Abstract
Mammary gland development, functional differentiation, and homeostasis are orchestrated and sustained by a balance of biochemical and biophysical cues from the organ’s microenvironment. The three-dimensional microenvironment of the mammary gland, predominantly ‘encoded’ by a collaboration between the extracellular matrix (ECM), hormones, and growth factors, sends signals from ECM receptors through the cytoskeletal intracellular matrix to nuclear and chromatin structures resulting in gene expression; the ECM in turn is regulated and remodeled by signals from the nucleus. In this chapter, we discuss how coordinated ECM deposition and remodeling is necessary for mammary gland development, how the ECM provides structural and biochemical cues necessary for tissue-specific function, and the role of the cytoskeleton in mediating the extra—to intracellular dialogue occurring between the nucleus and the microenvironment. When operating normally, the cytoskeletal-mediated dynamic and reciprocal integration of tissue architecture and function directs mammary gland development, tissue polarity, and ultimately, tissue-specific gene expression. Cancer occurs when these dynamic inter-actions go awry for an extended time.
Keywords: Acinar morphogenesis, Chromatin organization, Cytoskeleton, Extracellular matrix, Mammary-specific function, Microenvironment, Tissue architecture
1 Introduction
Tissue architecture is critical for cell homeostasis and tissue-specific functions [1]. Tissue microenvironments, which we define as the biochemical and biophysical cues a cell receives from the extracellular matrix (ECM), neighboring cells, the immune system and soluble factors (growth factors, hormones, and cytokines), plays an important role in regulating tissue structure and functions. Whereas the soluble factors have been know for decades to regulate tissue architecture and homeostasis, the insoluble ECM, neglected for much too long as a signaling entity [2], is gaining attention as another important regulator. Here we focus our discussion on the mechanism by which the ECM maintains tissue architecture and functions.
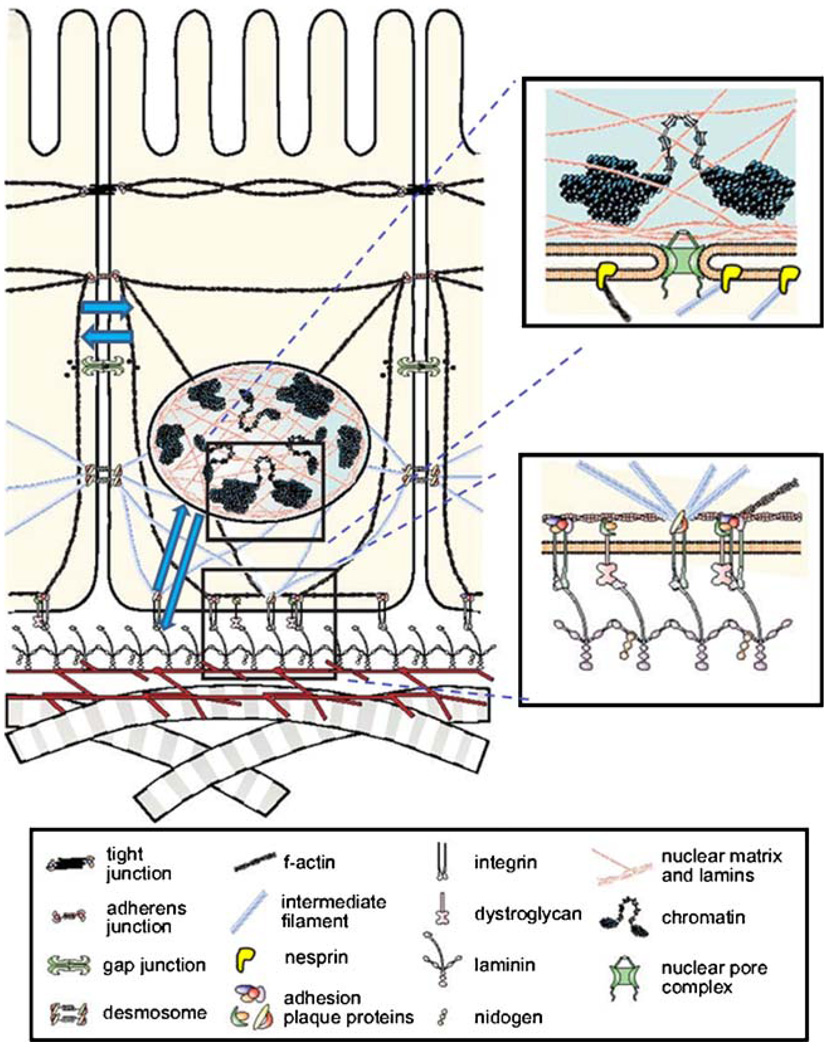
Epithelial cells within the organs are usually surrounded by a basement membrane (BM), a specialized form of ECM comprised largely of laminins, type IV collagen, entactin/nidogen, proteoglycans, and other glycoproteins. Cells interact with ECM molecules by specific receptors, of which integrins are the best understood (reviewed in [3]). Other ECM receptors include dystroglycans, syndecans, CD44 and Rhamm [4–6]. The engagement of ECM receptors induces a cascade of both physical and biochemical signals which transmit from the cell membrane to the nucleus (Fig. 1), accompanied by changes in cell and tissue morphology and architecture. These alterations involve dramatic reorganization of both the cytoskeleton and chromatin structures, leading to changes in cellular and tissue architecture and gene expression which in turn affect the microenvironment. This dynamic and reciprocal dialogue between the cells and their microenvironment acts as a circuitry by which tissue architecture and function become integrated. The wiring of this circuitry includes a major signaling axis that transmits through, and is regulated by, the cytoskeleton (Fig. 1).
