Abstract
Single neuron activity was recorded in the frontal eye field (FEF) of monkeys trained to perform a difficult luminance discrimination task. The appearance of a cue stimulus informed the monkeys of the locations of two gray luminance stimuli that would appear within 500–1500 ms. The monkeys were rewarded for making a saccade to the brighter of the two luminance stimuli, or if they were the same luminance, for making a saccade to the cue stimulus. Sixty percent (51/85) of FEF neurons exhibited elevated activity when the cue informed the monkeys that one of the luminance stimuli would appear in their response field (RF). This spatially selective anticipatory activity occurred without any visual stimulus appearing in their RF and was not related to saccade choice or latency. The responses of 27 of the anticipatory neurons (32% of the total sample) were also incompatible with the hypothesis that the activity represents saccade probability because they did not exhibit elevated activity for the cue stimulus which was the most probable saccade target. Behaviorally, monkeys exhibited improved perception at locations informed by cue than at unpredictable locations. These results provide physiological evidence that FEF serves an important role in endogenous spatial attention in addition to its well known role in saccade production.
Keywords: vision, saccade, attention, luminance, monkey, physiology
Introduction
People are better at perceiving a peripheral stimulus when they attend to its location before it appears (Carrasco, Ling & Read, 2004, Posner, 1980). In the past two decades, the effects of covert visual attention on visual processing has been studied widely (Ciaramitaro, Cameron & Glimcher, 2001, Corbetta & Shulman, 2002, Desimone & Duncan, 1995, Fries, Reynolds, Rorie & Desimone, 2001, Kastner & Ungerleider, 2000, Mitchell, Stoner & Reynolds, 2004). In general, attention enhances neuronal responses (Luck, Chelazzi, Hillyard & Desimone, 1997, McAdams & Maunsell, 1999, Reynolds, Pasternak & Desimone, 2000), thus improving stimulus discrimination and detection (Carrasco, Penpeci-Talgar & Eckstein, 2000).
Psychological studies suggest that there are two separate processes that guide the allocation of attention: reflexive (exogenous) and voluntary (endogenous) (Corbetta & Shulman, 2002, Kincade, Abrams, Astafiev, Shulman & Corbetta, 2005, Rosen, Rao, Caffarra, Scaglioni, Bobholz, Woodley, Hammeke, Cunningham, Prieto & Binder, 1999). Reflexive or exogenous attention refers to a bottom-up, or stimulus-driven, process in which external visual stimuli automatically capture attention. Voluntary or endogenous attention refers to a top-down, or cognitively-driven, process in which attention is willfully allocated to a specific location.
Functional imaging studies have revealed a fronto-parietal neural network, which includes the frontal eye field (FEF), that is involved in both endogenous and exogenous covert attention (Beauchamp, Petit, Ellmore, Ingeholm & Haxby, 2001, Corbetta & Shulman, 2002, Hopfinger, Buonocore & Mangun, 2000, Kastner, Pinsk, De Weerd, Desimone & Ungerleider, 1999, Kincade et al., 2005, Pessoa, Kastner & Ungerleider, 2003, Rosen et al., 1999, Serences & Yantis, 2006). Results from microstimulation and inactivation studies in monkeys and humans are consistent with the hypothesis that FEF is a source of top-down attentional modulation (Awh, Armstrong & Moore, 2006, Grosbras & Paus, 2002, Juan, Shorter-Jacobi & Schall, 2004, Moore & Armstrong, 2003, Moore, Armstrong & Fallah, 2003, Moore & Fallah, 2001, Schafer & Moore, 2007, Wardak, Ibos, Duhamel & Olivier, 2006), but these studies cannot identify or characterize the neurons involved in this modulation. Most single unit studies of monkey FEF have focused on its role in saccade production (Schall & Thompson, 1999). A few single unit recordings in FEF (Kodaka, Mikami & Kubota, 1997, Thompson, Biscoe & Sato, 2005) have revealed activity corresponding to exogenous attention following the presentation of visual stimuli. But in these studies, the attention-related activity could be attributed to modulation of stimulus-driven processes. There has been no single unit study in the frontal eye field describing attention-related activity in the absence of visual stimulation. Therefore, the neural mechanisms controlling the allocation of cognitively-directed endogenous spatial attention are still largely unknown.
We recorded single neuron activity in FEF while monkeys performed a task designed to isolate activity derived exclusively from cognitive processes without an exogenous, or stimulus derived influence. Monkeys performed a difficult luminance discrimination task in which the locations of two visual stimuli to be discriminated were known but the direction of the rewarded saccade response was unknown. The monkeys learned to associate the location of a cue to the locations at which two behaviorally important luminance stimuli would appear. The cue stimulus was far enough away from the luminance stimuli so that both would not appear in a neurons’ response field (RF) on the same trial. Thus, any spatially selective activity emerging in the absence of visual stimulation would have to be attributed to endogenous, cognitive processes.
Our data showed that a group of FEF neurons exhibited cognitively-driven spatially selective activity. This increased activation occurred without any visual stimulus appearing in the neurons’ RF and was not related to the direction or latencies of saccades following the presentation of the luminance stimuli. We hypothesize that FEF is a source of top-down spatial attention that influences ongoing visual processing. Some of the findings presented here have appeared in abstract form (Zhou & Thompson, 2004).
Materials and Methods
Data collection
Two male monkeys (Macaca mulatta), weighing 8 and 11 kg , were prepared for electrophysiological recordings. All surgical and experimental protocols were approved by the NEI Animal Care and Use Committee and complied with the NIH Guide for the Care and Use of Laboratory Animals. Sterile surgery was performed under ketamine and isofluorane anaesthesia to place a head-holding device, a plastic recording chamber over the right frontal eye field, and scleral search coils. Frontal eye field was localized within the recording chamber using low current microstimulation (< 50 µA) to evoke saccades, and by the presence of saccade-related movement neurons (Bruce & Goldberg, 1985). Recording sites were confirmed to be in the rostral bank of the arcuate sulcus by MRI.
Visual stimulation and behavioral control was done by a computer running REX. Visual stimuli were presented on a computer monitor (Hitachi, 26 cm X 21 cm, 1024 X 768 pixel resolution, 60 Hz frame rate) viewed at a distance of 57 cm. Action potential waveforms were recorded with tungsten microelectrodes, digitized, and saved using a computer-based data acquisition system (Plexon Inc.). Offline spike sorting (Plexon Offline Sorter) separated single units based on the size and shape of the spike waveforms. Analog eye position signals were digitized and sampled at 1 kHz.
Behavioral training and tasks
Monkeys were seated in a primate chair with their heads fixed. Using operant conditioning with liquid reward as positive reinforcement, the monkeys were trained to perform a memory-guided saccade task and a luminance discrimination task. The two tasks were run in separate blocks of trials. The peripheral target stimulus used in the memory-guided saccade task was exactly the same as the dim stimulus used in the luminance discrimination task. Four peripheral stimulus locations were used in both tasks: up, down, left and right at 10° eccentricity.
The memory-guided saccade task was used to distinguish visual activity from movement activity for cell classification, and to aid in mapping the spatial extent of each neuron’s response field (Bruce & Goldberg, 1985) . After the monkeys fixated a 0.2°×0.2° white spot on a gray background (4.1 cd/m²) for 300 ms, a 1.6°×1.6 ° gray square target (10.1 cd/m²) was flashed for 33 ms at 1 of 4 peripheral locations (the same locations used in the luminance discrimination task). The monkeys were required to maintain fixation on the central spot for a random interval ranging from 500 to 1500 ms. After the fixation spot disappeared, the monkeys were rewarded for making a saccade to the target within 500 ms. Once gaze shifted, the target reappeared to provide feedback and a fixation target for the monkeys.
In the luminance discrimination task, after the monkeys fixated a central blue spot on a gray background (4.1cd/m²), a bright 1°×1° green square appeared at 1 of 4 possible peripheral locations. This green square remained on throughout the rest of the trial. The monkeys were required to maintain fixation for a randomized period lasting between 500 and 1500ms. After this delay, two gray squares (1.6°×1.6°) were flashed for 33ms at the two isoeccentric stimulus locations positioned at right angles 90° clockwise (CW) and 90° counterclockwise (CCW) to the green stimulus (Fig. 1a). The luminance of the two gray squares were either the same dim luminance or one was brighter. ‘Same’ luminance and ‘different’ luminance trials were randomly interleaved. On ‘same’ trials the monkeys were rewarded for shifting gaze to the green stimulus. On ‘different’ trials the monkeys were rewarded for shifting gaze to the location of the brighter of the two flashed luminance stimuli. The locations of the two luminance stimuli were predictable based on the location of the green stimulus. The brighter luminance stimulus on ‘different’ trials occurred at either location with equal probability. The luminance of dim stimulus was 10.1 cd/m². The luminances of the four bright stimuli used on ‘different’ trials were 11.1, 12.1, 14.2, 18.2 cd/m²; which were about 10%, 20%, 40%, 80% brighter than the dim stimulus. Each luminance difference increment was presented in blocks of 20 trials in a pseudorandom sequence. Within each block, about 40% of the trials were ‘same’ trials in which both luminance stimuli were dim (10.1 cd/m²). In some later experimental sessions, larger blocks with about 100 trials were used. The results from these sessions did not differ from the sessions with 20 trials per block.
