Abstract
UVB (280–315 nm) in natural sunlight represents a major environmental challenge to the skin and is clearly associated with human skin cancer. Here we demonstrate that low doses of UVB induce keratinocyte proliferation and cell cycle progression of human HaCaT keratinocytes. Different from UVA, UVB irradiation induced extracellular signal-regulated kinase (ERK) and AKT activation and their activation are both required for UVB-induced cell cycle progression. Activation of epidermal growth factor receptor (EGFR) was observed after UVB exposure and is upstream of ERK/AKT/cyclin D1 pathway activation and cell cycle progression following UVB radiation. Furthermore, metalloproteinase (MP) inhibitor GM6001 blocked UVB-induced ERK and AKT activation, cell cycle progression, and decreased the EGFR phosphorylation, demonstrating that MPs mediate the EGFR/ERK/AKT/cyclin D1 pathways and cell cycle progression induced by UVB radiation. In addition, ERK or AKT activation is essential for EGFR activation because ERK or AKT inhibitor blocks EGFR activation following UVB radiation, indicating that EGFR/AKT/ERK pathways form a regulatory loop and converge into cell cycle progression following UVB radiation. Identification of these signaling pathways in UVB-induced cell cycle progression of quiescent keratinocytes as a process mimicking tumor promotion in vivo will facilitate the development of efficient and safe chemopreventive and therapeutic strategies for skin cancer.
INTRODUCTION
UV radiation in sunlight is clearly an important environmental factor in human skin carcinogenesis. Each year approximately one million new cases of skin cancer are diagnosed in the United States alone, making it the most common type of cancer in this country. In animal models, UV radiation is a complete carcinogen that can both initiate and promote skin carcinogenesis, resulting in squamous cell carcinoma (SCC), basal cell carcinoma and melanoma (1–3).
UV radiation in sunlight is composed of UVB (280–315 nm) and UVA (315–400 nm). UVB has been considered to be the major radiation in sunlight causing skin cancer. In an effort to prevent skin cancer caused by excessive sunlight exposure including sunscreen, it is still a concept for the public and the earlier scientific community that only inflammatory or sunburn-inducing high UV exposure is skin carcinogenic, while low doses of UV radiation could be safe. However, recent studies in the laboratories using mouse models have shown that low doses of UVB radiation cause skin tumorigenesis, particularly the formation of papilloma in mice (actinic keratosis in human) (4). While low doses of UVB radiation-induced gene mutations and immune suppression are critical processes in skin tumorigenesis, tumor promotion is essential for tumor growth following UVB radiation.
Cell proliferation as a consequence of cell cycle progression is the key process that leads to clonal expansion of initiated cells during tumor promotion. Cyclin D1 is a cell cycle regulatory protein that acts as a growth factor sensor to integrate extracellular signals with the cell cycle machinery, particularly during the G1 phase of the cell cycle (5). Increased cyclin D1 has been associated with mouse skin transformation (6–9). However, the effect of low-dose UVB radiation on cyclin D1 and cell proliferation and its underlying mechanisms are unknown. Understanding the signal transduction pathways by which the UVB signals to cyclin D1 and cell proliferation and identifying the critical elements in these pathways will provide novel essential targets for chemoprevention.
Epidermal growth factor receptor (EGFR), one of the receptor tyrosine kinases (RTKs), plays a pivotal role in regulating cell proliferation, differentiation and transformation. Binding of growth factors to RTKs promotes receptor dimerization and subsequent activation, which enhances autophosphorylation of RTKs, phosphorylation of numerous cellular proteins and recruitment of adaptor molecules, thus initiating signaling cascades, including phosphatidylinositol (PI) 3-kinase/AKT and extracellular signal-regulated kinase (ERK) pathways (10,11). Activation of the PI 3-kinase/AKT and/or ERK pathways can lead to cell proliferation and growth by regulating the cyclin D1 level (12,13).
Growing evidence points to the metalloproteinases (MPs), particularly the transmembrane MPs, as the key enzymes shedding growth factors and regulating EGFR signaling (14). The transmembrane MPs including certain matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinases (ADAMs) are zinc-dependent proteolytic enzymes. These enzymes are implicated in EGFR ligand shedding by 12-O-tetradecanoylphorbol 13-acetate (TPA) (15), and G-protein-coupled receptor signaling (16), and therefore the transactivation of EGFR. However, the role of MPs in UVB responses of keratinocytes is unknown.
We recently showed that low UVA radiation induces cell cycle progression in which EGFR/AKT but not ERK pathway is required (17). However, the effect of low-dose UVB radiation on cell cycle progression and the underlying mechanisms are unknown. It has been demonstrated that EGFR is activated by UVB radiation in mouse skin (18) and the activation of EGFR is a critical mediator for signal transduction induced by higher doses of UVB radiation (40 mJ cm−2) (19) and UVB-induced skin carcinogenesis (18). However, it is still unclear whether EGFR promotes cell proliferation and how EGFR is activated by low-dose UVB. To identify the mechanisms in low doses of UVB-induced cell proliferation, we studied the mechanisms in cell cycle progression induced by low, nonlethal doses of UVB in HaCaT cells, a human keratinocyte line. In this study, cyclin D1 upregulation and cell cycle progression were observed after UVB exposure. The MP-dependent ERK and AKT pathways transduced UVB signaling to cyclin D1 and induced a proliferative response in human keratinocytes. These data clearly demonstrate the mechanisms and the tumor promotional potential of UVB in human skin cells.