Fig. 1.
A scheme showing the principle of dynamic reciprocity between cells and their extracellular microenvironment. Cell-cell and cell-ECM interactions induce cascades of both physical and biochemical signals which transmit from the cell membrane to the nucleus via the cytoskeleton. These signals modulate organization of both the cytoskeleton and chromatin organization, leading to changes in cellular architecture and gene expression which in turn influence the microenvironment. Blue arrows represent the bidirectional transmission of physical and biochemical signals. (modified from [1])
In this chapter, we discuss the following issues pertinent to the ECM-cytoskeleton-nucleus signaling axis: how coordinated ECM deposition and remodeling may be necessary for tissue morphogenesis during development; how the ECM acts as an organizing unit by which tissue architecture, polarity, and specificity are maintained; and lastly, how the cytoskeleton acts as a conduit mediating the dynamic and reciprocal signals between the ECM and the nucleus to maintain correct tissue form and function. While we focus primarily on the mammary gland as a model, the general concepts and conclusions, in principle, should be applicable to many other organs. Understanding how the cytoskeleton integrates tissue architecture with function will help also to more clearly delineate how this balance becomes perturbed and eventually exploited by tumor cells during malignancy.
2 Coordinated ECM remodeling during tissue morphogenesis
The tissue microenvironment undergoes extensive remodeling during morphogenesis, including changes in the deposition, degradation, and structural organization of ECM components. This remodeling of the ECM within a changing microenvironment provides morphogenic cues to control cell survival, proliferation, migration, polarization, and differentiation [1, 7]. Since the normal mammary gland function is regulated by repeated cycles of branching, alveogenesis, lactation, and involution, where the ECM undergoes constant assembly and degradation [8–11], the mammary gland provides an elegant and versatile model by which to investigate how the ECM remodeling contributes to tissue morphogenesis and functional differentiation.
During all stages of mammary gland development, the expression of ECM proteins and remodeling enzymes is tightly regulated both temporally and spatially. In situ hybridization and immunofluorescence experiments demonstrate that expression and deposition of collagen I, collagen IV, and laminin-γ2 chain correlates with mammary epithelial cell proliferation and stromal invasion during puberty and pregnancy [8]. Consistent with its roles in ductal elongation, fibrillar collagen I is predominantly localized along mammary ducts, whereas collagen IV and laminins are concentrated near endbuds and around alveoli, suggesting these ECM components contribute to normal alveologenesis and functional differentiation [8]. Hyaluronan, a non-sulfated glycosaminoglycan, is localized at the tip of terminal end buds and is mainly produced by ‘cap cells’ [12, 13], which may contribute to cell proliferation and migration. Interestingly, the expression of α2β1-integrin, the receptor of collagen I, collagen IV, and laminins, correlates with levels of ligands during mammary gland development [8]. Conditional deletion of β1-integrin from basal cells abolishes the regenerative potential of epithelial cells and impairs ductal branching and lobuloalveolar development at pregnancy stage [14], indicating that integrin-mediated signals from the ECM are necessary for normal tissue morphogenesis. Deposition of fibronectin (FN) increases appreciably during ductal morphogenesis, and parallels the elevated expression of the FN receptor α5β1-integrin in myoepithelial cells at this stage of development [15]. In three-dimensional (3D) cultures, FN expression decreases during acinar morphogenesis as cells polarize and form a lumen. Addition of exogenous FN increases cell proliferation and colony size, and treatment with FN blocking antibody reverse malignant phenotypes in 3D cultures, suggesting that FN coordinates epithelial cell growth during mammary gland development [16, 17]. Taken together, these studies implicate ECM components as regulators of mammary gland morphogenesis.
Remodeling the ECM microenvironment requires the activity of ECM-degrading enzymes such as matrix metalloproteinases (MMPs) [7, 18, 19]. Analogous to the deposition of ECM components, ECM remodeling proteases have distinct temporal and spatial expression patterns during mammary gland development. MMP3 is highly expressed in fibroblasts lining the mammary ducts during ductal elongation, and becomes reactivated upon mammary gland involution [20–22], during which ECM degradation by MMPs coordinates the regression and reorganization of mammary structures to a state resembling that of a virgin gland. The expression pattern of MMP-2 is similar to MMP3 in the virgin gland, but MMP2 activity is reduced at terminal end buds [23]. MMP14 is expressed in both the stromal and epithelial compartments along the ducts, but is highly concentrated at terminal end buds [23]. We have found recently that MMP14 is expressed mainly in the myoepithelial cells and is crucial for branching morphogenesis [24]. MMP-dependent ECM remodeling breaks down a physical barrier which limits epithelial cell proliferation and migration, and genetic manipulation of MMP expression causes aberrant ECM remodeling which impairs epithelial morphogenesis [21, 25]. Targeted expression of constitutively-activated MMP3 in the mammary epithelium enhances branching morphogenesis and leads to precocious alveolar development in mice, accompanied with increased cell proliferation and the loss of basement membrane integrity [21, 25]. The numbers of ductal branches are significantly reduced during mid-puberty in MMP-3 null mice, while targeted deletion of MMP2 delays the invasion of mammary ducts into the fat pads during early puberty [23]. Degradation of the ECM can also generate or release cryptic fragments that act as growth stimulators or inhibitors to dictate tissue morphogenesis. Cleavage of laminin-γ2 chain with MMP14 generates an EGF-like fragment promoting epithelial cell migration and proliferation [26]; correspondingly, MMP14-deficient mice have significantly reduced processing of the γ2 chain and disrupted kidney epithelial morphogenesis [27]. Thus, MMP-dependent dynamic remodeling of ECM microenvironments generates both physical and biochemical cues necessary for tissue morphogenesis. These studies show the tight coordination of ECM deposition and turnover during development, but how ECM regulates tissue architecture and homeostasis remains unanswered.