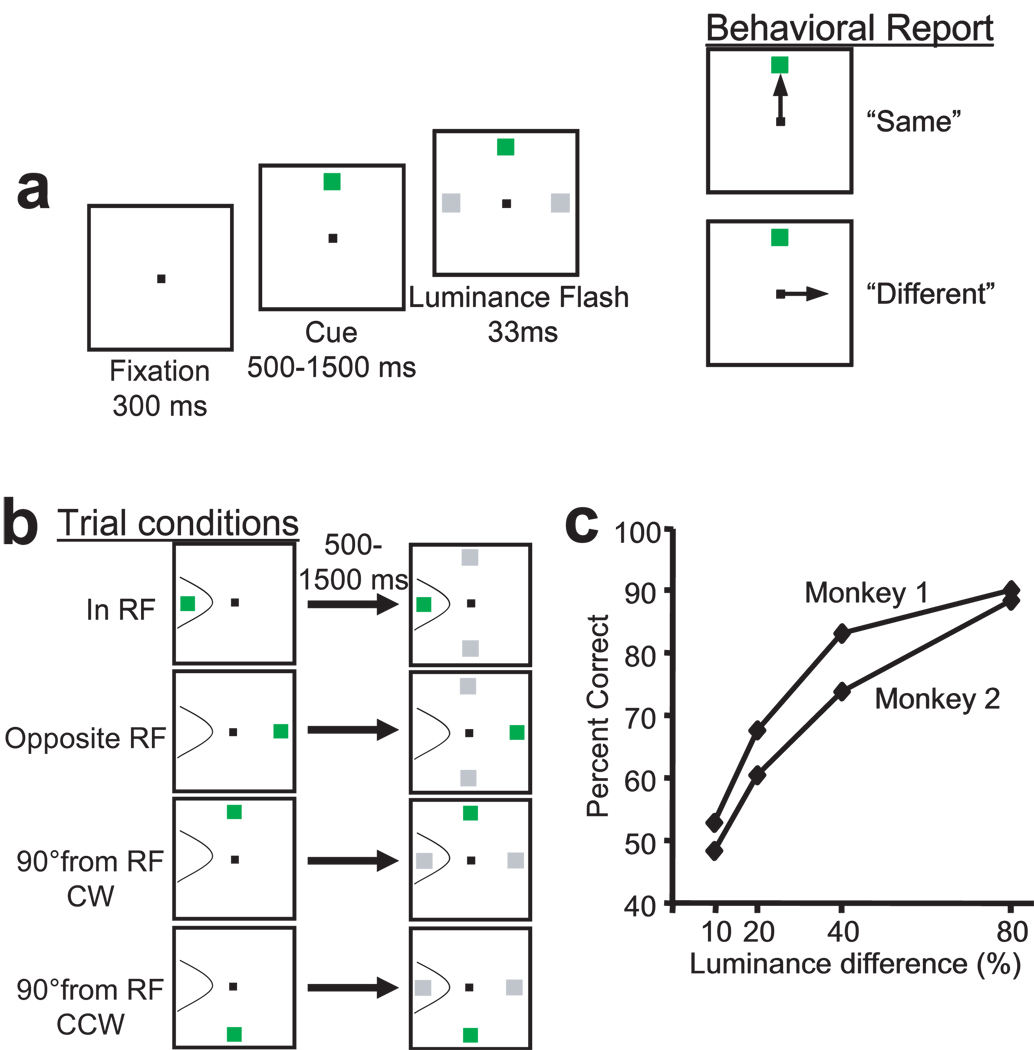
Figure 1.
The luminance discrimination task and behavioral performance. (a) Monkeys performed a luminance discrimination task. After the monkey fixated a central spot for 300 ms, a green cue stimulus appeared at one of four stimulus locations (left, right, up, or down at 10° eccentricity). After maintaining fixation for a random delay lasting between 500–1500 ms, two gray squares flashed for 33 ms at the two isoeccentric stimulus locations positioned at right angles 90° clockwise (CW) and 90° counterclockwise (CCW) to the green cue stimulus. Monkeys were rewarded for correctly reporting whether the two gray square were the same or different. A report of ‘same’ was a saccade to the green cue stimulus. A report of ‘different’ was a saccade to the location of the brighter of the two luminance stimuli. (b) The locations of two luminance stimuli were predicable. When the green cue stimulus appeared at the left or right locations, the luminance stimuli always appeared at the up and down locations. When the green cue stimulus appeared at the up or down location, the luminance stimuli always appeared at the left and right locations. A hypothetical neuron’s receptive field (RF) is outlined with the thin line. Four different trial conditions are labeled according to the position of the green cue stimulus in relation to the neuron’s RF: ‘In RF’; ‘Opposite RF’; ‘90° from RF-CW’; and ‘90° from RF-CCW’. (c) Behavioral performance of the two monkeys in typical recording sessions. Four luminance differences (10%, 20%, 40%, and 80%) were presented in blocks of 20 trials mixed with about 40% ‘same’ trials. Luminance difference is defined as the percent increase in luminance (cd/m2) of the brighter stimulus over the dimmer stimulus (see Methods). Percent correct was calculated from all trials (‘same’ and ‘different’) within blocks of the same luminance difference
Data analysis
All data were analyzed using custom-written programs in Matlab (The Mathworks, Inc.). Saccades were detected using an algorithm that searched for elevated eye velocity (> 20 deg/sec). Saccade initiations and terminations were then defined as the beginnings and ends of the monotonic changes in eye position that lasted at least 10 ms. Measurements of neural activity were derived from spike density functions generated by convolving the time of action potentials with a function that projects activity forward in time and approximates an EPSP (Thompson, Hanes, Bichot & Schall, 1996). The non-parametric Wilcoxon rank-sum test was used to test for significant differences in the magnitude of the spike density function across conditions averaged over the specific time intervals described below.
In this report we included only those cells that showed a response at only one of the four peripheral stimulus locations tested in the memory-guided saccade task. Neurons were classified as exhibiting one or more of the following responses when the target was flashed in its response field: 1) Visual activity - if there was a significant increase of activity between 50–150 ms following visual stimulus presentation compared to baseline (the average activation during the last 100 ms preceding the target flash), 2) Delay activity - if there was significantly elevated activity in the last 200 ms preceding the removal of the fixation spot relative to baseline, and 3) Movement activity – if there was a significant increase in activity from 50 ms before to 50 ms after saccade initiation as compared to the last 100 ms of the delay period.
A visuomovement index was calculated for each neuron that quantifies the relative contributions of visual and movement activity to a neuron’s response in the memory-guided saccade task. The visuomovement index was calculated as the contrast ratio between the visual response and the movement response in the memory-guided saccade task [(movement-visual)/(movement+visual)]. For this calculation, the visual response was obtained by subtracting baseline activity (average activity during the 100 ms preceding the target flash) from the average activity between 50 to 150 ms following the target flash, and the movement response was obtained by subtracting the late delay period activity (average activity during the 100 ms preceding the go cue) from the average activity between 50 ms before to 50 ms after saccade initiation. Negative visual or movement responses were rounded to zero.
The average firing rates on trials during the last 200ms of the delay period in both the memory-guided saccade task and the luminance discrimination task were used to compare delay period activation across trial conditions to characterize the anticipatory activity. The analysis was done in the following sequence. 1) Delay period activity in the memory-guided saccade task on trials in which the target appeared at the locations 90° CW and 90° CCW from the RF was compared to the activity on trials in which the target appeared at the location opposite the RF (opposite RF). The neurons showing no significant difference in activity were used in the subsequent analysis (see Fig. 3a). 2) Delay period activity in the luminance discrimination task from both 90° CW and 90° CCW trials were compared to activity on ‘opposite RF’ trials to determine which neurons exhibited significant anticipatory activity preceding the appearance of the luminance stimuli in the RF (Fig. 3b). Those that did not were classified as type III neurons. 3) The neurons that exhibited significant anticipatory activity were examined further (Fig. 3c). Those neurons that exhibited significantly greater delay period activity on ‘90° from RF’ trials than on trials in which the green stimulus was in the RF were classified as type I (Fig. 2b). The remaining neurons with anticipatory activity were classified as type II (Fig. 4b).