MATERIALS AND METHODS
Cell culture and UVB treatment
HaCaT cells were cultured in 60 mm dishes with normal culture medium. The cell culture medium was replaced with 0.1% fetal bovine serum (FBS) DMEM and cultured for 24–36 h. Cells were then exposed to UVB. For UVB treatment, the Stratalinker 2400 equipped with 312-nm UVB bulbs (Stratagene, La Jolla, CA) (UVC 0%, UVB 51% and UVA 49%) was used. Our data here and our recent paper have shown that UVB is 1000 times more potent than UVA because UVB at 2.5 mJ cm−2 induces cell cycle progression more than UVA at 4 J cm−2 (17). When cells are exposed to 10 mJ cm−2 of UVB, the UVA radiation in our exposure system is 9.6 mJ cm−2. Exposure of HaCaT cells to this UVA dose has no effect on cell cycle progression or ERK and AKT activation (data not shown). These findings confirm that our UVB exposure system is appropriate for determining UVB responses. The UV exposure was performed in PBS after washing the cells with PBS twice to avoid the photosensitization effect of components in culture medium on the cells. In selected experiments, cells were preincubated with inhibitors at 37°C for 1 h prior to irradiation. Inhibitors used were as follows: GM6001 (GM), AG1478 (AG), PD98059 (PD) and LY294002 (LY) from Biomol (Plymouth Meeting, PA). After treatment, cells were incubated at 37°C in medium containing 0.1% FBS with or without the inhibitors.
Animal treatments
Female outbred SKH-1 mice were obtained from Charles River Laboratories (Wilmington, MA). Irradiated mice were exposed to UVB (50 mJ cm−2) dorsally once a day for 4 days. Twenty-four hours after the final exposure, mice were killed and skin was collected for H&E staining. Skin histology was determined by light microscopy. Mice were housed three animals per cage, and there was no evidence of dorsal wounds caused by fighting or sunburn.
Cell cycle analysis
At predetermined time points after treatment, the cells were harvested and fixed with 5 mL of ice cold 70% ethanol. For cell cycle analysis, the fixed cells were then centrifuged (1000 rpm, 5 min) and washed with PBS. The cells were then incubated with propidium iodide (20 µg mL−1) containing RNase A (100 µg mL−1; Sigma Chemical, St. Louis, MO). The DNA content was determined by flow cytometry (BD Biosciences, Franklin Lakes, NJ) and the percentage of cells in the S phase was quantified by ModFit LT software (Verity Software House, Inc.).
Determination of cell proliferation
At different time points after UVB radiation, cell proliferation was measured using the MTS assay (CellTiter 96Aqueous Proliferation Assay; Promega) and monitored at 490 nm using a Tecan M200 plate reader according to the manufacturer's instructions.
Western blotting
Western blotting was performed as described previously (19–21). Antibodies used were as follows: cyclin D1 (Cell Signal), p-AKT (phospho-AKT-ser473; Cell Signal); p-ERK (phospho-ERK1/2; Santa Cruz); p-EGFR (phosphor-EGFR-tyrosine 1173; Santa Cruz); AKT (Santa Cruz), ERK (Santa Cruz), EGFR (Neo-marker) and β-actin (Santa Cruz). The Western blot signal was exposed to film. We then scanned the films into computer using a scanner. The optical density of the scanned blot was quantified using ImageJ (NIH) relative to a standard titration curve of PTEN protein. Data were expressed as a ratio with the protein level in the control taken to be 1.0.
Statistical analyses
Data were expressed as the mean of three independent experiments and were analyzed using Student's t-test. A two-sided value of P < 0.05 was considered significant in all cases.
RESULTS
Cell cycle progression induced by sublethal UVB radiation in human HaCaT keratinocytes
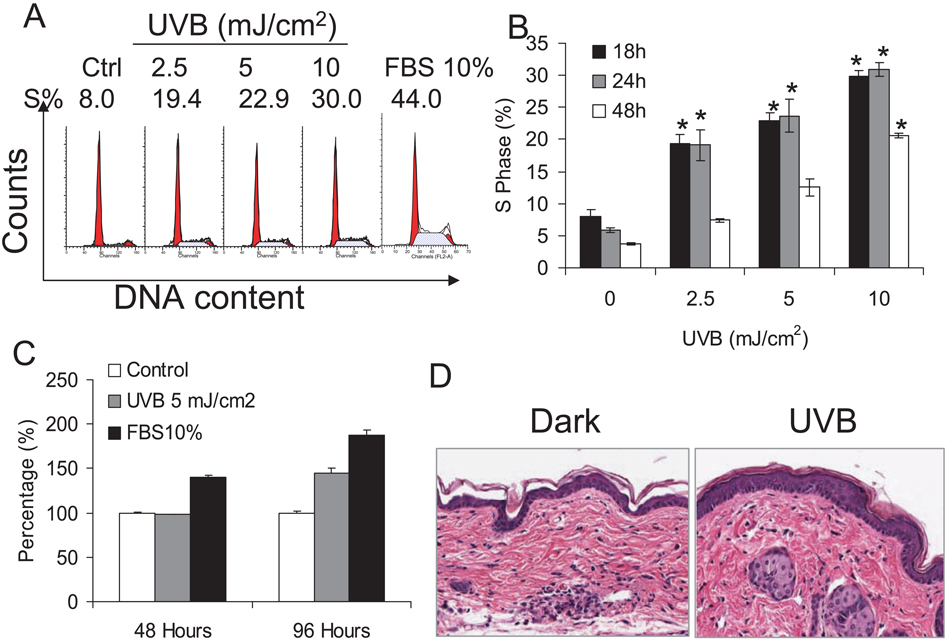
To study cell cycle progression induced by low-dose UVB exposure, human HaCaT keratinocytes were starved in 0.1% FBS for 24–36 h to synchronize the cells to the G0 phase. Cells were then exposed to different doses of UVB from 2.5 to 10 mJ cm−2. Eighteen hours after exposure cells were harvested for cell cycle analysis (Fig. 1A). ModFit LT was used to quantify percentage of the cell population in the S phase. As shown in Fig. 1A,B, 2.5 mJ cm−2 UVB exposure increased cell accumulation in the S phase (S) significantly (P < 0.05) while 5 and 10 mJ cm−2 induced higher proportions of cells in the S phase, indicating that UVB induces the G1-S cell cycle reentry of quiescent keratinocytes. It is noteworthy that at these doses no cell death was detected (data not shown). These data suggest that UVB-induced cell cycle progression is UVB dose dependent. To determine whether the accumulation of cells in the S phase following UVB radiation resembles serum addition, FBS (10%) was added back to starved cells. At 18 h after FBS, 44% of cells were accumulated in the S phase (Fig. 1A), indicating that serum induces cell cycle re-entry of quiescent keratinocytes.
