Abstract
Kidney cancer is not a single disease; it is made up of a number of different types of cancer, each with a different histology, a different clinical course, caused by a different gene, and responding differently to therapy. The VHL gene is the gene for the hereditary cancer syndrome, von Hippel-Lindau, as well as for the common form of sporadic, non-inherited, clear cell kidney cancer. Understanding the VHL-HIF pathway has provided the foundation for the development of a number of agents targeting this pathway, such as sunitinib, sorafenib and temsirolimus. Hereditary Papillary Renal Carcinoma (HPRC) is a hereditary renal cancer syndrome in which affected individuals are at risk for the development of bilateral, multifocal, type 1 papillary renal cell carcinoma. The genetic defect underlying HPRC is MET, the cell surface receptor for hepatocyte growth factor (HGF). Mutations of MET have also been found in a subset of tumors from patients with sporadic type 1 papillary renal cell carcinoma. Clinical trials targeting the MET pathway are underway in patients with HPRC as well as patients with sporadic (non-hereditary) papillary kidney cancer. The BHD (also known as FLCN) gene is the gene for Birt-Hogg-Dubé syndrome, an autosomal dominant genodermatosis associated with a hereditary form of chromophobe, and oncocytic hybrid RCC. Preclinical studies are underway targeting the BHD gene pathway in preparation for clinical trials in Birt-Hogg-Dubé and sporadic chromophobe RCC. Hereditary Leiomyomatosis Renal Cell Carcinoma (HLRCC) patients are at risk for the development of cutaneous and uterine leiomyomas and a very aggressive type of RCC. HLRCC is characterized by germline mutation of the Krebs cycle enzyme, fumarate hydratase (FH). Studies of the TCA cycle and VHL-HIF pathways have provided the foundation for therapeutic approaches in patients with HLRCC-associated kidney cancer, as well as other hereditary and sporadic forms of RCC.
Keywords: kidney, neoplasm, VHL, MET, BHD, fumarate hydratase
Kidney cancer affects over 54,000 Americans annually and 13,000 die of this disease in the U.S. each year. Kidney cancer is the 8th leading cause of cancer death in the U.S.(1) Patients who present with localized kidney cancer have a 95% survival rate for a 5–10 year period, whereas those who present with advanced disease have only an 18% chance for a two year survival.(2) Kidney cancer is not a single disease; it is made up of a number of different types of cancer that occur in the kidney. Each of these cancers has a different histology and clinical course, responds differently to therapy, and is caused by a different gene mutation.(3;4)
Inherited Forms of Kidney Cancer
Kidney cancer is similar to other cancers such as prostate, colon and breast cancer in that it occurs in both sporadic (non-familial) and hereditary forms. Study of families with specific types of inherited kidney cancer has led to the identification of four high risk susceptibility genes for kidney cancer and has provided the foundation for the development of therapies that target these cancer gene pathways.(5) There are four well described types of inherited kidney cancer: 1) von Hippel-Lindau, the inherited form of clear cell RCC; 2) Hereditary Papillary Renal Cell Carcinoma, the hereditary form of type 1 papillary RCC; 3) Birt-Hogg-Dubé, the inherited form of chromophobe, oncocytic hybrid RCC, and 4) Hereditary Leiomyomatosis Renal Cell Carcinoma.
von Hippel-Lindau: Inherited form of Clear Cell Kidney Cancer-VHL Gene
von Hippel-Lindau (VHL) is an inherited form of kidney cancer in which affected individuals are at risk for the development of tumors in a number of organs, including bilateral, multifocal clear cell kidney cancers,(6) pancreatic cysts and neuroendocrine tumors,(7) pheochromocytoma,(8) retinal angiomas,(9), CNS hemangioblastomas(10) and tumors in the inner ear (endolymphatic sac tumors).(11)
VHL patients are at risk of developing of up to 600 tumors per kidney.(6) The goal of surgical management of VHL-associated kidney tumors is to prevent metastasis while preserving renal function. An approach has been developed to recommend surgical removal of renal tumors(12–17) with partial nephrectomy when the largest tumor reaches 3 centimeters in size.(18) The use of laparoscopic partial nephrectomy as well as robotic-assisted partial nephrectomy(17) has the potential to significantly reduce morbidity for patients with VHL-associated renal tumors.
Identification of the VHL Gene
Genetic linkage analysis in VHL kindreds led to the localization and subsequent identification of the VHL gene.(19) VHL gene mutation is found consistently in the germline of affected patients.(20) The VHL gene is also the gene for the common form of sporadic, non-hereditary, clear cell kidney cancer.(21;22) The VHL protein forms a complex with other proteins and targets the hypoxia-inducible factors (HIF) for ubiquitin-mediated degradation. HIF is a transcription factor that drives the downstream expression of a number of growth and proangiogenic factors that are important in cancer, such as vascular endothelial growth factor (VEGF); transforming growth factor alpha (TGFα), platelet derived growth factor (PDGF) and the epidermal growth factor receptor (EGFR). When the VHL gene is mutated, both in VHL patients or sporadic clear cell RCC, the VHL complex cannot degrade HIF and HIF accumulates. The result is increased levels of VEGF, PDGF, EGFR and other downstream factors in clear cell RCC.