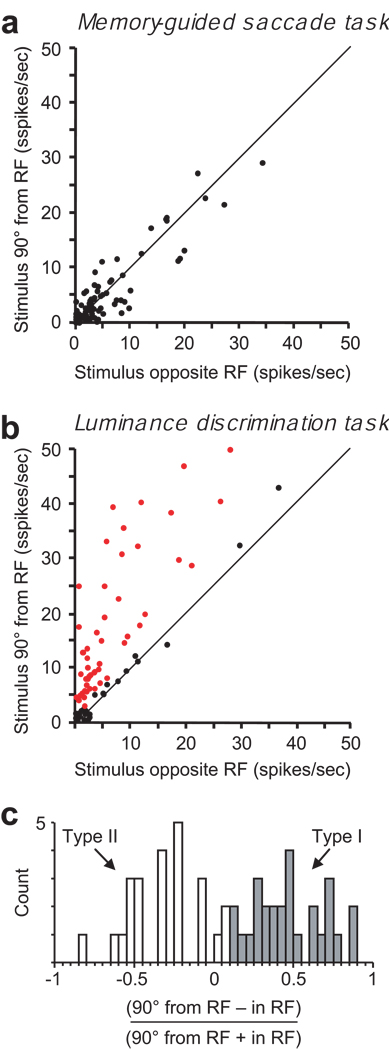
Figure 3.
Population analysis of anticipatory activity. (a) A comparison of activity in the memory-guided saccade task in the last 200 ms of the delay period on trials in which the target is flashed at different locations outside the receptive field – ‘Opposite the RF’ and ‘90° from RF’. The diagonal represents equal activation. None of the 85 neurons exhibited significantly different activity during the delay period for the two conditions (p > 0.05). (b) Corresponding comparison of delay period activity in the luminance discrimination task in which the green cue appears at different locations outside the receptive field. The red dots represent neurons in which the activity during last 200 ms of the delay period was significantly greater (p < 0.05) on ‘90° from RF’ trials than on ‘opposite the RF’ trials. Sixty percent (51/85) of the neurons exhibited significant anticipatory activity preceding the appearance of a luminance stimulus in their RF. (c) Differentiation of type I and type II neurons. The histogram shows the distribution of the contrast ratios of the 51 neurons identified in (b) comparing the anticipatory activity (90° from RF trials) to the activity when the green cue stimulus was in the RF (in RF trials) during the last 200 ms of the delay period. Type I neurons (gray bars) were defined as those in which the anticipatory activity preceding the appearance of the luminance stimuli was significantly greater than the activity related to the visible green cue stimulus (see Fig. 2). The remaining neurons with anticipatory activity (white bars) were defined as type II (see. Fig. 4).
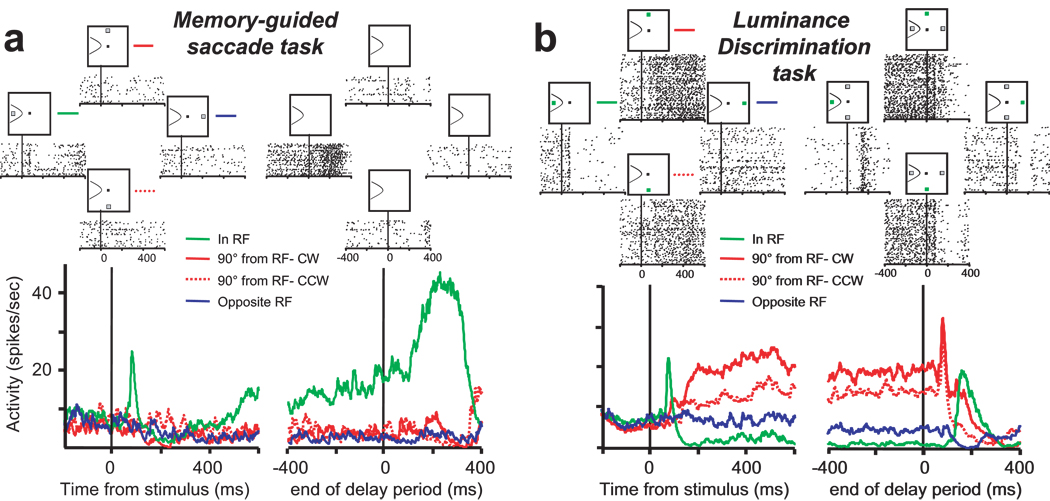
Figure 2.
A neuron that exhibits cognitively-driven spatially selective activity - Type I. (a) Activity of a visuomovement neuron recorded during the memory-guided saccade task aligned on the time of target presentation (left) and the time of the ‘go’ cue, which marks the end of the delay period (right). The upper rasters show the spike times on single trials and the lower spike density functions plot the average firing rate separately for the 4 stimulus locations. The green lines plot the activity when the target was presented in the response field (In RF). The red lines plot the activity when the target was presented at the stimulus location 90° CW (solid red) and 90° CCW (dotted red) from the RF. The blue line plots the activity when the target was presented at the stimulus location opposite the RF (Opposite RF). (b) Activity of the same neuron recorded during the luminance discrimination task aligned on the time of the presentation of the green cue (left) and the time of the appearance of the luminance stimuli, which marks the end of the delay period (right). The upper rasters and lower spike density functions correspond to the activity on trials in which the green cue stimulus appeared at the same stimulus locations as the flashed target in the memory-guided saccade task. Note the similar initial visual response in the two tasks when the visual stimulus is presented in the RF (green activity traces). This neuron exhibited clear anticipatory activity during the delay period on trials in which the green stimulus informed the monkey that a luminance stimulus will appear in its RF (solid and dotted red activity traces). In contrast, there was no activity modulation on trials in which the green stimulus was presented opposite its RF and informed the monkey that both luminance stimuli will appear outside its RF (blue activity trace).
Figure 4.
Another neuron that exhibits cognitively-driven spatially selective activity – Type II. Activity of a neuron recorded in (a) the memory-guided saccade task and (b) the luminance discrimination task. Conventions are the same as in Figure 2. Type II neurons are distinguished from type I neurons (see Fig. 2) by the presence of maintained activity during the delay period on trials in which the green stimulus remains present in the RF (green activity trace).
Results
In the luminance discrimination task, each trial began with the presentation of a peripheral green cue stimulus at one of four positions (up, down, left, right, at 10° eccentricity) (see Fig. 1a and Methods for details). The location of the green cue stimulus informed the monkey that two gray stimuli would be flashed for 33 ms at the two isoeccentric stimulus locations positioned at right angles, 90° clockwise (CW) and 90° counterclockwise (CCW), to the green cue stimulus (Fig. 1b). The monkeys’ task was to judge whether the two gray stimuli had the same or different luminance. On trials with a luminance difference, the monkeys were rewarded for making a saccade to the location of the brighter of the two gray stimuli. On trials in which the two gray stimuli had the same luminance, the monkeys were rewarded for making a saccade to the green cue stimulus which remained on throughout the trial. About 40% of trials were ‘same’ trials in which the green cue stimulus was the correct target.
For both monkeys, task performance improved as the luminance difference increased from about 50% correct in the 10% luminance difference condition to more than 85% correct in the 80% luminance difference condition (Fig. 1c; see also Fig. 9). The monkeys had three choices in this task; they performed above chance (33% correct) even in the most difficult luminance difference condition.