3 ECM directs tissue polarity and function
Disruption of tissue structure usually parallels the loss of tissue-specific differentiation, suggesting that tissue architecture is intimately linked to function [28, 29]. Mammary epithelial cells that are cultured on conventional, two-dimensional (2D) plastic fail to form acinar-like structures and lose tissue-specific milk protein expression [30]. We showed that culturing mammary epithelial cells in 3D laminin-rich (lr) ECM gels (usually composed of a reconstituted lrECM derived from EHS tumors [31]) can help reconstruct the acinar structure seen in vivo and restore a number of mammary-specific functions; these processes require the orchestrated action of laminin in a gel and lactogenic hormones [32, 33]. Basal epithelial cells are tightly adherent to BM in mammary gland, which not only provides mechanical support and segregates epithelial cells from the stroma, but also directs cell polarity, proliferation, differentiation, and gene expression (for review see [29, 34]). Culturing cells in different 3D models allows delineation of the mechanisms of how the ECM microenvironment regulates tissue architecture and tissue specific function.
Normal epithelial cells in culture and in vivo have a defined apical-basal polarity, which is established by cell-ECM and cell-cell adhesions and which contributes to induction and maintenance of tissue specificity [35]. In the mammary gland, myoepithelial and luminal epithelial cells form polarized and bilayered acini, which secrete milk into the lumen of the acini during lactation. Laminin-111, a major component of BM, is secreted by myoepithelial cells, which is necessary for polarization of luminal epithelial cells [36]. Even in the absence of myoepithelial cells, luminal epithelial cells cultured in 3D lrECM establish apical-basal polarity and express milk proteins in response to lactogenic hormones [33]. The same cells cultured in an improper 3D context, such as in collagen gels lacking laminin, have reversed polarity and lose mammary-specific gene expression. Polarity and tissue-specific functions can be rescued by co-culturing luminal epithelial cells with either myoepithelial cells or with purified laminin-111 [36], confirming that the critical role of the ECM in guiding mammary specific function [37]. Accordingly, deletion of two laminin-111 receptors, β1-integrin or dystroglycan, impairs polarization of mammary epithelial cells and inhibits milk protein expression in 3D cultures [38, 39]. These studies establish the importance of correct cell polarity and physiological context in determining tissue specific function.
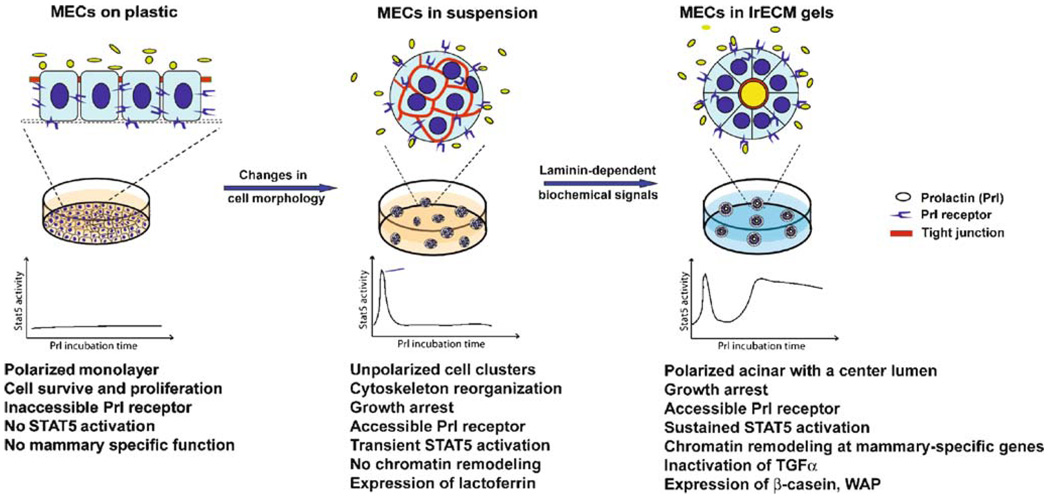
Why is polarity an important integrator of tissue-specificity? By definition, polarized cells have an asymmetrical distribution of proteins within the cell, including transmembrane receptors and other factors mediating tissue differentiation and function. We show that the receptor for the lactogenic hormone, prolactin, localizes to the basolateral surface of mammary epithelial cells [40]. Since prolactin is secreted into the interstitium and blood supply during pregnancy and lactation [41] and the prolactin receptor is available at the basal surface of the ducts and acini in vivo, ligand binding and subsequent signaling events occur rapidly upon prolactin release. However, in 2D culture, the cells do not express milk proteins in response to lactogenic hormone treatment because the basolaterally localized receptor is inaccessible to apically presented prolactin (Fig. 2). The asymmetric distribution of proteins within a polarized cell also sequesters inhibitory ligands from their effectors to regulate tissue-specific gene expression. An example of this is illustrated with whey acidic protein (WAP), a protein released into milk during pregnancy and lactation, and its inhibitor TGFα. Unlike β-casein, WAP is not expressed even in cells treated with lrECM and lactogenic hormones, unless cells are able to form completely polarized acini with functional tight junctions; this complete polarization separates TGFα from its receptor to allow WAP expression in epithelial cells [42, 43]. In lung epithelial cells, the EGF receptors erbB2 and erbB4 are basolaterally localized, but their ligand heregulin is secreted from the apical membrane [44]. This physical segregation limits heregulin-mediated receptor activation to periods where the epithelial integrity is disrupted such as during tissue injury and cancer progression. Cytoplasmic molecules are also asymmetrically localized in polarized epithelial cells, for example, PIP3 and PI3K, a key integrator of signaling events downstream of integrins and receptor tyrosine kinases, localize predominantly to the basal surface of polarized acinar structures in 3D cultures [45]. Perturbing the basal PI3K distribution or blocking its activity significantly inhibits milk protein expression (manuscript in preparation). Together these studies suggest that the asymmetrical localization of important signaling modulators in tissues is crucial for the activation and maintenance of mammary-specific functions.