Hereditary Non-VHL Clear Cell RCC
A number of families have been reported in which affected individuals bearing a balanced, constitutional translocation of a segment of chromosome 3 (to chromosome 8(23); chromosome 6(24) or chromosome 2(25)) are at risk for the development of bilateral, multifocal clear cell kidney cancer. This represents an example of a “3-hit” model of kidney cancer: the first alteration being the germline balanced translocation of chromosome 3, the second alteration being somatic loss of the chromosome 3 translocate (bearing one copy of the VHL gene), and the third alteration being the somatic mutation of the sole remaining allele of the VHL gene.(26) Clear cell kidney cancer in the chromosome 3 translocation families tends to be later onset than in von Hippel-Lindau, most likely due to the requirement for 3 events (non-VHL familial Clear Cell RCC) versus the need for two mutations in von Hippel Lindau. In a non-VHL family in which multiple members are affected with clear cell kidney cancer, a germline karyotype is recommended to rule out the possibility of a chromosome 3 translocation.
Targeting the VHL Gene Pathway in Clear Cell RCC
Understanding the VHL gene pathway has provided the foundation for the development of targeted approaches in the treatment of patients with advanced kidney cancer. In a phase 3 randomized study in patients with untreated metastatic clear cell RCC, treatment with Sunitinib has been shown to result in superior progression free survival and overall survival compared to interferon. Sunitinib, a tyrosine kinase inhibitor which targets the VEGF and PDGF receptors, has been associated with nearly a 35% response rate in patients with advanced clear cell RCC and an increased progression free survival over interferon.(27;28) Sorafenib, another tyrosine kinase inhibitor which targets the VEGF and PDGF receptors as well as Raf, has been found to have a 2–10% partial response and in patients with cytokine-refractory disease demonstrates a doubling in time to progression versus placebo.(29) The mammalian target of rapamycin (mTOR) inhibitor, temsirolimus, which indirectly targets the VHL pathway by inhibiting the transcription of HIF, has been found in patients with advanced clear cell kidney cancer to have a 9% partial response rate and an increased progression free survival as well as survival versus interferon in patients with advanced RCC who demonstrate 3 or more pre-defined ‘poor prognosis’ features.(30) While there are few reported complete responses with these agents, and most patients eventually develop progressive disease, these initial approaches offer proof of principle of the efficacy of targeting the VHL pathway in VHL −/− clear cell kidney cancer.
Hereditary Papillary Renal Cell Carcinoma: Type 1 Kidney Cancer-MET gene
Hereditary Papillary Renal Carcinoma (HPRC) is a hereditary cancer syndrome in which affected individuals are at risk for the development of bilateral, multifocal, type 1 papillary RCC.(31) To identify the HPRC gene, families were studied and genetic linkage analysis was performed to localize the HPRC gene to the long arm of chromosome 7. The MET gene was subsequently found to be the gene for HPRC.(32) The MET gene encodes the cell surface receptor for hepatocyte growth factor (HGF). MET is a proto-oncogene; hitherto identified HPRC-associated mutations constitutively activate the tyrosine kinase domain of MET. HPRC is autosomal dominant and highly penetrant; i.e., affected individuals are highly likely to develop bilateral, multifocal, papillary kidney cancer.(33) HPRC kidney cancer is uniformly type 1 papillary RCC;(34) the estimated prevalence of renal tumors is 1,100 to 3,400 microscopic papillary tumors in a single kidney.(35) Although HPRC is typically late onset (age of onset 5th, 6th and 7th decade), an early onset-form has been recently been described in which the disease appears in the second and third decade.(36) MET gene mutations have also been detected in a subset of tumors from patients with sporadic, type 1 papillary RCC.(37)
Clinical Evaluation of Patients with Bilateral, Multifocal Papillary RCC
Germline MET mutation analysis is recommended for patients with bilateral, multifocal papillary RCC as well as for those with a family history of type 1 papillary RCC. Family history should be obtained, although this disease can be occult and late onset. Imaging assessment includes contrast enhanced CT or MRI. HPRC renal tumors typically are relatively hypovascular, enhance uniformly and grow slowly. CT is preferable to ultrasound for screening because ultrasound can easily miss HPRC tumors.(38)
Clinical Management of Patients with HPRC
HPRC kidney cancers tend to be occult and, if not detected and treated, can spread to other organs. Parenchymal sparing surgery (partial nephrectomy) is recommended when the largest renal tumor approaches 3 cm in size. Patients whose tumors are smaller than 3 cm are generally managed with observation.(13;39) The goal of management is to maintain the patient’s renal function while minimizing the risk for metastasis.