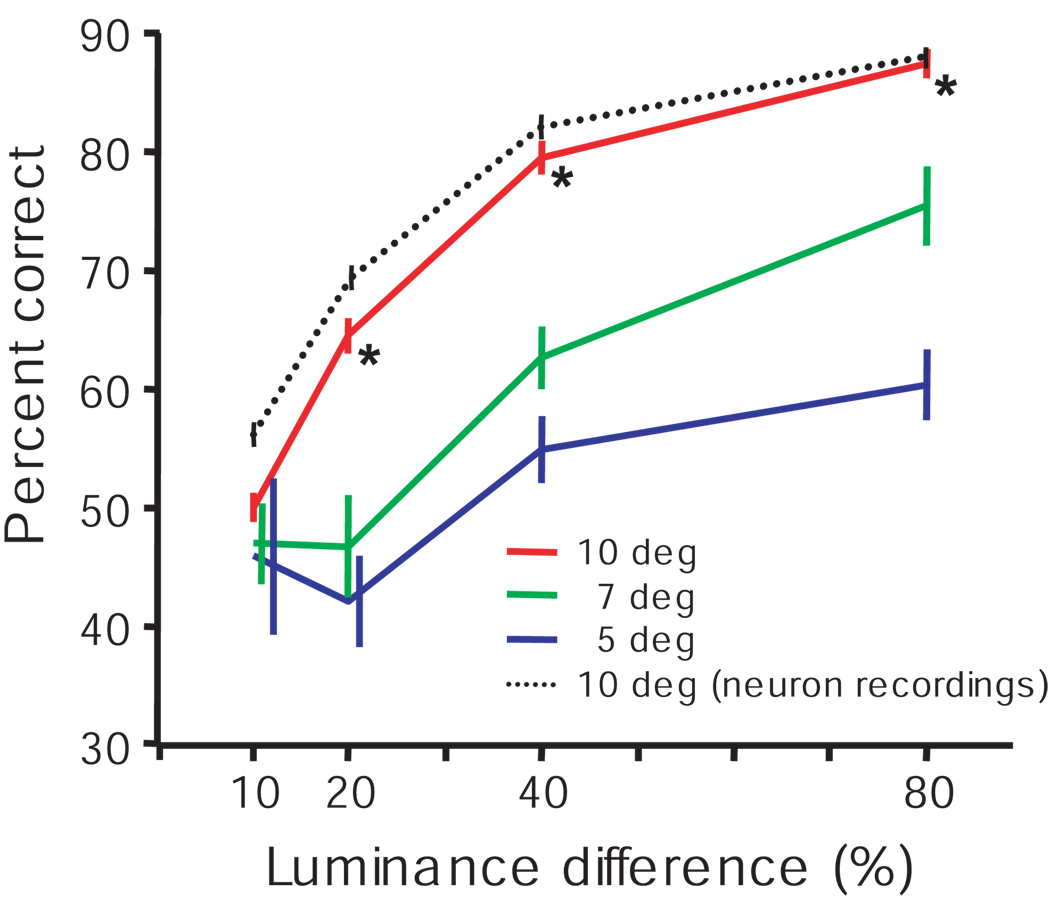
Figure 9.
Performance was better when luminance stimuli appeared at expected locations. Behavioral data from 12 daily sessions, 6 from each monkey, were averaged. In these sessions, the green cue stimulus always appeared at 10° eccentricity. The luminance stimuli appeared at either 7° or 5° eccentricities unexpectedly on 10% of trials. The averaged percent corrects (±SEM across sessions) as function of luminance differences are plotted for stimuli presented at three different eccentricities: Red – the expected 10° eccentricity; Green and Blue – unexpected 7° and 5° eccentricities, respectively. For comparison, the average behavioral performance across all neuron recording sessions for both monkeys is also shown (black dotted line). Some of the error bars are shifted to the right to clearly present the data. Asterisks (*) indicate that the performance at 10° eccentricity in the behavior only sessions was significantly better than the performance at both 7° and 5° eccentricities (p < 0.05).
Neuron activity
We recorded the activity of 268 FEF neurons in the two monkeys; 205 of these neurons exhibited clear visual or saccade related activity that was spatially tuned in the memory-guided saccade task. To isolate spatially selective activity in the absence of a visual stimulus in the luminance discrimination task, it was essential to compare the delay period activity before the appearance of the luminance stimuli on trials when a stimulus did not appear in the RF to the activity on trials when a luminance stimulus appeared in the RF. If a neuron had a RF that encompassed more than one of the four stimulus locations, one of the luminance stimuli appeared in the RF on every trial, which made the essential comparison impossible. Therefore, it was necessary to limit the pool of neurons to those that responded to a stimulus presented at only one of the four stimulus locations. A total of 85 neurons in our sample fit this criterion. In a memory-guided saccade task, these neurons showed increased visual activity, delay period activity, saccade-related activity, or any combination of the three at one of the four stimulus locations; but not at the other three locations. To determine whether cognitively-driven spatially selective activity is present in FEF, we examined the activity occurring during the delay period of the luminance discrimination task following the appearance of the green cue stimulus and ending at the onset of the luminance stimuli. The results from the two monkeys were the same so they are combined.
Figure 2 shows the activity of an FEF neuron that exhibited cognitively-driven spatially selective activation preceding the presentation of the luminance stimuli. In the memory-guided saccade task (Fig. 2a) this neuron exhibited a brief phasic visual response, some late delay period activity, and eye movement related activation when the target stimulus was flashed in its response field (RF), but did not exhibit activity above baseline at the other three locations. In the luminance discrimination task (Fig. 2b), when the green cue stimulus appeared in its RF, this neuron showed a similar phasic visual response as was observed in the memory-guided saccade task. However, instead of an increase in activity during the delay period, this neuron exhibited a decrease in activation. Importantly, when the green cue appeared outside the neuron’s RF and its position informed the monkey that a luminance stimulus would appear inside the RF (Fig. 2b, red traces) there was no initial phasic visual response, but its activity began growing after about 100 ms and continued throughout the delay period. In contrast, on trials in which the green cue appeared outside the neuron’s RF and its position informed the monkey that the luminance stimuli would appear outside the RF (Fig. 2b, blue trace), the neuron maintained a baseline level of activation throughout the delay period. In the last two conditions (90° from RF and Opposite RF) there was no visual stimulus in the neuron’s RF. Therefore, the increased activity that occurred later in the delay period on ‘90° from RF’ trials is due to the cognitive influence of anticipating the appearance of a behaviorally relevant luminance stimulus in the RF.
To determine the frequency with which FEF neurons exhibit cognitively-driven anticipatory activity, we measured firing rates recorded during the last 200 ms of the delay periods in the memory-guided saccade and luminance discrimination tasks. In the memory-guided saccade task, all 85 neurons exhibited equivalent activity (Wilcoxon rank sum test, p > 0.05) during the delay period on trials when the visual stimulus was presented at the stimulus locations 90° from the RF and opposite the RF (Fig. 3a). For this analysis we combined the activity on 90° CW and 90° CCW trials. In the luminance discrimination task, 60% (51/85) of the neurons exhibited significantly higher activity when the green cue stimulus was presented 90° from the RF than when the green cue stimulus was presented opposite the RF (Fig. 3b). For these neurons the pooled average firing rate was 177% higher during the last 200 ms of the delay period on trials before a luminance stimulus appeared in the RF (‘90° from RF’ trials = 17.26 spikes/s) than on trials when no stimulus appeared in the RF (‘Opposite RF’ trials = 6.22 spikes/s). None of the neurons showed significantly higher activity on trials in which the cue stimulus was presented opposite of the RF than on trials when cue stimulus appeared 90° from the RF.
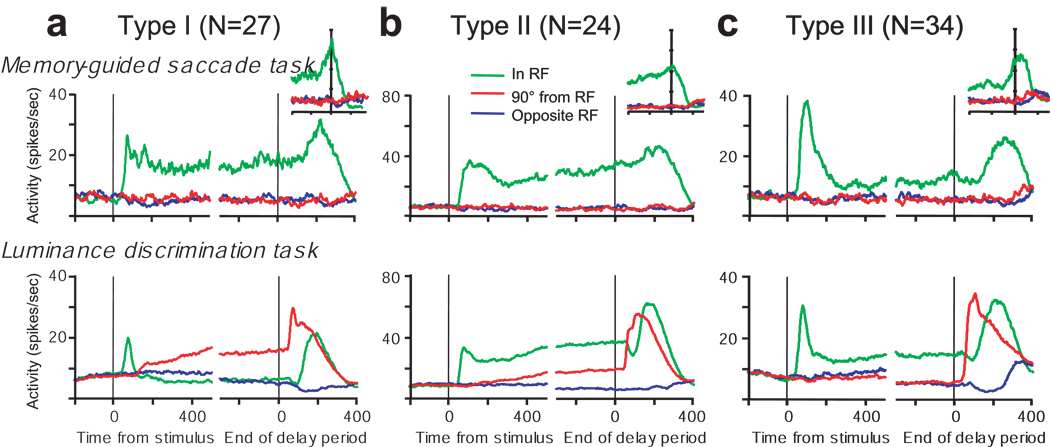
Next, the 51 neurons that exhibited spatially specific anticipatory activity were subdivided into two subtypes (Fig. 3c). We designated them type I and type II. The neuron in Figure 2 is an example of a type I neuron and the neuron in Figure 4 is an example of a type II neuron. Both type I and type II neurons exhibited significant anticipatory activity preceding the appearance of a task-relevant visual stimulus in the RF. The difference between type I and type II neurons is that type II neurons also exhibited sustained delay period activation when the green cue stimulus was present in the RF (compare the green traces in Fig. 2b and Fig. 4b). Type I (n = 27) neurons were distinguished from type II (n = 24) as those neurons that exhibited significantly higher activity (Wilcoxon rank sum test, P < 0.05) when the green stimulus was outside of their RF (90° CW or CCW from RF) than when the green stimulus was inside their RF. The remaining subset of neurons (n = 34), designated as type III, did not exhibit significant anticipatory activation before the luminance stimuli appeared in their RFs and are represented by the black dots in Figure 3b.