Fig. 2.
A cartoon showing that the ECM provides architectural and biochemical cues necessary for tissue-specific function. Aggregating mammary epithelial cells in suspension exposes prolactin receptor and allows transient STAT5 activation in response to prolactin stimulation, but this is not sufficient to induce casein and WAP expression. Laminin-dependent biochemical signals, in conjunction with prolactin signaling, induce the sustained STAT5 activation required for casein expression. Additionally, laminin-induced reorganization of cellular architecture is necessary for the formation of polarized acini, which allows for the expression of WAP
As discussed above, mammary epithelial cells can recapitulate some features of apical-basal polarity when cultured on 2D substrata, but the cells cannot functionally differentiate and lose their capacity to express milk proteins in response to lactogenic hormone treatment [40]. We have shown that the coordination of continuous signals from both laminin-111 and prolactin receptor are required for β-casein expression [40, 46, 47]. The discovery of an ECM-regulated transcriptional element in the promoter of a casein gene provides a mechanistic rationale for how laminin-111 may coordinate functional differentiation [48, 49]. Induction of cis-regulated DNA elements requires the activation of trans-acting transcription factors to bind at such sites; as such, the identification of ECM-response elements suggested the existence of ECM-regulated transcription factors. In fact, a number of transcription factors, including STAT5 and C/EBPβ, are modulated by ECM to regulate tissue-specific gene expression [46, 50, 51]. The Bissell laboratory previously demonstrated that establishment of tissue polarity induces the relocalization of several other factors, such as the nuclear protein NuMA, the RNA splicing factor SRm160, NFκB, TIN2, and the cell cycle regulator Rb in mammary epithelial cell line HMT-3522 S1 [52–54]. Treatment of cells with a NuMA antibody leads to the disruption of NuMA foci, which in turn reverses the lrECM-induced chromatin reorganization and breaks down the endogenous BM [52]. Therefore, the integrity of ECM to nucleus dynamic reciprocity is necessary to establish normal tissue architecture and function. We hypothesized long ago that this dynamic reciprocity is coordinated along the signaling axis that is channeled through the cytoskeleton [2].
4 Cytoskeleton is a signaling axis between microenvironment and genome which sustains dynamic reciprocity
Eukaryotic cell contains three types of cytoskeletal components: microfilaments, intermediate filaments, and microtubules. Cytoskeletal proteins form macromolecular complexes at sites of cell-ECM adhesions with adaptors and signaling modulators [55], and engagement of integrins by the ECM induces reorganization of both actin and intermediate filaments (Fig. 1). Lamins, which are structural proteins comprising the nuclear envelope, are connected with cytoskeletal actin filaments through nesprin (Fig. 1), which serves to anchor the nucleus to the cytoskeleton in order to regulate nuclear localization, movement, and possibly other functions [56, 57]. As such, the cytoskeleton acts as a conduit connecting the ECM to the nucleus, allowing dynamic and reciprocal interactions between the extracellular environment and the nucleus to coordinate gene expression and tissue homeostasis.
Substantial evidence supporting the model of dynamic reciprocity includes elegant work done by Maniotis in the laboratory of Don Ingber, where it was shown that inducing mechanic strain on integrins immediately changes the shape and organization of nuclei, a mechanotransduction process which is dependent on intermediate filaments and microfilaments [58]. Culturing mammary epithelial cells in 3D lrECM reorganizes actin to have a predominantly cortical distribution similar to mammary acini in vivo, whereas actin frequently forms stress fibers when cells are cultured on plastic [59] (our unpublished data). Disruption of stress fibers with cytochalasin D (CytoD), an inhibitor of actin polymerization, leads to global histone deacetylation and cell rounding in 2D cultures, suggesting that the actin cytoskeleton mediates the ECM-induced changes to cellular architecture and chromatin organization, and correspondingly, tissue-specific gene expression [59].