Systemic Therapy: Targeting the MET in Papillary RCC
There are a number of potential approaches to targeting the MET gene pathway which are currently under study. One approach involves the use of tyrosine kinase inhibitors (TKIs) that target the tyrosine kinase domain of MET. Other approaches include antibodies that target HGF, the ligand of the MET cell surface receptor, or antibodies that target the extracellular domain of MET and inactivate the receptor. A Phase II study of the dual MET-VEGFR2 TK inhibitor, GSK1363089 (formerly XL880) in patients with papillary RCC is currently underway and is the first trial to evaluate MET inhibition in this disease. In an interim analysis of this clinical trial, GSK1363089 demonstrated antitumor efficacy in patients with papillary kidney cancer.(40)
Birt-Hogg-Dubé: Chromophobe Kidney Cancer-BHD Gene
Birt-Hogg-Dubé is an inherited renal cancer syndrome in which affected individuals are at risk for the development of cutaneous lesions (fibrofolliculoma),(41) pulmonary cysts and spontaneous pneumothorax, and bilateral, multifocal kidney cancer.(42) In describing the renal tumors in BHD patients, Pavlovich, et al found 34% (44 of 130) of the tumors were chromophobe kidney cancer, 50% were hybrid oncocytic tumors (areas reminiscent of chromophobe kidney cancer as well as oncocytomas), and 9% were clear cell RCC. Fifty eight percent of the patients were found to have microscopic oncocytosis in the remaining, grossly normal renal parenchyma.(43) In this study, the size of the tumors at presentation correlated with increasing malignant potential of the histologic type. Hybrid tumors averaged 2.2 cm, chromophobe renal carcinomas averaged 3.0 cm in diameter, and clear cell renal carcinomas averaged 4.7 cm. These varying histologies can be found in the same family, in the same patient, and sometimes even in the same kidney. In one patient, two oncocytomas, seven hybrid tumors, chromophobe renal carcinomas, and one clear cell renal carcinoma were found. Less than 15% of this patient’s kidney was found to be unaffected by tumor nodules.(43) In an initial report of 127 patients from 45 BHD families who were screened at NCI, 27% were found to have renal tumors.(44) Because of the bilateral, multifocal nature of BHD-associated kidney cancer, a management approach involving observation of small tumors until they reach 3 cm in size is often recommended.(44) When surgical intervention is recommended, an attempt is made to remove all or most of the visible tumors. Although surgical management is not considered “curative” for BHD-associated RCC, BHD-associated renal tumors are often slow growing and only infrequently are multiple surgical procedures during a patient’s lifetime required to control the tumor burden and prevent the development of metastasis.
Identification of the BHD Gene
In order to identify the susceptibility gene for Birt-Hogg-Dubé syndrome, affected individuals and their family members were evaluated by a multidisciplinary team at the Clinical Center, NIH. Genetic linkage analysis was performed and the BHD gene was localized(45) and then identified on the short arm of chromosome 17.(46) Sequence analysis of the BHD gene (also known as FLCN) in the germline of affected individuals from BHD families has identified mutation of this gene in nearly 90% of affected families.(46;47) A CLIA genetic test is available for individuals or families who are at risk for BHD syndrome.
BHD (FLCN) Gene Function
When BHD was identified, it was a novel gene with an unknown function. Subsequent studies which have identified binding partners to the protein product of the BHD gene (called folliculin (FLCN))(48;49) have placed this gene in a pathway involving AMPK, LKB1, TSC1/TSC2 and the mammalian target of rapamycin (mTOR). These studies suggested that FLCN may be involved in nutrient and/or energy sensing through the AMPK/mTOR pathway.(48)
Vocke and co-workers evaluated seventy-seven renal tumors from 12 patients with germline mutations of the BHD gene by DNA sequencing to identify somatic mutations in the second (wild-type) copy of the gene.(50) Mutations or loss of heterozygosity of the second allele were identified in 70% of renal tumors, suggesting that BHD is a loss-of-function, tumor suppressor gene.(51)
Targeting the BHD Gene Pathway
In order to asses the phenotypic effect of BHD gene mutations in the kidney and to provide a model for the evaluation of potential molecular therapeutic approaches for treatment of Birt-Hogg-Dubé, a kidney targeted BHD knockout mouse model was developed by Baba, et al.(52) In this rodent model, the BHD knockout mice developed enlarged, polycystic kidneys and died of renal failure at a very early age. Treatment with rapamycin had a significant impact on reducing the kidney size and increasing the median survival of the animals.(52) These studies provide the foundation for a potential therapeutic approach to BHD-associated kidney cancer.