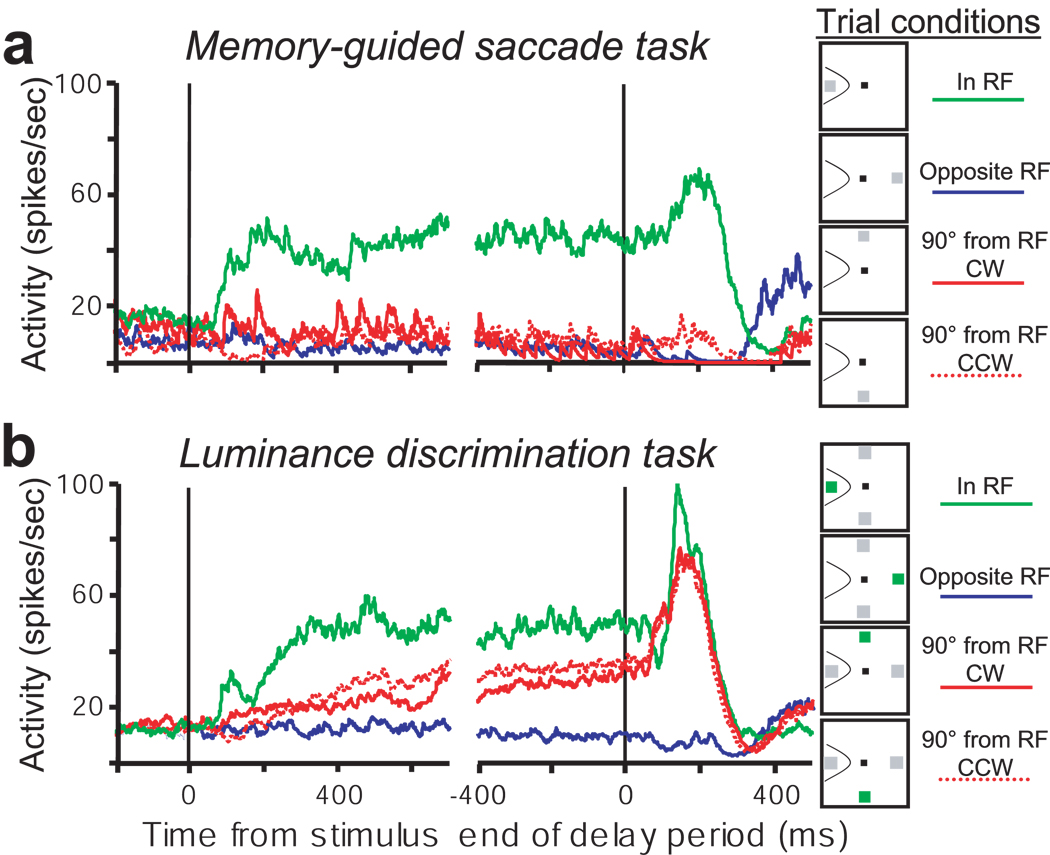
The different activity patterns of type I, II, and III neurons during the luminance discrimination task are evident in the pooled activity shown along the bottom row of plots in Figure 5. To summarize, type I (Fig. 5a) and type II (Fig. 5b) neurons exhibit anticipatory activity during the delay period in the absence of visual input. Type II neurons, but not type I neurons, also exhibit sustained activity when the green cue stimulus is present in the RF. Type III neurons (Fig. 5c) do not exhibit anticipatory activity in the luminance discrimination task. The overall pattern of activity of type III neurons in the luminance discrimination task is similar to their activity in the memory guided saccade task; they are active during the delay period only on trials in which a visual stimulus was presented in the RF.
Figure 5.
Pooled average activity of FEF (a) type I neurons, (b) type II neurons, (c) type III neurons in the memory-guided saccade task (top row) and the luminance discrimination task (bottom row). Conventions are the same as in Figure 2. The insets plot the pooled average activity in the memory guided saccade task aligned on the time of saccade initiation.
FEF neuron classification: Task comparison
FEF neurons are usually classified based on activity recorded during memory-guided saccade tasks (Bruce & Goldberg, 1985, Lawrence, White & Snyder, 2005). We differentiated four different patterns of activity in the memory-guided saccade task. 1) Phasic visual neurons exhibited a brief visual response after the stimulus was flashed in their RF and were silent for the remainder of the trial. 2) Visual-delay neurons exhibited a visual response followed by elevated activity during the delay period, and no increase in activity around the time of the saccade. 3) Visuomovement neurons exhibited a visual response and an increase in activity before the monkey made a saccade into the RF. Most visuomovement neurons also exhibited delay activity (see Fig. 2a and Fig. 4a). 4) Movement neurons exhibited no visual response following the stimulus flash and exhibited increased activity immediately before the saccade into the RF.
Table 1 compares our neuron classifications based on activity recorded in the luminance detection task to the classifications based on the activity recorded in the memory-guided saccade task. Three main points are evident in Table 1. First, none of the phasic visual neurons (0/14 – 0%) exhibited anticipatory activity. Evidently, a neuron must be able to carry information that is not directly related to the physical presence of a visual stimulus (e.g., memory- or saccade-related activity) to exhibit anticipatory activation preceding a predictable visual stimulus. Second, most of the neurons that exhibited delay period or movement activity in the memory-guided saccade task also exhibited anticipatory activity in the luminance discrimination task (overall: 51/71 = 72%; visual-delay neurons: 8/12 = 67%; visuomovement neurons: 36/44 = 82%; movement neurons: 7/15 = 47%). Third, most of the type I neurons (20/27 = 74%) and all of the type II neurons (24/24 = 100%) were visually responsive.
Table 1.
A comparison of neuron classification based on the memory-guided saccade (columns) and the luminance discrimination task (rows).
| Visual: phasic | Visual: with delay | Visuo-movement | Movement | Total | |
|---|---|---|---|---|---|
| Type I | 0 | 2 | 18 | 7 | 27 |
| Type II | 0 | 6 | 18 | 0 | 24 |
| Type III | 14 | 4 | 8 | 8 | 34 |
| Total | 14 | 12 | 44 | 15 | 85 |
The responses of FEF neurons in memory-guided saccade tasks lie on a visual-movement continuum (Lawrence et al., 2005). At one end of the continuum are the visual neurons and at the other end are the movement neurons. Visuomovement neurons connect the two extremes exhibiting varying degrees of both visual and saccade related activity. To quantify the visual-movement continuum, a visuomovement index was calculated for each neuron as the contrast ratio between the visual response and the movement response in the memory-guided saccade task [(movement-visual)/(movement+visual)] (see Methods for details). For this calculation, the baseline activity of each neuron was subtracted from its visual response and late delay period activity was subtracted from its movement response.