ECM-dependent cytoskeletal reorganization contributes to transduction of biochemical signals which establish and maintain tissue architecture and function. In mammary cells, laminin-111 induces transmission of mechanical and biochemical signals first through dystroglycan which leads to formation of an organized BM and cell shape changes, and subsequently through β1-integrins to further alter cytoskeletal structure [37, 38, 60, 61]. These events are essential for activation of STAT5, a regulator of mammary-specific function [40]. Canonical STAT5 signal transduction is initiated by binding of prolactin to the receptor, which activates transient JAK2-mediated phosphorylation and nuclear translocation of STAT5. However, in mammary epithelial cells, the transient STAT5 activation fails to induce and maintain milk protein expression. We have shown recently that laminin-dependent biochemical signals induce a sustained activation of STAT5, and that blocking the sustained activation inhibits chromatin remodeling and milk protein expression (Fig. 2) [40]. Chromatin immunoprecipitation and photobleaching techniques have demonstrated that the association of transcription factors with cis-regulatory elements is very transient, inducing dynamic and cyclic chromatin remodeling [62, 63]. Thus, the ECM-dependent sustained activation of transcription factors is critical in order to allow the dynamic chromatin remodeling necessary for tissue homeostasis. It has been shown that integrity of the cytoskeleton is necessary for transcription factor activation and tissue-specific gene expression in mammary epithelial cells at defined stages in signal transduction pathways, as disruption of cortical actin filaments with CytoD inhibits the laminin-induced STAT5 activation and milk protein expression in 3D lrECM cultures [64]. Therefore, the cytoskeleton may provide a conduit for the transmission of transcription factor from ECM to nucleus, which in turn regulates tissue-specific gene expression.
The ECM-induced cytoskeletal rearrangement appears to occur through the Rho family of small GTPases, a diverse family of signaling molecules which are known to regulate cell migration, polarization and differentiation by influencing actin dynamics and organization [45, 65–67]. Binding of laminin-111 to β1-integrin induces Rac1 activation in mammary epithelial cells, which correlates with acinar morphogenesis and activation of mammary-specific function. Overexpression of dominant-negative Rac1 disturbs acinar morphogenesis and lumen formation, and inhibits the laminin-induced STAT5 activation and milk protein expression in 3D lrECM cultures [64, 65]. Cytoskeletal-regulated signals also regulate expression of chemokines and MMPs, which in turn influence the tissue microenvironment [68]. In rabbit synovial fibroblasts, perturbing the adhesion of α5β1-integrin with a functional blocking antibody disrupts actin microfilaments and activates Rac1, which leads to increased expression of interleukin (IL)-1α and collagenase-1[68]. These studies provide additional support to the hypothesis that the ECM and nucleus regulate each other dynamically and reciprocally, that this balance occurs through the cytoskeleton, and that it is necessary for normal tissue architecture and function.
In addition to the biochemical signals originating from ECM receptors, the biophysical and mechanical properties of the tissue microenvironment are necessary for cell differentiation, function, and maintenance of architecture [69]. In vivo, tissues have intimate contact with their tissue-specific ECM, which in turn can greatly vary in ‘stiffness’ from one organ to another; for example, the ECM to which mammary epithelial cells adhere is much softer than the bone matrix where osteocytes reside [70]. Manipulating the ECM stiffness in culture dramatically changes gene expression profiles and rapidly reprograms cell differentiation. In sparse cultures of mesenchymal stem cells, matrices which mimic the stiffness of brain, muscle, and bone shift cell differentiation towards neuronal, myoblast, and osteoblast fates, respectively [69]. In mammary epithelial cells, endogenous milk protein expression is induced to an appreciable level when cells are cultured in floating collagen gels versus attached collagen gels [71], and we have recently demonstrated that the elasticity of floating collagen gels mimics the stiffness of the mammary gland in vivo, while attached collagen gels are greater than 3 fold stiffer [72]. Stiffness regulates acinar morphogenesis, as culturing mammary epithelial cells in ECM gels of comparable stiffness to the mammary gland leads to polarized acini with a central lumen, while increasing the gel rigidity enhances cell spreading and proliferation, inhibits cell polarization, and impedes tubulogenesis and lumen formation independent of altered matrix concentration [70, 73]. These studies elegantly demonstrate that tissue-specific architecture and function are regulated by the biophysical properties of the ECM, in particular matrix stiffness, independently and in addition to biochemical properties of the microenvironment.
Epithelial cell mechanics are largely determined by the biophysical and biochemical composition of the ECM substrata [74]. Mammary epithelial cells cultured on soft polyacrylamide gels acquire an elastic modulus similar to mammary tissue in vivo, and correspondingly express β-casein. However, increasing the matrix rigidity causes cell stiffening and spreading, which is accompanied by a dramatic reduction in β-casein expression, increased actin stress fibers, and increased phosphorylation of myosin II light chain [72]; this is in agreement with previous studies demonstrating that ECM-integrin signals are directly linked to the actinomyosin cytoskeleton [70]. Perturbing either actin polymerization or myosin II ATPase activity significantly blocks mechanotransduction from the substrata to cytoplasm and reduces cellular elasticity, indicating that actinomyosin contraction is a major contributor to this transmission [72]. An increase in cytoskeletal tension and substrata stiffness coordinates the activation of Rho/ROCK signaling, and treatment with a ROCK inhibitor reduces cellular elasticity and promotes acinar morphogenesis [70, 72]. Cell mechanics, in turn, also have a profound influence on the ECM microenvironment through regulating gene expression and ECM assembly: cytoskeletal tension can be transmitted through integrins to influence both fibronectin assembly [75, 76] and MMPs transcription [77], indicating that the cytoskeletal tension can actively regulate remodeling of the ECM microenvironment. This reciprocal mechanotransduction between the ECM and the cytoskeleton coordinates gene expression and tissue architecture, and serves as another plausible means by which the ECM regulates tissue structure and function dynamically and reciprocally. This balance is necessary for normal tissue homeostasis and tissue-specific function.