Hereditary Leiomyomatosis Renal Cell Carcinoma: the Fumarate Hydratase Gene
Hereditary Leiomyomatosis Renal Cell Carcinoma (HLRCC) is a hereditary cancer syndrome in which affected individuals are at risk for the development of cutaneous and uterine leiomyomas and kidney cancer.(53) Cutaneous leiomyomas are most often present on the trunk or extremities. The lesions are often unilateral and from 10 to 100 have been observed in a single individual. Most patients will experience pain or paresthesia in association with these lesions. A high percentage (nearly 100%) of female patients with either cutaneous leiomyoma or who are FH mutation carriers develop uterine leiomyomas (fibroids). The uterine leiomyomas are often multiple and early onset; a high percentage of affected individuals reported hysterectomy or myomectomy in their 20’s.(54) Cutaneous and uterine leiomyosarcoma have been reported rarely.(54;55)
HLRCC-Associated Kidney Cancer
HLRCC associated kidney cancer is an aggressive form of kidney cancer that has a predisposition to metastasize early.(56) The morphologic spectrum of HLRCC renal cancers is unique and may be tubulo-papillary, solid, often with microcystic features. The most notable histologic features may include the presence of large nuclei with prominent orangiophilic nucleoli.(57) The histologic pattern can be suggestive of either type 2 papillary kidney cancer or collecting duct carcinoma. In a patient with a “collecting duct carcinoma” or “type 2 papillary kidney cancer” who has a family history of early onset uterine leiomyomas or cutaneous leiomyomas, HLRCC should be considered in the differential diagnosis. HLRCC patients are at risk for developing renal cysts and tumors that appear to develop either independently or within the renal cysts. The renal carcinomas can be early onset and, infrequently, bilateral and multifocal. When HLRCC renal tumors are detected, early surgical intervention is strongly recommended.
Fumarate Hydratase: the HLRCC Gene
The susceptibility gene for HLRCC encodes the Krebs cycle enzyme, fumarate hydratase (FH).(58) Mutations of the fumarate hydratase (FH) gene have been detected in over 90% of HLRCC kindreds.(54;59) Loss of heterozygosity is frequently found in HLRCC-associated kidney cancers, (53;57) suggesting that the FH gene has the characteristics of a tumor suppressor gene. In patients with a history of multiple cutaneous leiomyomas and/or early onset uterine leiomyomas, germline FH mutation analysis is often recommended.(56) Isaacs, et al. demonstrated a novel mechanism whereby inactivation of fumarate hydratase was associated with a VHL-independent dysregulation of HIF, providing the foundation for a potential therapeutic approach to treatment of HLRCC-associated kidney cancer.(60) Further studies are underway to understand the relationship between dysregulation of the Krebs cycle and tumorigenesis and the role of FH mutation in RCC.
Hereditary Paraganglioma/Pheochromocytoma
Hereditary paraganglioma/pheochromocytoma, an autosomal dominant hereditary condition in which affected individuals are at risk for the development of pheochromocytoma and extra-adrenal pheochromocytomas (paragangliomas) (PGL), is characterized by germline mutation of three (SDHB, SDHC, and SDHD) of the four genes that encode the Krebs cycle gene, succinate dehydrogenase.(61;62) In 2004 Vanharanta, et al. identified kidney cancer as a component of the hereditary PGL complex.(62) Recently Ricketts, et al. reported three patients with either early onset or bilateral, multifocal clear cell or chromophobe renal carcinoma who had germline mutations of SDHB.(63) In patients with early onset or bilateral, multifocal clear cell or chromophobe kidney cancer consideration should be given to succinate dehydrogenase germline mutation testing.