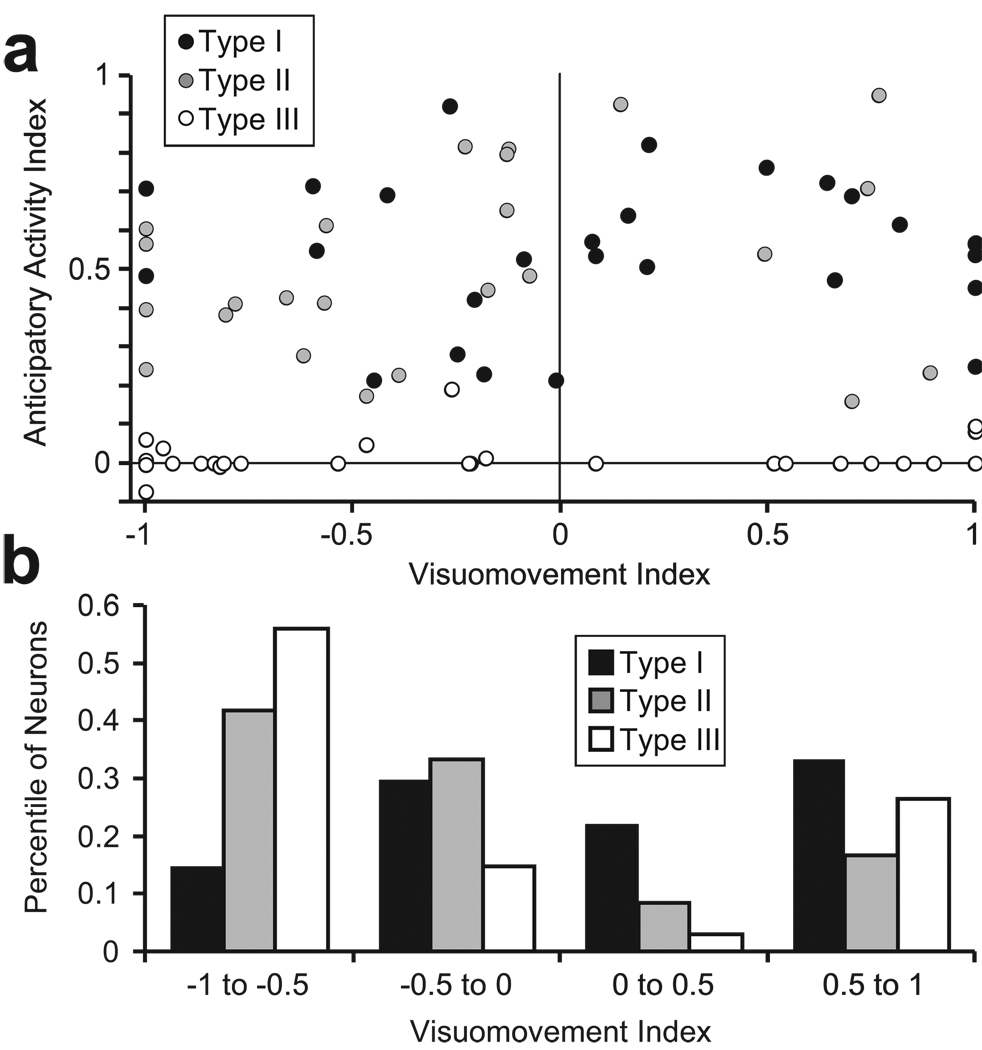
We examined whether a neuron’s placement on the visuomovement axis was related to anticipatory spatial selectivity in the luminance discrimination task (Figure 6a). An anticipatory activity index was calculated as a contrast ratio of the activity during the last 200 ms of the delay period between the trials in which the green cue stimulus appeared 90° from the RF and opposite the RF (90°RF-OppRF)/( 90°RF+OppRF). Because this measure is unreliable at extremely low firing rates, it was necessary to set the index to 0 for neurons in which the denominator (90°RF+OppRF) was less than 5 spikes/second. This only affected the selection index for type III neurons; those neurons in which these two activity measures were not statistically different (see Figure 3B, black dots). Figure 6 shows that strong anticipatory selection was observed across the entire visuomovement axis for both type I and type II neurons, and the magnitude of the anticipatory index was not different between the two groups (type I: average = 0.54; type II: average = 0.51; p = 0.59). However, the distributions in Figure 6b shows that a higher proportion of type II and type III neurons had stronger visual than movement responses in the memory-guided saccade task (type II: 75%, chi-square = 5.04, p = 0.02; type III: 71%, chi-square = 4.98, p = 0.03). Type I neurons did not exhibit a significant trend of having stronger visual or motor responses (44% visual > movement; p = 0.7).
Figure 6.
Relationship between anticipatory activity in the luminance discrimination task and FEF neuron classification along a visuomovement continuum. (a) The anticipatory activity index is plotted as a function of visuomovement index for type I neurons (black circles), type II neurons (gray circles) and type III neurons (white circles). The anticipatory activity index is a contrast ratio that quantifies the magnitude of anticipatory activity in the last 200 ms of the delay period recorded during the luminance discrimination task; (R90°-Ropp)/ (R90°+Ropp), where R90° is the response on ‘90° from RF’ trials and Ropp is the response on ‘opposite RF’ trials. Values near 0 indicate no anticipatory activity and values near +1 indicate strong anticipatory activity. The visuomovement index is a contrast ratio of the visual and saccade-related responses recorded during the memory-guided saccade task. Neurons with values near −1 are dominated by a visual response, and neurons near +1 are dominated by saccade-related activity. Values near 0 indicate nearly equivalent visual and saccade related activity. (b) Distribution of proportions of type I (black bars), type II (gray bars), and type III (white bars) neurons along the visuomovement continuum.
Relationship of anticipatory activity to saccade production
To determine whether the activity preceding the luminance stimulus appearing in the RF was related to the monkeys’ anticipation or preparation of a saccade to the RF (Bruce & Goldberg, 1985, Coe, Tomihara, Matsuzawa & Hikosaka, 2002) we examined the relationship of anticipatory neural activity to reaction time (Basso & Wurtz, 1998, Dorris & Munoz, 1998) and to motor choice on error trials (Cisek & Kalaska, 2005).
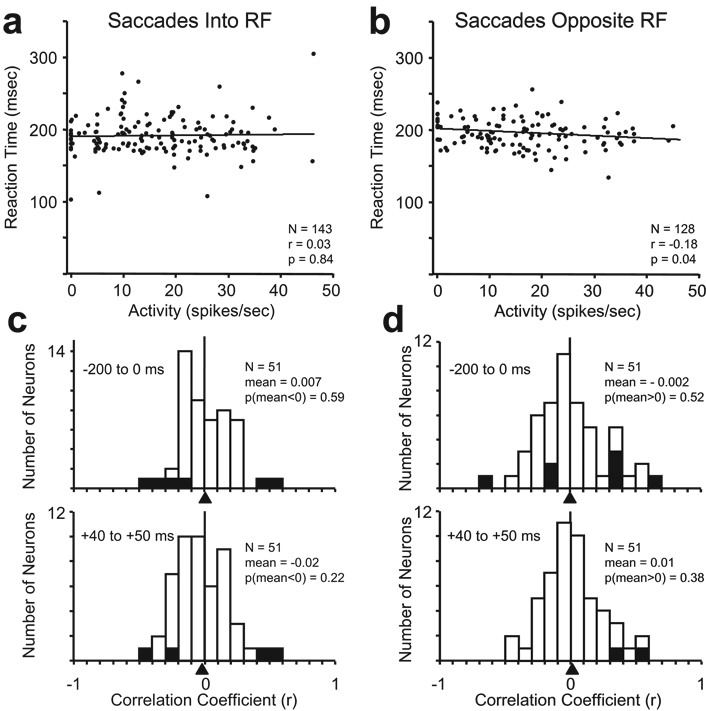
Studies in superior colliculus (Basso & Wurtz, 1998, Dorris & Munoz, 1998) and FEF (Ding & Hikosaka, 2006) have used correlations between anticipatory activity observed in other tasks and reaction time to argue that the anticipatory activity is related to saccade production. However, the anticipatory activity in the luminance discrimination task was not correlated with saccadic reaction time (Fig. 7). We calculated the Pearson correlation coefficients for activity versus reaction time on trials when the saccade was made into the RF and opposite the RF for the anticipatory activity in type I and type II neurons. For the neuron shown in Figure 2, there was no correlation between reaction time and level of anticipatory activity for saccades into the RF (p = 0 84; Fig. 7a); and there was a slight negative correlation for saccades away from the RF (p = - 0.04; Fig. 7b). Overall, however, there was not a systematic relationship between anticipatory activity and reaction time. Histograms of the correlation coefficients for all anticipatory neurons are shown in Figure 7c and 7d during two separate time intervals. The upper histograms show the results from the average activity measured during the last 200 ms of the delay period before the presentation of the luminance stimuli. The lower histograms in Figure 7c and 7d show the results from the average activity measured between 40 and 50 ms following the presentation of the luminance stimuli. This time range is similar to that used by Dorris and Munoz (1998); it is the last 10 ms of the anticipatory activity before neurons began to respond to the appearance of the luminance stimuli in their RFs. Although a few of the neurons showed significant positive or negative correlations (p<0.05; hatched bars), the mean correlation coefficients were not significantly less than 0 for saccades into the RF or greater than 0 for saccades away from the RF (one-tailed z-tests) for any of the distributions.
Figure 7.
Correlation analysis of saccadic reaction time versus delay period activity in the luminance discrimination task. Saccadic reaction times plotted as a function of anticipatory activity from the neuron shown in Figure 2 for (a) saccades into the RF, and (b) saccades opposite the RF. Activity was averaged over last 200 ms of the delay period. Each data point represents a single trial. The best fit regression lines are shown. The Pearson correlation coefficient (r), number of trials (N), and probability of significant correlation (p) are shown in the plots for each set of trials. The distributions of correlation coefficients of activity versus reaction time are shown obtained from all 51 anticipatory neurons for (c) saccades into the RF, and (d) saccades opposite the RF for the average activity during the last 200 ms before the appearance of the luminance stimuli (upper histogram), and for the average activity between 40 and 50 ms following the appearance of the luminance stimuli (lower histogram). Triangles below the abscissas indicate the mean correlation coefficients for each condition. The number of neurons (N), the mean correlation coefficient (mean), and the statistical probability (one-tailed z-tests) that the mean is less than 0 for saccades into the RF (c) and greater than 0 for saccades opposite the RF (d) are shown in each histogram. Hatched bars represent individual neurons with statistically significant correlations (p<0.05).