5 Conclusions
Individual tissues and organs within an organism vary dramatically in their cellular composition and overall architecture, and clearly they have different functional specificity. Even the most common types of cells from two discrete tissues display unique pattern of gene expression leading to distinct biological behavior despite identical genomic information. For instance, fibroblasts from two different locations (cutaneous versus visceral tissues) have distinct and characteristic transcriptional patterns [78]. Two recent studies from the laboratory of Gill Smith and his colleagues show that testicular cells and neuronal cells can differentiate into functional mammary epithelial cells after being transplanted into the mammary gland [79, 80]. These experiments strongly support the concept that the tissue microenvironment directs organ development and tissue specificity [81].
In this chapter, we have presented several lines of evidence illustrating the importance of the ECM in establishing the cellular architecture necessary for tissue-specificity. Biochemical and biophysical signals from the ECM polarize cells to induce tissue-specific differentiation by establishing an asymmetrical distribution of receptors and signal transduction components within cells, by activating transcription factors acting at ECM-response elements, and by remodeling the nuclear and chromatin structure globally and at specific loci. These signals depend on a cytoskeleton-regulated bidirectional signaling axis to bridge the ECM and nuclear compartments, as disruption of the cytoskeletal integrity prevents these ECM-induced events, and correspondingly, disrupts functional gene expression. In return, cells are able to influence the physical and biochemical properties of their microenvironment by regulating expression, assembly, and remodeling of their ECM. This dynamic and reciprocal interaction between the ECM and cells depends on the cytoskeleton to maintain the architecture necessary for tissue-specific function.
The deposition of ECM components and expression of ECM remodeling enzymes is subject to tight spatiotemporal regulation, reflecting the importance of finely-tuned ECM microenvironment in establishing the architecture necessary for proper function. ECM remodeling enzymes such as MMPs are able to modulate the tissue architecture within the context of normal organ development and biology, we and others have shown that forced expression of MMPs disrupts the normal tissue microenvironment leading to tumorigenesis in vivo [82–85], suggesting that altering the fine balance of ECM and cell homeostasis by disrupting tissue architecture is sufficient in the long run not only to disrupt function, but also to induce tumorigenesis. Clearly the cytoskeleton is a critical player regulating cell and ECM homeostasis, but much more needs to be learned about the exact role and mechanism by which this complex and dynamic intracellular ‘matrix’ helps regulate tissue-specificity.
Acknowledgements
We apologize to those whose work could not be cited due to space limitations. This work was supported by the Office of Biological and Environmental Research of the Department of Energy (DOE-AC03-76SF00098), the National Institutes of Health (CA112970-01), (R01CA057621) to Zena Werb and M.J.B., (R01CA064786) to M.J.B. and the Breast Cancer Research Program (BCRP) of the Department of Defense (DOD) (Innovator Award) to M.J.B. M.JB. is a Distinguished Scientist of the OBER Office of the DOE. Support was also provided by a DOD BCRP postdoctoral fellowship DAMD17-02-1-0441 to R.X., a predoctoral fellowship W81XWH-05-1-0339 to A.T.B, and by a California BCRP Dissertation Award to ATB.
Abbreviations
- 2D
two-dimensional
- 3D
three-dimensional
- BM
basement membrane
- C/EBP, CAAT/
enhancer-binding protein
- DG
dystroglycan
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- FN
fibronectin
- JAK
Janus kinase
- lrECM
laminin-rich ECM
- MMP
matrix metalloproteinases
- PI3K
Phosphoinositide-3 kinase
- polyHEMA
poly(2-hydroxyethyl methacrylate)
- STAT5
signal transducers and activators of transcription protein 5
- TGF-α
transforming growth factor-α
- WAP
whey acidic protein
Contributor Information
Ren Xu, Email: RXu@lbl.gov.
Mina J. Bissell, Email: MJBissell@lbl.gov.