Tuberous Sclerosis Complex
Tuberous Sclerosis Complex (TSC) is a hereditary condition in which affected individuals are at risk for the development of a number of manifestations, including bilateral, multifocal renal lesions. While most TSC renal lesions are angiomyolipoma, renal cell carcinoma has been reported in 1–3%.(64) The predominant management issue with TSC-associated angiomyolipoma revolves around the potential for spontaneous hemorrhage. Most clinicians manage TSC-associated renal angiomyolipomas with observation, surgical removal or embolization. The management approach depends on the size and location of the tumor and the concern about the potential for sponataneous hemorrhage. Many clinicians recommend emoblization of TSC-associated renal angiomyolipomas, particularly those over 4–8 centimeters in size.(64;65) In a recent report of an open-label, phase 1–2 trial of sirolimus in patients with TSC, or the related condition, lymphangioleiomyomatosis. Treatment with sirolimus, which suppresses mTOR signaling, was associated with reduction of angiomyolipoma volume of 30% in 5 patients on this study.(66)
Summary
Understanding the genetic basis of kidney cancer has provided the foundation for the development of therapeutic approaches targeting the kidney cancer genes. Encouraging progress has been made with agents such as sunitinib, sorafenib and temsirolimus targeting the VHL gene pathway in clear cell kidney cancer. Studies are currently underway evaluating the role of agents which target the MET gene, in both Hereditary Papillary Renal Carcinoma as well as sporadic, non-inherited papillary RCC. Encouraging preclinical studies have provided a basis for potential therapeutic trials in BHD-associated kidney cancer as well as chromophobe renal carcinoma. Study of the fumarate hydratase pathway has provided an approach to the treatment of HLRCC-associated kidney cancer that may also be of benefit to patients with non-familial type 2 papillary kidney cancer. Knowledge of the mutations and pathways underlying each form of RCC has enabled targeted approaches to be developed to treat the various types of kidney cancer, both hereditary and sporadic.
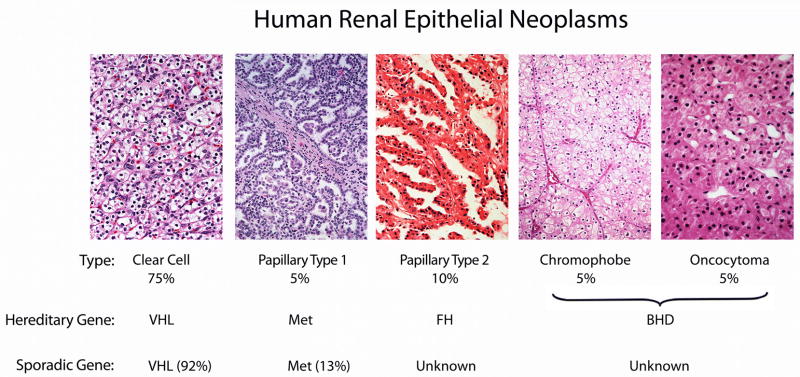
Figure 1.
Kidney cancer is made up of a number of different types of cancer, each with a different histologic type, with a different clinical course, responding differently to therapy and caused by different genes. The VHL gene is the gene for von Hippel-Lindau and for sporadic clear cell kidney cancer. The MET gene is the gene for Hereditary Papillary Renal Cell Carcinoma (HPRC) and has been found to be mutated in a subset of type 1 papillary renal tumors (13%).(37) The fumarate hydratase gene is the gene for hereditary leiomyomatosis renal cell carcinoma (HLRCC); the genetic basis of sporadic, type 2 papillary kidney cancer remains to be determined. The BHD gene is the gene for the hereditary form of chromophobe kidney cancer and oncocytoma associated with Birt Hogg-Dubé. BHD gene mutations have been identified in sporadic chromophobe kidney cancer,(67) however, the primary genetic basis of sporadic chromophobe kidney cancer and oncocytomas remains to be determined. Adapted from Linehan, et al.(5)
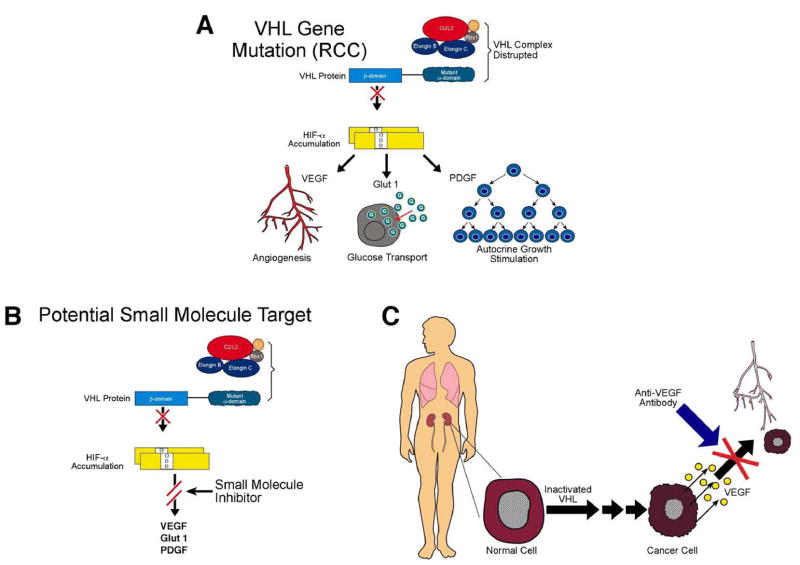
Figure 2.
