, Bombino M, Pelosi P, et al. Lung structure and function in different stages of severe adult respiratory distress syndrome. JAMA. 1994;271(22):1772–1779. [PubMed] [Google Scholar]
- 20.Argiras EP, Blakeley CR, Dunnill MS, et al. High PEEP decreases hyaline membrane formation in surfactant deficient lungs. Br J Anaesth. 1987;59(10):1278–1285. doi: 10.1093/bja/59.10.1278. [DOI] [PubMed] [Google Scholar]
- 21.Corbridge TC, Wood LD, Crawford GP, et al. Adverse effects of large tidal volume and low PEEP in canine acid aspiration. Am Rev Respir Dis. 1990;142(2):311–315. doi: 10.1164/ajrccm/142.2.311. [DOI] [PubMed] [Google Scholar]
- 22.Tremblay L, Valenza F, Ribeiro SP, et al. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchida S, Engelberts D, Peltekova V, et al. Atelectasis causes alveolar injury in non-atelectatic lung regions. Am J Resp Crit Care Med. 2006;174(3):279–289. doi: 10.1164/rccm.200506-1006OC. [DOI] [PubMed] [Google Scholar]
- 24.Quinn DA, Moufarrej RK, Volokhov A, et al. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol. 2002;93(2):517–525. doi: 10.1152/japplphysiol.00570.2001. [DOI] [PubMed] [Google Scholar]
- 25.Bailey TC, Martin EL, Zhao L, et al. High oxygen concentrations predispose mouse lungs to the deleterious effects of high stretch ventilation. J Appl Physiol. 2003;94(3):975–982. doi: 10.1152/japplphysiol.00619.2002. [DOI] [PubMed] [Google Scholar]
- 26.Goodman LR. Congestive heart failure and adult respiratory distress syndrome. New insights using computed tomography. Radiol Clin North Am. 1996;34(1):33–46. [PubMed] [Google Scholar]