References
- 1.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annual review of cell and developmental biology. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? Journal of theoretical biology. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 3.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell CA, McCarthy J, Turley E. Cell-surface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions? Journal of Cell Science. 2008;121:925–932. doi: 10.1242/jcs.022038. [DOI] [PubMed] [Google Scholar]
- 5.Sanderson RD, Yang Y, Suva LJ, Kelly T. Heparan sulfate proteoglycans and heparanase–partners in osteolytic tumor growth and metastasis. Matrix biol. 2004;23:341–352. doi: 10.1016/j.matbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Current opinion in cell biology. 2004;16:572–579. doi: 10.1016/j.ceb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast cancer research. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keely PJ, Wu JE, Santoro SA. The spatial and temporal expression of the alpha 2 beta 1 integrin and its ligands, collagen I, collagen IV, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation. 1995;59:1–13. doi: 10.1046/j.1432-0436.1995.5910001.x. [DOI] [PubMed] [Google Scholar]
- 9.Talhouk RS, Chin JR, Unemori EN, Werb Z, Bissell MJ. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991;112:439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schedin P, Mitrenga T, McDaniel S, Kaeck M. Mammary ECM composition and function are altered by reproductive state. Molecular carcinogenesis. 2004;41:207–220. doi: 10.1002/mc.20058. [DOI] [PubMed] [Google Scholar]
- 11.Wicha MS, Liotta LA, Vonderhaar BK, Kidwell WR. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Developments in biologicals. 1980;80:253–256. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]
- 12.Silberstein GB, Daniel CW. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Developmental biology. 1982;90:215–222. doi: 10.1016/0012-1606(82)90228-7. [DOI] [PubMed] [Google Scholar]
- 13.Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: epithelial-mesenchymal transition–does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol. 2008;5:280–290. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, et al. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nature cell biology. 2008;10:716–722. doi: 10.1038/ncb1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodward TL, Mienaltowski AS, Modi RR, Bennett JM, Haslam SZ. Fibronectin and the alpha(5)beta(1) integrin are under developmental and ovarian steroid regulation in the normal mouse mammary gland. Endocrinology. 2001;142:3214–3222. doi: 10.1210/endo.142.7.8273. [DOI] [PubMed] [Google Scholar]
- 16.Williams CM, Engler AJ, Slone RD, Galante LL, Schwarzbauer JE. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer research. 2008;68:3185–3192. doi: 10.1158/0008-5472.CAN-07-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandal T, Valyi-Nagy K, Spencer VA, Folberg R, Bissell MJ, Maniotis AJ. Epigenetic reversion of breast carcinoma phenotype is accompanied by changes in DNA sequestration as measured by AluI restriction enzyme. American journal of pathology. 2007;170:1739–1749. doi: 10.2353/ajpath.2007.060922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werb Z, Chin JR. Extracellular matrix remodeling during morphogenesis. Ann N Y Acad Sci. 1998;857:110–118. doi: 10.1111/j.1749-6632.1998.tb10111.x. [DOI] [PubMed] [Google Scholar]
- 19.Lochter A, Sternlicht MD, Werb Z, Bissell MJ. The significance of matrix metalloproteinases during early stages of tumor progression. Ann N Y Acad Sci. 1998;857:180–193. doi: 10.1111/j.1749-6632.1998.tb10116.x. [DOI] [PubMed] [Google Scholar]
- 20.Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. Journal of cell biology. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Molecular biology of the cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen O, et al. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. American journal of pathology. 1998;153:457–467. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, et al. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. Journal of cell biology. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori H, Nelson CM, Alcaraz J, Chen CS, Lo AT, Fata JE, et al. The catalytic and non-catalytic domains of MMP14 and stromal collagen density regulate signaling loops that direct invasion and branching of mammary epithelial cells. (Submitted) [Google Scholar]
- 25.Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, et al. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. Journal of cell biology. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koshikawa N, Minegishi T, Sharabi A, Quaranta V, Seiki M. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is a processing enzyme for human laminin gamma 2 chain. Journal of biological chemistry. 2005;280:88–93. doi: 10.1074/jbc.M411824200. [DOI] [PubMed] [Google Scholar]
- 27.Koshikawa N, Schenk S, Moeckel G, Sharabi A, Miyazaki K, Gardner H, et al. Proteolytic processing of laminin-5 by MT1-MMP in tissues and its effects on epithelial cell morphology. FASEB journal. 2004;18:364–366. doi: 10.1096/fj.03-0584fje. [DOI] [PubMed] [Google Scholar]
- 28.Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Current opinion in cell biology. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagios C, Lochter A, Bissell MJ. Tissue architecture: the ultimate regulator of epithelial function? Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1998;353:857–870. doi: 10.1098/rstb.1998.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- 31.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 32.Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bissell MJ, Kenny PA, Radisky DC. Micro-environmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harbor Symposia on Quantitative Biology. 2005;70:343–356. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bissell MJ, Bilder D. Polarity determination in breast tissue: desmosomal adhesion, myoepithelial cells, and laminin 1. Breast cancer research. 2003;5:117–119. doi: 10.1186/bcr579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. Journal of Cell Science. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. Journal of cell biology. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weir ML, Oppizzi ML, Henry MD, Onishi A, Campbell KP, Bissell MJ, et al. Dystroglycan loss disrupts polarity and beta-casein induction in mammary epithelial cells by perturbing laminin anchoring. Journal of Cell Science. 2006;119:4047–4058. doi: 10.1242/jcs.03103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. Journal of cell biology. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu R, Nelson CM, Muschler J, Veiseh M, Vonderhaar BK, Bissell MJ. Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. Journal of cell biology. 2008 doi: 10.1083/jcb.200807021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocrine reviews. 