The VHL gene is the gene for clear cell kidney cancer. The VHL protein targets hypoxia-inducible factor (HIF) for ubiquitin-mediated degradation. When the VHL gene is mutated in clear cell kidney cancer, the VHL protein cannot target and degrade HIF. HIF over-accumulates and causes increased transcription of downstream genes such as vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF) (A). Current therapeutic approaches include antibodies such as bevacizumab, which target VEGF, as well as agents such as sunitinib and sorafenib, which target the VEGF and PDGF receptors.(C) Future approaches could include agents which target HIF directly.(B) From Linehan, et al.(5)
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project was funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Reference List
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer Journal for Clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Linehan WM, Yang JC, Rini B. Cancer of the Kidney. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 8. New Haven: Lippincott Williams & Wilkins; 2008. pp. 1331–58. [Google Scholar]
- 3.Linehan WM, Vasselli J, Srinivasan R, Walther MM, Merino MJ, Choyke P, et al. Genetic Basis of Cancer of the Kidney: Disease-Specific Approaches to Therapy. Clinical Cancer Research. 2004;10:6282S–9S. doi: 10.1158/1078-0432.CCR-050013. [DOI] [PubMed] [Google Scholar]
- 4.Linehan WM, Zbar B. Focus on kidney cancer. Cancer Cell. 2004;6:223–8. doi: 10.1016/j.ccr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. Journal of Urology. 2003;170:2163–72. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 6.Walther MM, Lubensky IA, Venzon D, Zbar B, Linehan WM. Prevalence of microscopic lesions in grossly normal renal parenchyma from patients with von Hippel-Lindau disease, sporadic renal cell carcinoma and no renal disease: clinical implications. Journal of Urology. 1995;154:2010–4. [PubMed] [Google Scholar]
- 7.Blansfield JA, Choyke L, Morita SY, Choyke PL, Pingpank JF, Alexander HR, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine tumors (PNETs) Surgery. 2007;142:814–8. doi: 10.1016/j.surg.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walther MM, Reiter R, Keiser HR, Choyke PL, Venzon D, Hurley K, et al. Clinical and genetic characterization of pheochromocytoma in von Hippel-Lindau families: comparison with sporadic pheochromocytoma gives insight into natural history of pheochromocytoma. Journal of Urology. 1999;162:659–64. doi: 10.1097/00005392-199909010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Wong WT, Agron E, Coleman HR, Reed GF, Csaky K, Peterson J, et al. Genotype-phenotype correlation in von Hippel-Lindau disease with retinal angiomatosis. Arch Ophthalmol. 2007;125:239–45. doi: 10.1001/archopht.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonser RR, Glenn GM, Walther MM, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–67. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 11.Choo D, Shotland L, Mastroianni M, Glenn G, van WC, Linehan WM, et al. Endolymphatic sac tumors in von Hippel-Lindau disease. J Neurosurg. 2004;100:480–7. doi: 10.3171/jns.2004.100.3.0480. [DOI] [PubMed] [Google Scholar]
- 12.Walther MM, Choyke PL, Weiss G, Manolatos C, Long J, Reiter R, et al. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma. Journal of Urology. 1995;153:913–6. [PubMed] [Google Scholar]
- 13.Herring JC, Enquist EG, Chernoff A, Linehan WM, Choyke PL, Walther MM. Parenchymal sparing surgery in patients with hereditary renal cell carcinoma: 10-year experience. Journal of Urology. 2001;165:777–81. [PubMed] [Google Scholar]
- 14.Drachenberg DE, Mena OJ, Choyke PL, Linehan WM, Walther MM. Parenchymal sparing surgery for central renal tumors in patients with hereditary renal cancers. Journal of Urology. 2004;172:49–53. doi: 10.1097/01.ju.0000130930.70356.28. [DOI] [PubMed] [Google Scholar]
- 15.Grubb RL, III, Choyke PL, Pinto PA, Linehan WM, Walther MM. Management of von Hippel-Lindau-associated kidney cancer. Nat Clin Pract Urol. 2005;2:248–55. doi: 10.1038/ncpuro0179. [DOI] [PubMed] [Google Scholar]
- 16.Bratslavsky G, Liu JJ, Johnson AD, Sudarshan S, Choyke PL, Linehan WM, et al. Salvage Partial Nephrectomy for Hereditary Renal Cancer: Feasibility and Outcomes. Journal of Urology. 2007;179:67–70. doi: 10.1016/j.juro.2007.08.150. [DOI] [PubMed] [Google Scholar]