Figure 1.
UVB exposure-induced cell cycle progression in human HaCaT cells. HaCaT cells were seeded in 60 mm dishes, starved with 0.1% FBS DMEM medium for 24–36 h and then exposed to UVB at the doses indicated. (A) Cells were exposed to 2.5, 5 or 10 mJ cm−2 or kept in the dark. Eighteen hours after UVB exposure, cells were harvested and prepared for cell cycle analysis by flow cytometry as described in the Materials and Methods section. The left peak on the left indicates the G1 phase and the right peak indicates the G2 phase in each histogram. The S phase is in the middle. The histograms of dark, UVB (2.5, 5 or 10 mJ cm−2) and FBS (10%) are shown. (B) Cells were exposed to UVB as in (A) and harvested at 18, 24 and 48 h after the exposure. Data are expressed as percentages and represent means (± standard deviation, n = 3); *Significant differences from control cells (P < 0.05). (C) Starved HaCaT cells were exposed to UVB once or twice every other day or incubated with 10% FBS. Cell proliferation was determined by the MTS assay. (D) SKH-1 mice were exposed to UVB (50 mJ cm−2) once a day for 4 days. Skin was collected at 24 h after the final UVB exposure and skin thickness was evaluated after H&E staining.
When later time points were examined, including 24 and 48 h after UVB exposure, the proportion of cells in the S phase decreased at 48 h following UVB exposure at each UVB dose compared with the proportion at 18 h after UVB exposure while a similar percentage of cells remained in the S phase at 24 h following UVB radiation (Fig. 1B). At 48 h after UVB exposure, most of the cells went back to the G0-G1 phase (G1) following 2.5 mJ cm−2 of UVB radiation, because no significant increase in the G2-M (G2) phase was observed (data not shown). This suggests that the cells were able to finish the cell cycle and return to the G1 phase after UVB-induced G1-S progression. Following 5 and 10 mJ cm−2 of UVB radiation, more cells in the S phase were detected at 48 h, possibly due to prolonged G1-S cell cycle progression and/or slower S-G2 cell cycle progression.
To determine whether UVB exposure induces cell proliferation, HaCaT cells were exposed to UVB (5 mJ cm−2) once or twice every other day. Cell proliferation was evaluated using the MTS assay (CellTiter 96 Aqueous Proliferation Assay; Promega). Cell proliferation at 48 h after the first UVB exposure remained similar to that of the control cells, while 10% FBS induced significant increase in proliferation (Fig. 1C, P < 0.05). In contrast, a significant increase in proliferation was observed at 48 h after the second UVB exposure with a lesser extent to FBS (10%) (Fig. 1C). These data indicate that UVB-induced cell cycle progression observed in Fig. 1A,B results in cell proliferation, similar to FBS addition.
To test whether UVB exposure will cause keratinocyte proliferation in vivo in mouse skin, SKH-1 hairless mice were exposed to UVB (50 mJ cm−2, three mice per group) every day for 4 days. This exposure regime did not cause erythema in the skin because the total dose used here (200 mJ cm−2) is below the minimal erythemal UVB doses (1 MED) for SKH-1 mice (approximately 224 mJ cm−2) (20). Twenty-four hours after the final exposure, mouse skin was collected and skin histology was determined after H&E staining. Compared with control skin, UVB-irradiated skin showed significantly increased epidermal thickness (P < 0.05) (Fig. 1D). This hyperplasia may be due to keratinocyte proliferation induced by low-dose UVB radiation, implying that low-dose UVB induces keratinocyte proliferation in vivo.