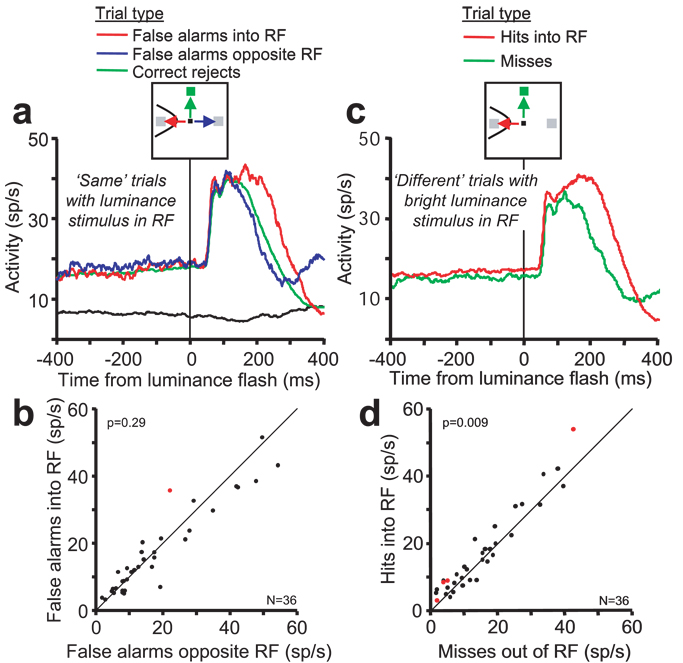
A recent study in premotor cortex of monkeys performing a delayed matching task showed that pre-cue anticipatory activity on error trials reliably predicts the location of the goal of erroneous reach movements (Cisek & Kalaska, 2005). Using a similar analysis, we examined activity on error trials to determine if the anticipatory activity in FEF influenced the choice of the saccade goal. On ‘same’ trials, the monkeys occasionally made an incorrect saccade to the location of one of the luminance stimuli. On these ‘false alarm’ error trials, the sensory evidence did not support the monkeys’ choice; the luminance stimuli were physically identical. Therefore, any motor bias or predisposition in favor of one stimulus location over the other should play a larger role in the monkeys’ choice. If the anticipatory activity was related to a motor choice, then the activity on false alarm trials in which the saccade was made into the RF should be greater than on false alarm trials in which the saccade was in the direction opposite the RF. The data recorded from 36 neurons (19 type I and 17 type II) contained enough false alarm error trials to analyze (at least 10 trials for each trial condition). The pooled responses of these neurons are shown in Figure 8a. The activity of the individual neurons on false alarm trials is compared in Figure 8b. Across the population, there was not a significant difference in the average activation preceding the luminance stimuli for the two saccade directions on false alarm trials (paired t-test, p = 0.29). Only one neuron exhibited activity (p < 0.05) that was significantly higher on trials in which the errant saccade was made into the RF. In summary, the results of the reaction time analysis and the saccade choice analysis indicate that the anticipatory activity was not related to saccade production.
Figure 8.
Comparisons of activity across trial conditions in which a luminance stimulus appeared in the neurons’ RF. (a) Pooled average activity of 36 type I and type II neurons on ‘same’ trials in which the luminance stimuli appear in the RF. Activity is aligned on the time of the onset of the luminance stimuli. The different trial conditions and the saccade directions are indicated by the arrows and line colors in the cartoon at the top. The red and blue traces show the average activity on false alarm trials in which the saccade was made incorrectly to the luminance stimulus located in the RF and opposite the RF, respectively. The green activity trace shows the average activity on trials in which the saccade was correctly made to the green cue stimulus located 90° from the RF (correct rejects). For comparison, the black line shows the activity on ‘Opposite RF’ trials in which both luminance stimuli were flashed outside the RF. (b) A comparison of the activity for type I and II neurons during the last 200 ms of the delay period on false alarm trials ending with an incorrect saccade into the RF and opposite the RF (represented by the red and blue activity traces). Only one neuron exhibited a significant activity difference (red data point, p < 0.05). The responses of the remaining 35 neurons were distributed along the diagonal representing equal activation for saccades into the RF and opposite the RF. The probability that the entire sample of neurons exhibited equal activity based on a paired t-test is shown at the top left corner. (c,d) Pooled average activity and comparison of 36 type I and type II neurons on correct and error ‘different’ trials in which the bright luminance stimulus appeared in the RF. Conventions are the same as in (a,b). Four neurons exhibited a significant activity difference and, overall, the activity during the last 200 ms of the delay period was greater on hits than on misses.
Relationship of anticipatory activity to performance accuracy
We examined whether variations in the anticipatory activity in type I, and II neurons were correlated with differences in performance accuracy. On ‘same’ trials, the level of anticipatory activity when the monkeys incorrectly reported that one of the luminance stimuli was ‘bright’ (false alarms) was not different from the activity on trials when the monkey correctly reported ‘same’ (correct rejections) (p = 0.35; Fig. 8a). On ‘different’ trials, however, there was evidence suggesting that small changes in anticipatory activity affected perceptual sensitivity in the neuron’s RF. The anticipatory activity on ‘miss’ trials (average = 15.45 spikes/sec) when the monkeys failed to report the bright stimulus in the RF was 9.6% lower than on ‘hit’ trials (average = 17.08 spikes/sec) when the monkeys correctly reported the bright stimulus in the RF (paired t-test, p = 0 009; Fig. 8c and d). The same result was obtained when the analysis was done separately on those neurons with greater visual than eye movement activity in the memory-guided saccade task (‘miss’ = 16.28 spikes/sec, ‘hit’ = 18.27 spikes/sec, p = 0.08), and on those neurons with greater movement than visual activity in the memory guided saccade task (‘miss’ = 14.83 spikes/sec, ‘hit’ = 16.19 spikes/sec, p = 0.06). Although the difference is slight, it suggests that the amount of anticipatory activity has an affect on perceptual choice behavior. There was no activity difference between ‘miss’ and ‘hit’ trials when the bright stimulus was presented outside the RF (p = 0.68), or between any other comparison of the activity recorded on correct and error trials in which a luminance stimulus appeared in the neurons’ RF.
Behavioral evidence for spatial attention
We assessed whether the monkeys’ attention was allocated to the stimulus locations informed by the green stimulus. In separate behavior only sessions the monkeys performed the luminance discrimination task using the same rule; make a saccade to the brighter of the two flashed luminance stimuli or, if they are of equal luminance, make a saccade to the green cue stimulus. Trials began with the appearance of the green cue at the usual 10° eccentricity. However, on 10% of the trials, the two luminance stimuli were presented unexpectedly at 5° or 7° eccentricities instead of the predicted 10° eccentricity. The higher spatial resolution and the increased neural resources dedicated to visual processing at eccentricities closer to the fovea could lead to the prediction that the monkeys would perform better on trials in which the luminance stimuli were flashed at eccentricities closer to the fovea (Virsu & Rovamo, 1979, Wassle, Grunert, Rohrenbeck & Boycott, 1990). However, the monkeys’ performance was actually worse when the luminance stimuli were flashed at the closer eccentricities. Figure 9 shows the average performance across 12 experimental sessions, 6 sessions for each of the two monkeys. The monkeys made a valid saccade choice within 500 ms on 99.4% and 98.7% of the expected and unexpected eccentricity trials, respectively. Performance improved with increasing luminance difference for all eccentricities indicating that the monkeys were using the luminance stimuli to guide their behavior during the expected and unexpected eccentricity trials. During the behavior only sessions the monkeys’ performance accuracy was similar to but not quite as good as during the recording sessions. This performance difference at the expected eccentricity may be due to the uncertainty introduced by the inclusion of the unexpected eccentricity probe trials. But most importantly, the performance accuracy during the behavior only sessions was much better at the expected eccentricity than at the closer, unexpected eccentricities. This is evidence that the monkeys directed their attention to the locations informed by the green cue stimulus.
Discussion
We have described a spatially selective anticipatory response in 60% of FEF neurons in the absence of direct visual input. This activity is a cognitively-driven signal derived from the monkeys’ expectation of the appearance of visual stimuli to be discriminated. It identified spatial locations important for performing the task. The lack of a correlation with saccadic reaction time and saccade choice suggests that this anticipatory activity was not directly related to saccade production. The monkeys’ behavior suggests that the monkeys attended the locations informed by the cue. We propose that this activity is related to a shift of endogenous attention and provides a top-down signal that biases visual processing at the locations of the visual stimuli to be discriminated. The remaining 40% of neurons did not exhibit anticipatory activity and therefore may represent a population of neurons in FEF that do not contribute to the allocation of attention in this task.