- 17.Rogers CG, Singh A, Blatt AM, Linehan WM, Pinto PA. Robotic Partial Nephrectomy for Complex Renal Tumors: Surgical Technique. Eur Urol. 2008;53:514–23. doi: 10.1016/j.eururo.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffey BG, Choyke PL, Glenn GM, Grubb RL, Venzon D, Linehan WM, et al. The Relationship Between Renal Tumor Size and Metastases in Patients with von Hippel-Lindau Disease. Journal of Urology. 2004;172:63–5. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 19.Latif F, Tory K, Gnarra JR, Yao M, Duh F-M, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–20. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 20.Stolle C, Glenn GM, Zbar B, Humphrey JS, Choyke P, Walther MM, et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Human Mutation. 1998;12:417–23. doi: 10.1002/(SICI)1098-1004(1998)12:6<417::AID-HUMU8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nature Genetics. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 22.Nickerson ML, Jaeger E, Durocher JA, Walters KB, Shi Y, Mahurkar S, et al. Improved Identification of von Hippel-Lindau Gene Alterations in Clear Cell Renal Tumors. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-07-4921. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen AJ, Li FP, Berg S, Marchetto DJ, Tsai S, Jacobs SC, et al. Hereditary renal-cell carcinoma associated with a chromosomal translocation. New England Journal of Medicine. 1979;301:592–5. doi: 10.1056/NEJM197909133011107. [DOI] [PubMed] [Google Scholar]
- 24.Eleveld MJ, Bodmer D, Merkx G, Siepman A, Sprenger SH, Weterman MA, et al. Molecular analysis of a familial case of renal cell cancer and a t(3;6)(q12;q15) Genes Chromosomes Cancer. 2001;31:23–32. doi: 10.1002/gcc.1114. [DOI] [PubMed] [Google Scholar]
- 25.Podolski J, Byrski T, Zajaczek S, Druck T, Zimonjic DB, Popescu NC, et al. Characterization of a familial RCC-associated t(2;3)(q33;q21) chromosome translocation. Journal of Human Genetics. 2001;46:685–93. doi: 10.1007/s100380170001. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt L, Li F, Brown RS, Berg S, Chen F, Wei MH, et al. Mechanism of tumorigenesis of renal carcinomas associated with the constitutional chromosome 3;8 translocation. Cancer J Sci Am. 1995;1:191–5. [PubMed] [Google Scholar]
- 27.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–24. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 28.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. New England Journal of Medicine. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 29.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. New England Journal of Medicine. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 30.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 31.Zbar B, Tory K, Merino MJ, Schmidt L, Glenn GM, Choyke P, et al. Hereditary papillary renal cell carcinoma. Journal of Urology. 1994;151:561–6. doi: 10.1016/s0022-5347(17)35015-2. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt L, Duh FM, Chen F, Kishida T, Glenn GM, Choyke P, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nature Genetics. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt L, Junker K, Weirich G, Glenn G, Choyke P, Lubensky I, et al. Two North American families with hereditary papillary renal carcinoma and identical novel mutations in the MET proto-oncogene. Cancer Research. 1998;58:1719–22. [PubMed] [Google Scholar]
- 34.Lubensky IA, Schmidt L, Zhuang Z, Weirich G, Pack S, Zambrano N, et al. Hereditary and sporadic papillary renal carcinomas with c-met mutations share a distinct morphological phenotype. American Journal of Pathology. 1999;155:517–26. doi: 10.1016/S0002-9440(10)65147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ornstein DK, Lubensky IA, Venzon D, Zbar B, Linehan WM, Walther MM. Prevalence of microscopic tumors in normal appearing renal parenchyma of patients with hereditary papillary renal cancer. Journal of Urology. 2000;163:431–3. [PubMed] [Google Scholar]
- 36.Schmidt LS, Nickerson ML, Angeloni D, Glenn GM, Walther MM, Albert PS, et al. Early onset Hereditary Papillary Renal Carcinoma: germline missense mutations in the tyrosine kinase domain of the Met proto-oncogene. Journal of Urology. 2004;172:1256–61. doi: 10.1097/01.ju.0000139583.63354.e0. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt L, Junker K, Nakaigawa N, Kinjerski T, Weirich G, Miller M, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999;18:2343–50. doi: 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- 38.Choyke PL, Walther MM, Glenn GM, Wagner JR, Venzon DJ, Lubensky IA, et al. Imaging features of hereditary papillary renal cancers. J Comput Assist Tomogr. 1997;21:737–41. doi: 10.1097/00004728-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Chernoff A, Choyke PL, Linehan WM, Walther MM. Parenchymal sparing surgery in a patient with multiple bilateral papillary renal cancer. Journal of Urology. 2001;165:1623–4. [PubMed] [Google Scholar]
- 40.Srinivasan R, Choueiri TK, Vaishampayan U, Rosenberg JE, Stein MN, Logan T, et al. A phase II study of the dual MET/VEGFR2 inhibitor XL880 in patients (pts) with papillary renal carcinoma (PRC) Journal of Clinical Oncology. 2008 May 26;20(Suppl):5103. [Google Scholar]
- 41.Birt AR, Hogg GR, Dube WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Archives Dermatology. 1977;113:1674–7. [PubMed] [Google Scholar]
- 42.Zbar B, Alvord WG, Glenn GM, Turner M, Pavlovich CP, Schmidt L, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dube syndrome. Cancer Epidemiology, Biomarkers and Prevention. 2002;11:393–400. [PubMed] [Google Scholar]