Cyclin D1/AKT/ERK/EGFR signaling following low-dose UVB radiation in human HaCaT keratinocytes
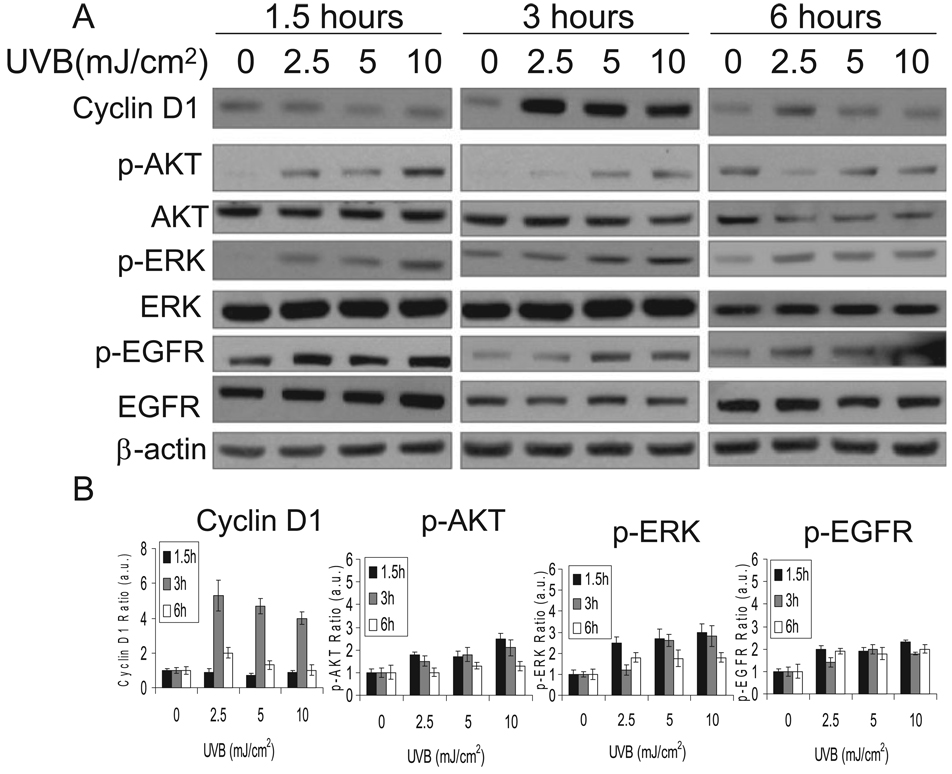
To delineate the mechanisms and pathways involved in UVB-induced cell cycle progression from the G1 to the S phase, we exposed cells to UVB at different doses, harvested them at different times and analyzed protein samples by Western blot. The cyclin D1 levels, AKT phosphorylation, ERK phosphorylation and EGFR phosphorylation were determined at different time points after UVB exposure or kept in the dark. It is interesting that in HaCaT keratinocytes, ERK2 is the more dominant form than ERK1. Only when the blot is exposed to longer time when p-ERK2 signal is saturated, p-ERK1 can be detectable. A similar molecular composition has been detected in normal human epidermal keratinocytes.
As shown in Fig. 2A,B, UVB exposure (2, 5 or 10 mJ cm−2) increased cyclin D1 accumulation at 3 h following the exposure but not at 1.5 or 6 h, indicating UVB-induced cyclin D1 accumulation is time dependent. The cyclin D1 accumulation peaked at 2.5 mJ cm−2 of UVB radiation and slightly decreased with UVB doses. In contrast, dose-dependent ERK, AKT and EGFR activation peaked as early as 1.5 h and then decreased with time up to 6 h following UVB exposure compared to that in the dark. These data indicate that low-dose UVB increases cyclin D1 accumulation and activates AKT, ERK and EGFR pathways.
Figure 2.
Cells were exposed to UVB as in Fig. 1. Cells were harvested at 1.5, 3 or 6 h following UVB radiation for Western blot analysis using specific antibodies against cyclin D1, p-AKT, AKT, p-ERK, ERK, p-EGFR, EGFR and beta-actin (an equal loading control) (A). The Western blots were quantified with ImageJ (B).
Role of ERK activation in UVB-induced cell cycle progression
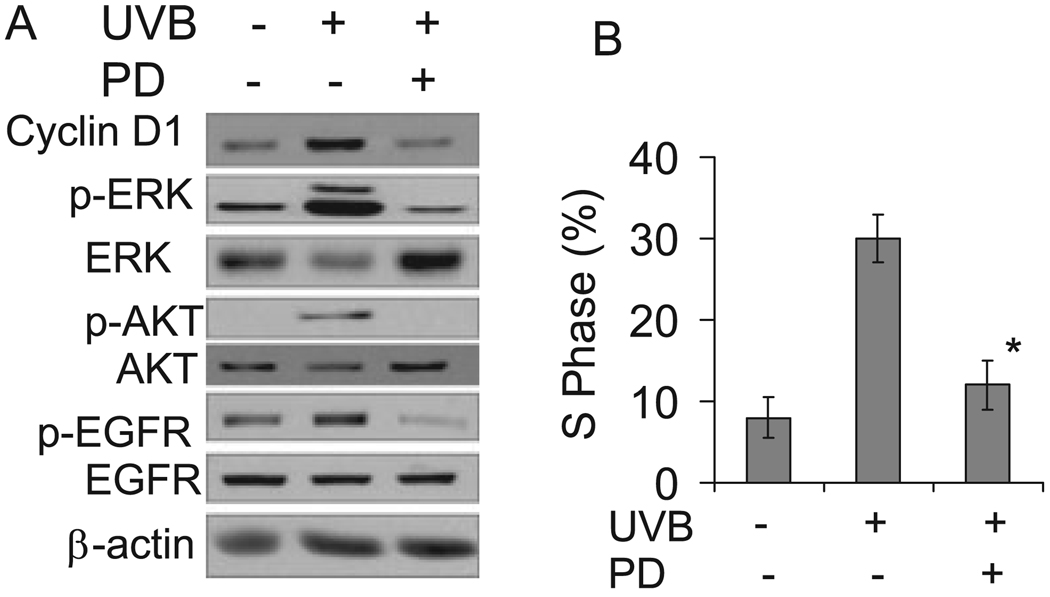
To determine whether ERK activation is involved in the cell cycle progression induced by UVB exposure, cells were incubated with MEK/ERK activation inhibitor PD98059 (PD, 20 µM) to block ERK activation. The presence of PD inhibited UVB-induced ERK phosphorylation and cyclin D1 accumulation (Fig. 3A), indicating that ERK activation mediates cyclin D1 upregulation. Inhibition of ERK also abolished AKT and EGFR activation. Compared with cells incubated with vehicle alone, the proportion of cells in the S phase decreased significantly in cells treated with PD after UVB exposure (Fig. 3B). This suggests that the ERK pathway is an essential element in UVB-induced cyclin D1 upregulation and cell cycle progression. In addition, the ERK pathway is also required for UVB-induced AKT and EGFR activation.