1996;17:639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 42.Lin CQ, Dempsey PJ, Coffey RJ, Bissell MJ. Extracellular matrix regulates whey acidic protein gene expression by suppression of TGF-alpha in mouse mammary epithelial cells: studies in culture and in transgenic mice. Journal of cell biology. 1995;129:1115–1126. doi: 10.1083/jcb.129.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen LH, Bissell MJ. A novel regulatory mechanism for whey acidic protein gene expression. Cell regulation. 1989;1:45–54. doi: 10.1091/mbc.1.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, et al. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. Journal of cell biology. 2004;164:603–612. doi: 10.1083/jcb.200306090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu R, Spencer VA, Bissell MJ. Extracellular matrix-regulated gene expression requires cooperation of SWI/SNF and transcription factors. Journal of biological chemistry. 2007;282:14992–14999. doi: 10.1074/jbc.M610316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, et al. Laminin mediates tissue-specific gene expression in mammary epithelia. Journal of cell biology. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin—and extracellular matrix-dependent regulation of beta-casein gene expression. Molecular biology of the cell. 1992;3:699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, Casperson G, et al. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Molecular and cellular biology. 1998;18:2184–2195. doi: 10.1128/mcb.18.4.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clayton DF, Harrelson AL, Darnell JE., Jr Dependence of liver-specific transcription on tissue organization. Molecular and cellular biology. 1985;5:2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Streuli CH, Edwards GM, Delcommenne M, Whitelaw CB, Burdon TG, Schindler C, et al. Stat5 as a target for regulation by extracellular matrix. Journal of biological chemistry. 1995;270:21639–21644. doi: 10.1074/jbc.270.37.21639. [DOI] [PubMed] [Google Scholar]
- 52.Lelievre SA, Weaver VM, Nickerson JA, Larabell CA, Bhaumik A, Petersen OW, et al. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14711–14716. doi: 10.1073/pnas.95.25.14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaminker P, Plachot C, Kim SH, Chung P, Crippen D, Petersen OW, et al. Higher-order nuclear organization in growth arrest of human mammary epithelial cells: a novel role for telomere-associated protein TIN2. Journal of Cell Science. 2005;118:1321–1330. doi: 10.1242/jcs.01709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berrier AL, Yamada KM. Cell-matrix adhesion. Journal of cellular physiology. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, et al. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. Journal of Cell Science. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- 57.Hetzer MW, Walther TC, Mattaj IW. Pushing the envelope: structure, function, and dynamics of the nuclear periphery. Annual review of cell and developmental biology. 2005;21:347–380. doi: 10.1146/annurev.cellbio.21.090704.151152. [DOI] [PubMed] [Google Scholar]
- 58.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Beyec J, Xu R, Lee SY, Nelson CM, Rizki A, Alcaraz J, et al. Cell shape regulates global histone acetylation in human mammary epithelial cells. Experimental cell research. 2007;313:3066–3075. doi: 10.1016/j.yexcr.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muschler J, Lochter A, Roskelley CD, Yurchenco P, Bissell MJ. Division of labor among the alpha6beta4 integrin, beta1 integrins, and an E3 laminin receptor to signal morphogenesis and beta-casein expression in mammary epithelial cells. Molecular biology of the cell. 1999;10:2817–2828. doi: 10.1091/mbc.10.9.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McNally JG, Muller WG, Walker D, Wolford R, Hager GL. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science. 2000;287:1262–1265. doi: 10.1126/science.287.5456.1262. [DOI] [PubMed] [Google Scholar]
- 63.Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, et al. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 64.Zoubiane GS, Valentijn A, Lowe ET, Akhtar N, Bagley S, Gilmore AP, et al. A role for the cytoskeleton in prolactin-dependent mammary epithelial cell differentiation. Journal of Cell Science. 2004;117:271–280. doi: 10.1242/jcs.00855. [DOI] [PubMed] [Google Scholar]
- 65.Akhtar N, Streuli CH. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. Journal of Cell Biology. 2006;173:781–793. doi: 10.1083/jcb.200601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jou TS, Nelson WJ. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. Journal of Cell Biology. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annual review of cell and developmental biology. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 68.Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- 69.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 70.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 71.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. Journal of Cell Biology. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alcaraz J, Xu R, Mori H, Nelson CM, Mroue R, Spencer VA. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO journal. 2008;27:2829–2838. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. Journal of Cell Biology. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alcaraz J, Nelson CM, Bissell MJ. Biomechanical approaches for studying integration of tissue structure and function in mammary epithelia. Journal of mammary gland biology and neoplasia. 2004;9:361–374. doi: 10.1007/s10911-004-1406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feral CC, Zijlstra A, Tkachenko E, Prager G, Gardel ML, Slepak M, et al. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. Journal of Cell Biology. 2007;178:701–711. doi: 10.1083/jcb.200705090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lambert CA, Colige AC, Munaut C, Lapiere CM, Nusgens BV. Distinct pathways in the over-expression of matrix metalloproteinases in human fibroblasts by relaxation of mechanical tension. Matrix Biol. 2001;20:397–408. doi: 10.1016/s0945-053x(01)00156-1. [DOI] [PubMed] [Google Scholar]
- 78.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3871–3876. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14891–14896. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bissell MJ, Inman J. Reprogramming stem cells is a microenvironmental task. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15637–15638. doi: 10.1073/pnas.0808457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ha HY, Moon HB, Nam MS, Lee JW, Ryoo ZY, Lee TH, et al. Overexpression of membrane-type matrix me0talloproteinase-1 gene induces mammary gland abnormalities and adenocarcinoma in transgenic mice. Cancer research. 2001;61:984–990. [PubMed] [Google Scholar]
- 84.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCawley LJ, Wright J, LaFleur BJ, Crawford HC, Matrisian LM. Keratinocyte expression of MMP3 enhances differentiation and prevents tumor establishment. American journal of pathology. 2008;173:1528–1539. doi: 10.2353/ajpath.2008.080132. [DOI] [PMC free article] [PubMed] [Google Scholar]