- 43.Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, et al. Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol. 2002;26:1542–52. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Pavlovich CP, Grubb RL, Hurley K, Glenn GM, Toro J, Schmidt LS, et al. Evaluation and Management of Renal Tumors in the Birt-Hogg-Dube Syndrome. Journal of Urology. 2005;173:1482–6. doi: 10.1097/01.ju.0000154629.45832.30. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt LS, Warren MB, Nickerson ML, Weirich G, Matrosova V, Toro JR, et al. Birt-Hogg-Dube syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2. American Journal of Human Genetics. 2001;69:876–82. doi: 10.1086/323744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn GM, Turner ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell. 2002;2:157–64. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt LS, Nickerson ML, Warren MB, Glenn GM, Toro JR, Merino MJ, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dube syndrome. American Journal of Human Genetics. 2005;76:1023–33. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baba M, Hong SB, Sharma N, Warren MB, Nickerson ML, Iwamatsu A, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552–7. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasumi H, Baba M, Hong SB, Hasumi Y, Huang Y, Yao M, et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene. 2008;415:60–7. doi: 10.1016/j.gene.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vocke CD, Yang Y, Pavlovich CP, Schmidt LS, Nickerson ML, Torres-Cabala CA, et al. High Frequency of Somatic Frameshift BHD Gene Mutations in Birt-Hogg-Dube-Associated Renal Tumors. Journal of the National Cancer Institute. 2005;97:931–5. doi: 10.1093/jnci/dji154. [DOI] [PubMed] [Google Scholar]
- 51.Hasumi H, Baba M, Hong SB, Hasumi Y, Huang Y, Yao M, et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene. 2008;415:60–7. doi: 10.1016/j.gene.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baba M, Furihata M, Hong SB, Tessarollo L, Haines DC, Southon E, et al. Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys. Journal of the National Cancer Institute. 2008;100:140–54. doi: 10.1093/jnci/djm288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Launonen V, Vierimaa O, Kiuru M, Isola J, Roth S, Pukkala E, et al. Inherited Susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–2. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toro JR, Nickerson ML, Wei MH, Warren MB, Glenn GM, Turner ML, et al. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. American Journal of Human Genetics. 2003;73:95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiuru M, Launonen V. Hereditary leiomyomatosis and renal cell cancer (HLRCC) Curr Mol Med. 2004;4:869–75. doi: 10.2174/1566524043359638. [DOI] [PubMed] [Google Scholar]
- 56.Grubb RL, III, Franks ME, Toro J, Middelton L, Choyke L, Fowler S, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. Journal of Urology. 2007;177:2074–80. doi: 10.1016/j.juro.2007.01.155. [DOI] [PubMed] [Google Scholar]
- 57.Merino MJ, Torres-Cabala C, Pinto P, Linehan WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578–85. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 58.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–10. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 59.Wei MH, Toure O, Glenn GM, Pithukpakorn M, Neckers L, Stolle C, et al. Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet. 2006;43:18–27. doi: 10.1136/jmg.2005.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–53. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–51. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 62.Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peczkowska M, Morrison CD, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. American Journal of Human Genetics. 2004;74:153–9. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ricketts C, Woodward ER, Killick P, Morris MR, Astuti D, Latif F, et al. Germline SDHB mutations and familial renal cell carcinoma. Journal of the National Cancer Institute. 2008;100:1260–2. doi: 10.1093/jnci/djn254. [DOI] [PubMed] [Google Scholar]
- 64.Lane BR, Aydin H, Danforth TL, Zhou M, Remer EM, Novick AC, et al. Clinical correlates of renal angiomyolipoma subtypes in 209 patients: classic, fat poor, tuberous sclerosis associated and epithelioid. Journal of Urology. 2008;180:836–43. doi: 10.1016/j.juro.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 65.Seyam RM, Bissada NK, Kattan SA, Mokhtar AA, Aslam M, Fahmy WE, et al. Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urology. 2008;72:1077–82. doi: 10.1016/j.urology.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 66.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. New England Journal of Medicine. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gad S, Lefevre SH, Khoo SK, Giraud S, Vieillefond A, Vasiliu V, et al. Mutations in BHD and TP53 genes, but not in HNF1beta gene, in a large series of sporadic chromophobe renal cell carcinoma. Br J Cancer. 2007;96:336–40. doi: 10.1038/sj.bjc.6603492. [DOI] [PMC free article] [PubMed] [Google Scholar]