Figure 3.
Inhibition of ERK pathway blocked cyclin D1 accumulation and cell cycle progression following UVB exposure. (A) Cells were incubated with or without PD98059 (PD, 20 µM), an ERK activation inhibitor, and then exposed to UVB radiation (10 mJ cm−2). Three hours after UVB exposure, cells were collected for Western blotting as described in Fig. 2. (B) Cells were treated as in (A) except that cells were harvested for cell cycle analysis at 18 h following UVB radiation. Data are expressed as percentages and represent means (± standard deviation, n = 3); *Significant differences from cells treated with UVB alone (P < 0.05).
Role of AKT activation in UVB-induced cell cycle progression
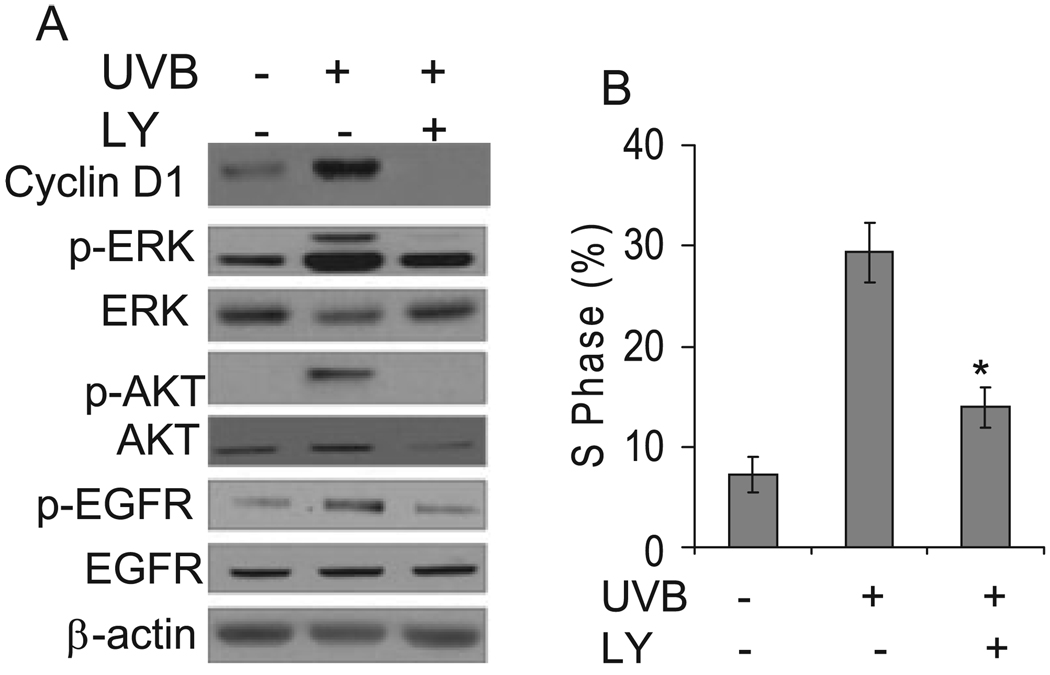
To determine whether AKT activation is involved in the cell cycle progression induced by UVB exposure, cells were incubated with the PI3K inhibitor LY (LY294002, 10 µM) to block AKT activation. The presence of LY inhibited UVB-induced AKT phosphorylation and cyclin D1 accumulation (Fig. 4A), indicating that AKT activation is required for cyclin D1 upregulation. Inhibition of AKT abolished ERK and EGFR phosphorylation. Compared with cells incubated with vehicle alone, the proportion of cells in the S phase decreased significantly in cells treated with LY after UVB exposure (Fig. 4B). This suggests that the AKT pathway is required for UVB-induced cyclin D1 upregulation and cell cycle progression. Taken together, it appears that ERK and AKT pathways cross talk with each other and both are essential for EGFR activation and cell cycle progression following UVB radiation.
Figure 4.
Blocking AKT signaling reduced cyclin D1 upregulation and cell cycle progression induced by UVB irradiation. (A) Cells were incubated with or without PI3K inhibitor (LY) to block AKT activation and then exposed to UVB radiation (10 mJ cm−2). Three hours after UVB exposure, cells were collected for Western blotting analysis as described in Fig. 2. (B) Cells were treated as in (A) except that cells were harvested 18 h after UVB exposure for cell cycle analysis. Percentage of cells in the S phase was quantified as in Fig. 1. Data are expressed as percentages and represent means (± standard deviation, n = 3); *Significant differences from cells treated with UVB alone (P < 0.05).
Role of EGFR activation in UVB-induced cell cycle progression
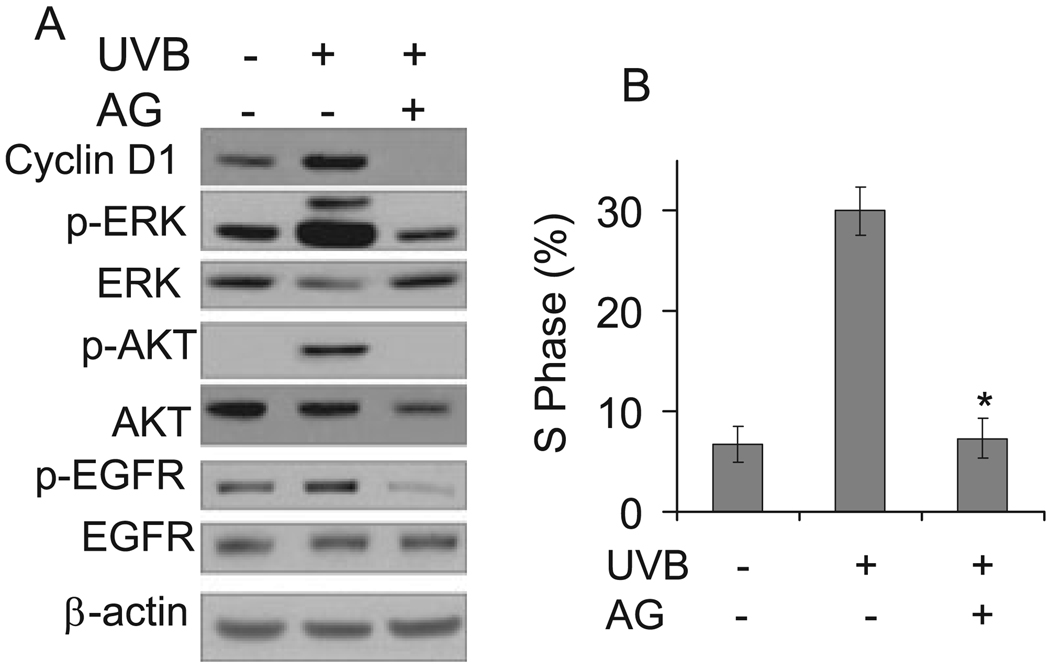
To determine whether EGFR activation is required for UVB-induced ERK and AKT activation, cyclin D1 accumulation and cell cycle progression, cells were incubated with the EGFR kinase inhibitor AG (AG1478, 1 µM). The presence of AG blocked EGFR phosphorylation and UVB-induced ERK and AKT activation (Fig. 5A). Compared with cells incubated with vehicle alone, AG significantly (P < 0.05) reduced the cell proportions in the S phase (Fig. 5B). These findings indicate that EGFR activity is required for ERK and AKT activation, cyclin D1 accumulation and cell cycle progression from the G1 to the S phase induced by UVB radiation.
Figure 5.
Inhibition of EGFR pathway blocked ERK/AKT/cyclin D1 pathways and cell cycle progression induced by UVB exposure. (A) Cells were incubated with or without AG1478 (1 µM), an EGFR kinase inhibitor, and then exposed to UVB radiation (10 mJ cm−2). Three hours after UVB exposure, cells were collected for Western blotting as in Fig. 2. (B) Cells were treated as in (A) and harvested 18 h after UVB exposure for cell cycle analysis. Data are expressed as percentages and represent means (± standard deviation, n = 3); *Significant differences from cells treated with UVB alone (P < 0.05).
Role of MPs in UVB-induced cell cycle progression
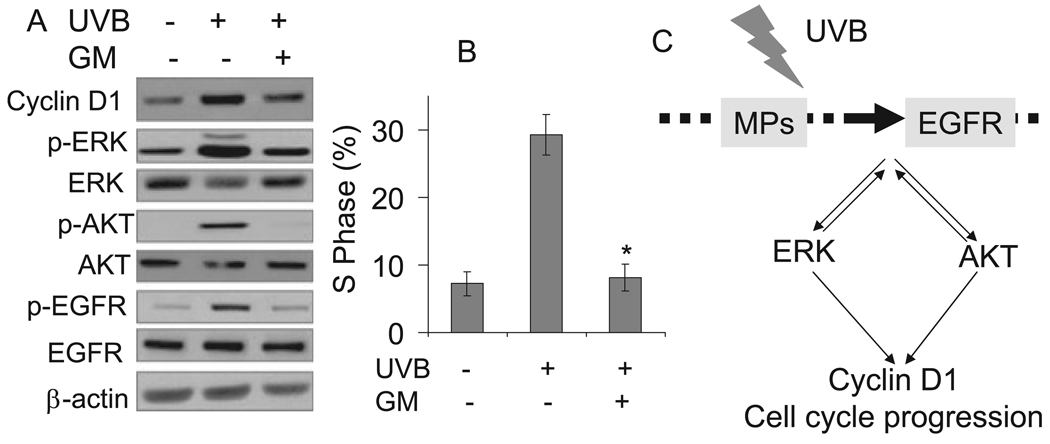
We found that EGFR is required for cell cycle progression by UVB exposure at a lower nonlethal dose. However, the mechanism of EGFR activation is unknown. To determine whether MPs are required for EGFR activation and cell cycle progression induced by low-dose UVB irradiation, we incubated cells with the MP inhibitor GM6001 (GM, 25 µM) and exposed them to UVB or kept them in the dark. Pretreatment with GM reduced EGFR phosphorylation and abolished ERK and AKT activation and cyclin D1 accumulation following UVB exposure (Fig. 6A). Compared with cells incubated without GM, the presence of GM reduced the proportion of cells in the S phase significantly (P < 0.05) after UVB exposure (Fig. 6B). These findings indicate that the MPs are the upstream targets of low-dose UVB radiation mediating the signaling transduction from UVB to EGFR/ERK/AKT activation, cyclin D1 upregulation and cell cycle progression.
Figure 6.
Metalloproteinases (MPs) mediated UVB-induced ERK/AKT/cyclin D1 pathways and cell cycle progression. (A) Cells were incubated with or without metalloproteinase inhibitor GM6001 (25 /µM) and then exposed to UVB irradiation (10 mJ cm−2). Three hours after exposure, cells were collected for Western blotting as in Fig. 2. (B) Cells were treated as in (A) except that cells were harvested 18 h after UVB exposure for cell cycle analysis. Data are expressed as percentages and represent means (± standard deviation, n = 3); *Significant differences from cells exposed to UVB in the absence of GM (P < 0.05). (C) Mechanisms of UVB-induced cell cycle progression in human HaCaT keratinocytes.
DISCUSSION
In the present work, cell cycle progression in response to low-dose UVB irradiation was investigated using the HaCaT human keratinocyte cell line. HaCaT cells are spontaneously immortalized through p53 mutation, a modification similar to that initiated in cells during skin carcinogenesis, thereby providing a valuable model system for the investigation of tumor promotion events associated with skin carcinogenesis (21). Using this model, we have shown that nonlethal doses of UVB irradiation induce cyclin D1 upregulation and cell cycle progression, and that this process is mediated via ERK and AKT activation. In this process the MP/EGFR pathway transduced the UVB signal to ERK and AKT activation causing cyclin D1 upregulation and cell proliferation, which might be equivalent to inducing tumor promotion in skin carcinogenesis.
AKT activation has been shown to be one of the key molecular determinants of keratinocyte transformation (22). AKT has been shown to increase the upregulation of cyclin D1 in AKT-transformed keratinocytes via both transcriptional and posttranscriptional processes (12). In our study, we show that AKT activation is mediated by MP/EGFR pathways following UVB exposure and is required for cyclin D1 accumulation and cell cycle progression. The accumulation of cyclin D1 by UVB exposure could be due to both transcriptional and posttran-scriptional regulation by AKT activation. It is possible that AKT activation as a central signaling pathway mediates the proliferative effect of UVB through several downstream targets. We are currently in the process of identifying those targets to understand the complex mechanisms of UVB-induced cell proliferation and skin carcinogenesis.
The ERK/MAPK pathway is activated by low-dose UVB exposure and required for UVB-induced cyclin D1 accumulation and cell cycle progression. We previously detected sustained and prolonged ERK/MAPK activation after apoptosis-inducing UVB (23). In this study, it appears that ERK signaling is clearly UVB dose dependent and transient, which is expected because the low doses of UVB used in this study did not induce cell death. High-dose UVB-induced sustained ERK activation may protect cells from cell death and allow more cells to survive (23) while transient ERK activation is involved in cell proliferation.
AKT and ERK activation appear to interact reciprocally following low-dose UVB radiation. Inhibition of AKT blocks ERK activation to a lesser extent than the ERK inhibitor itself. Inhibition of ERK activation decreases AKT activation. Both ERK and AKT activation seem to originate from MPs/EGFR and converge into cyclin D1 accumulation and cell proliferation. Inhibition of either ERK or AKT activation is sufficient to prevent EGFR activation and cell proliferation. This interaction between ERK, AKT and EGFR activation appears to be unique for the responses of keratinocytes to low-dose UVB radiation based on the facts that (1) ERK and AKT do not cross talk following UVA radiation using the same concentration of PD or LY (data not shown); and (2) AKT activation by insulin-like growth factor has been shown to phosphorylate Raf and therefore inhibit ERK activation as shown by the increased ERK activation in the presence of the AKT inhibitor LY (15 µM) (24). The mechanisms of the cross talk between these signaling pathways following UVB radiation are under investigation in our laboratory.
Interestingly low-dose UVB radiation is more than one thousand times as potent as low nonlethal UVA radiation, because 2.5 mJ cm−2 of UVB in this study induces a percentage of the G1-S cell cycle progression similar to 4 J cm−2 of UVA radiation (17). The potency difference resembles their physical property difference including radiation energy between UVB and UVA. It is also noteworthy that the molecular mechanisms of UVB-induced cell cycle progression differ from those for UVA. UVB induces transient activation of both ERK and AKT pathways whereas UVA only activates the AKT pathway. Most likely UVB and UVA target distinct molecular mechanisms of epidermal keratinocytes in their mitogenic effects.
The involvement of EGFR activation was first seen in EGFR activation induced by UVB radiation and further confirmed by the effect of EGFR inhibition on ERK and AKT activation, cyclin D1 accumulation and cell cycle progression following UVB irradiation. This is further confirmed by a similar inhibitory effect of the MP inhibitor GM on these UVB responses in which GM inhibits EGFR phosphorylation. This EGFR activation is sufficient to activate the downstream ERK/AKT/cyclin D1 signaling, and transduce a proliferative signal to induce cell cycle progression.
Metalloproteinases seem to play a pivotal role in cell cycle progression following low-dose UVB radiation, because the presence of the MP inhibitor GM reduced EGFR phosphorylation, ERK and AKT activation, cyclin D1 upregulation and cell proliferation. The MPs can be subdivided into MMPs and ADAMs. Although several MMPs are released into the extracellular space, they are still candidates for EGFR ligand cleavage. The transmembrane MMPs may be involved in the EGFR/ERK/AKT/cyclin D1 pathways, although the mechanisms remain an open question.
Recently it has been reported that environmental stress signaling, including oxidative and osmotic stress, is mediated by ADAM proteases and heparin-binding epidermal growth factor (25). The MP signaling appears to be essential for ERK/AKT/cyclin D1 pathways and cell proliferation following low-dose UVB radiation in this study. We would predict that there may be multiple MMPs and/or ADAMs including ADAM17 and ADAM10 (14) responsible for the UVB responses investigated in this study. Identifying these MPs in the near future will advance our understanding of the novel roles of the MPs in skin tumor promotion caused by low-dose UVB exposure. Currently we are working on elucidating the MPs involved in UVB-induced cell cycle progression and its underlying mechanisms.
In summary, nonlethal doses of UVB have been shown to induce cell cycle (G1 to S) progression of human HaCaT keratinocytes. Different from UVA, UVB-induced cell cycle progression requires both MPs/EGFR/ERK and AKT pathways. Given the complexity and interactions of these signaling pathways, it is entirely possible that other pathways may also play a role in UVB-induced cell proliferation. This study provides compelling evidence that UVB alone, at low, nonlethal doses, has the potential to be a human skin tumor promoter. The acquisition of proliferation signaling in UVB-irradiated keratinocytes may be an important factor in the formation of premalignant skin lesions including actinic keratoses and malignant SCC in the clinical setting. Identification of the fundamental mechanisms of the effect of UVB on tumor promotion will facilitate the development of safe and efficient chemopreventive and therapeutic strategies for skin cancer.
Acknowledgements
This research was supported by the Department of Medicine and the Section of Dermatology at the University of Chicago, the American Skin Association and the University of Chicago Cancer Research Center.
REFERENCES
- 1.de Gruijl FR, Sterenborg HJ, Forbes PD, Davies RE, Cole C, Kelfkens G, van Weelden H, Slaper H, van der Leun JC. Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice. Cancer Res. 1993;53:53–60. [PubMed] [Google Scholar]
- 2.Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, De Fabo EC, Merlino G. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- 3.Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc. Natl Acad. Sci. USA. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halliday GM, Lyons JG. Inflammatory doses of UV may not be necessary for skin carcinogenesis. Photochem. Photobiol. 2008;84:272–283. doi: 10.1111/j.1751-1097.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- 5.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 6.Robles AI, Conti CJ. Early overexpression of cyclin D1 protein in mouse skin carcinogenesis. Carcinogenesis. 1995;16:781–786. doi: 10.1093/carcin/16.4.781. [DOI] [PubMed] [Google Scholar]
- 7.Zhang SY, Liu SC, Goodrow T, Morris R, Klein-Szanto AJ. Increased expression of G1 cyclins and cyclin-dependent kinases during tumor progression of chemically induced mouse skin neoplasms. Mol. Carcinog. 1997;18:142–152. [PubMed] [Google Scholar]
- 8.Rodriguez-Puebla ML, LaCava M, Gimenez-Conti IB, Johnson DG, Conti CJ. Deregulated expression of cell-cycle proteins during premalignant progression in SENCAR mouse skin. Oncogene. 1998;17:2251–2258. doi: 10.1038/sj.onc.1202131. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Puebla ML, Robles AI, Conti CJ. ras activity and cyclin D1 expression: An essential mechanism of mouse skin tumor development. Mol. Carcinog. 1999;24:1–6. [PubMed] [Google Scholar]
- 10.Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 11.Stephens LR, Jackson TR, Hawkins PT. Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: A new intracellular signalling system? Biochim. Biophys. Acta. 1993;1179:27–75. doi: 10.1016/0167-4889(93)90072-w. [DOI] [PubMed] [Google Scholar]
- 12.Segrelles C, Moral M, Lara MF, Ruiz S, Santos M, Leis H, Garcia-Escudero R, Martinez-Cruz AB, Martinez-Palacio J, Hernandez P, Ballestin C, Paramio JM. Molecular determinants of Akt-induced keratinocyte transformation. Oncogene. 2006;25:1174–1185. doi: 10.1038/sj.onc.1209155. [DOI] [PubMed] [Google Scholar]
- 13.Mirza AM, Kohn AD, Roth RA, McMahon M. Oncogenic transformation of cells by a conditionally active form of the protein kinase Akt/PKB. Cell Growth Differ. 2000;11:279–292. [PubMed] [Google Scholar]
- 14.Blobel CP. ADAMs: Key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 15.Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umata T, Miyado K, Tamai Y, Kurisaki T, Sehara-Fujisawa A, Ohno S, Mekada E. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 1998;17:7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 17.He YY, Council SE, Feng L, Chignell CF. UVA-induced cell cycle progression is mediated by a disintegrin and metalloprotease/epidermal growth factor receptor/AKT/Cyclin D1 pathways in keratinocytes. Cancer Res. 2008;68:3752–3758. doi: 10.1158/0008-5472.CAN-07-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Abaseri TB, Fuhrman J, Trempus C, Shendrik I, Tennant RW, Hansen LA. Chemoprevention of UV light-induced skin tumorigenesis by inhibition of the epidermal growth factor receptor. Cancer Res. 2005;65:3958–3965. doi: 10.1158/0008-5472.CAN-04-2204. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Voorhees JJ, Fisher GJ. Epidermal growth factor receptor is a critical mediator of ultraviolet B irradiation-induced signal transduction in immortalized human keratinocyte HaCaT cells. Am. J. Pathol. 2006;169:823–830. doi: 10.2353/ajpath.2006.050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas-Ahner JM, Wulff BC, Tober KL, Kusewitt DF, Riggenbach JA, Oberyszyn TM. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res. 2007;67:3468–3474. doi: 10.1158/0008-5472.CAN-06-3798. [DOI] [PubMed] [Google Scholar]
- 21.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segrelles C, Ruiz S, Perez P, Murga C, Santos M, Budunova IV, Martinez J, Larcher F, Slaga TJ, Gutkind JS, Jorcano JL, Paramio JM. Functional roles of Akt signaling in mouse skin tumorigenesis. Oncogene. 2002;21:53–64. doi: 10.1038/sj.onc.1205032. [DOI] [PubMed] [Google Scholar]
- 23.He YY, Huang JL, Chignell CF. Delayed and sustained activation of extracellular signal-regulated kinase in human keratinocytes by UVA: Implications in carcinogenesis. J. Biol. Chem. 2004;279:53867–53874. doi: 10.1074/jbc.M405781200. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 25.Fischer OM, Hart S, Gschwind A, Prenzel N, Ullrich A. Oxidative and osmotic stress signaling in tumor cells is mediated by ADAM proteases and heparin-binding epidermal growth factor. Mol. Cell Biol. 2004;24:5172–5183. doi: 10.1128/MCB.24.12.5172-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]