In neurophysiological studies it is often difficult to attribute neural activity to a specific cognitive process such as spatial attention (Maunsell, 2004). Alternative explanations need to be considered. Spatially selective anticipatory responses preceding visual stimulation have been observed previously in visuomotor structures, including FEF, of behaving monkeys and were attributed to motor planning (Bruce & Goldberg, 1985, Dorris & Munoz, 1998), reward anticipation (Ding & Hikosaka, 2006, Takikawa, Kawagoe & Hikosaka, 2002), and choosing a saccade target (decision-making) (Coe et al., 2002, Platt & Glimcher, 1999). However, the anticipatory activity we described in this study is inconsistent with any of these alternative hypotheses.
The green cue in the luminance discrimination task informed the monkeys about the future locations of the luminance stimuli to be compared; and it was also the most probable rewarded saccade target on each trial. But, in spite of the higher probability of a reward for making a saccade to the green stimulus in the response field, the type I neurons (53% of all anticipatory neurons) did not exhibit delay activity on trials when the green target was in the response field. Instead, the delay activity of these neurons anticipated the appearance of the visual stimuli to be discriminated. This activity is consistent with the hypothesis that it represents the allocation of attention in anticipation of the luminance stimuli, but not with the hypotheses that it is related to the probability that a saccade to the neurons’ RF was the correct behavior to obtain reward.
The type II neurons, however, also exhibited elevated activity for the green cue stimulus in addition to the anticipatory activity for the luminance stimuli (Fig. 4 and Fig. 5b). Consequently, there is a possibility that the activity of type II neurons represents the probability that the stimulus in the RF was the saccade goal or rewarded stimulus (Basso & Wurtz, 1998). However, if this were the case, it would be saccade- or reward-related activity that is unlike any described previously. In previous studies, anticipatory activity related to the probability of reward or to saccade target selection was correlated with saccade reaction time and to the choice of saccade target. (Basso & Wurtz, 1998, Coe et al., 2002, Ding & Hikosaka, 2006, Dorris & Munoz, 1998, Takikawa et al., 2002). However, we found no evidence of a relationship between the anticipatory activity in type I or in type II neurons and saccadic reaction time (Fig. 7).
The lack of a relationship to saccade reaction time suggests that the activity associated with the green target in type II cells is most likely a visual response to a stimulus in the receptive field. All of the type II neurons were visually responsive. Neurons that exhibit sustained visual responses as long as a visual stimulus remains in the RF are commonly found in FEF (Bruce & Goldberg, 1985, Schall, 1991). In addition, there was no relationship between the anticipatory activity of type I or type II neurons and saccade choice on error trials (Fig. 8). In a recent study of anticipatory activity in premotor cortex related to two alternative hand movements, the activity reliably predicted the monkey’s motor choice on error trials. This result supported their conclusion that the activity in question was related to motor preparation (Cisek & Kalaska, 2005). In contrast, we did not find evidence suggesting that the anticipatory activity observed in our study was related to the selection of the saccade target. The results of both the reaction time (Fig. 7) and saccade choice (Fig. 8) analyses indicate that the anticipatory activity in both type I and type II neurons observed in the luminance discrimination task is not directly related to anticipatory saccade planning. We think the most likely interpretation is that the anticipatory activation in both type I and type II neurons reflect endogenous spatial attention and the activity related to the green cue in type II neurons is a sustained visual response signaling the presence of a visual stimulus in the neurons’ receptive field. However, we cannot completely rule out the possibility that type II neurons represent saccade probability. Therefore, if we count only the type I neurons, then at least 32% (27/85) of FEF neurons exhibit activity that is consistent with representing endogenous spatial attention and completely incompatible with a saccade preparation explanation.
Recently, Hasegawa et al. (2004) described neurons in FEF that exhibited increased activity related to the suppression of saccades in a specific direction. Although we did not test neurons in a condition that required the suppression of saccades, all neurons in this study exhibited increased visual, delay or saccade related activity in the memory-guided saccade task and therefore would not be classified as “don’t look” neurons.
There was enhanced perceptual sensitivity at the expected stimulus locations relative to the less eccentric unexpected stimulus locations (Fig. 9), suggesting that the monkeys were attending the locations they learned to associate with the location of the green cue. This is consistent with previous studies showing that monkeys, like humans, can and do shift attention according to target probability and behavioral relevance (Ciaramitaro et al., 2001, Corbetta & Shulman, 2002, Desimone & Duncan, 1995, Fries et al., 2001, Kastner & Ungerleider, 2000, Luck et al., 1997, McAdams & Maunsell, 1999). The enhanced perceptual sensitivity supports the hypothesis that the anticipatory activity preceding the appearance of the luminance stimuli represents the allocation of endogenous attention.
We examined whether small variations in the anticipatory activity was correlated with differences in performance accuracy. On ‘same’ trials, the level of anticipatory activity when the monkeys incorrectly reported that one of the luminance stimuli was ‘bright’ (false alarms) was not different from the activity on trials when the monkey correctly reported ‘same’ (correct rejections) (Fig. 8a). On ‘different’ trials, however, there was evidence suggesting that small changes in anticipatory activity affected perceptual sensitivity in the neuron’s RF. The anticipatory activity on ‘miss’ trials when the monkeys failed to report the bright stimulus in the RF was slightly lower than on ‘hit’ trials when the monkeys correctly reported the bright stimulus in the RF (Fig. 8b). The lower activity on ‘miss’ trials is consistent with the hypothesis that anticipatory activity contributes to perceptual performance; especially if detecting the brighter stimulus was the most important factor for the monkeys in performing the task.
In neurophysiological studies of visual cortex the effect of endogenous spatial attention is enhanced visual responses (McAdams & Maunsell, 1999, Mitchell et al., 2004, Reynolds et al., 2000) and small (~40%) increases in baseline activity (Luck et al., 1997). These attention effects in visual cortex have been attributed to a top-down modulation from higher-order control areas to lower-order processing areas. Converging lines of evidence point to the FEF as an important source of top-down spatial attentional control (Awh et al., 2006, Corbetta & Shulman, 2002, Hamker, 2005, Moore et al., 2003, Pessoa et al., 2003, Schall, 2004, Thompson & Bichot, 2005) in addition to its well-known role in saccade production (Bruce & Goldberg, 1985, Schall, 1991, Schall, 2004) . FEF is reciprocally connected with prefrontal areas thought to be involved in executive control and working memory (Huerta, Krubitzer & Kaas, 1987, Miller & D'Esposito, 2005), and with both the ventral and dorsal visual processing streams (Schall, Morel, King & Bullier, 1995), putting it in an ideal position to modulate visual processing. Numerous human imaging studies show strong activation of FEF during voluntary shifts of attention (Hopfinger et al., 2000, Kastner et al., 1999, Kincade et al., 2005, Serences & Yantis, 2006). Electrical microstimulation of FEF improves perceptual performance and enhances neuronal responses in visual cortex in a manner that mirrors the effects of directed spatial attention (Moore & Armstrong, 2003, Moore & Fallah, 2001). Inactivation of FEF causes spatially selective deficits in visual attention (Wardak et al., 2006). In a particularly relevant study, Schafer and Moore (2007) recently showed that attentional effects of subthreshold microstimulation of FEF during the presentation of the visual stimuli dominates the oculomotor effects. The results of our study provide physiological evidence that FEF neurons represent the locus of endogenous covert spatial attention in the absence of visual input. The FEF neurons with anticipatory activity are ideally suited to convey a top-down spatial attention signal to visual cortex that enhances the processing of behaviorally important visual stimuli.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Eye Institute. We thank J. Trageser, I. Monosov, L. Ding, J. Schall, R. Wurtz and B. Richmond for helpful comments on earlier versions of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10(3):124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J. Neurosci. 1998;18:7519–7534. doi: 10.1523/JNEUROSCI.18-18-07519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage. 2001;14(2):310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields: I. Single neurons discharging before saccades. J. Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nat Neurosci. 2004;7(3):308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Penpeci-Talgar C, Eckstein M. Spatial covert attention increases contrast sensitivity across the CSF: support for signal enhancement. Vision Res. 2000;40(10–12):1203–1215. doi: 10.1016/s0042-6989(00)00024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramitaro VM, Cameron EL, Glimcher PW. Stimulus probability directs spatial attention: an enhancement of sensitivity in humans and monkeys. Vision Res. 2001;41(1):57–75. doi: 10.1016/s0042-6989(00)00203-0. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45(5):801–814. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Coe B, Tomihara K, Matsuzawa M, Hikosaka O. Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J. Neurosci. 2002;22:5081–5090. doi: 10.1523/JNEUROSCI.22-12-05081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Ann. Rev. Neurosci. 1995;18:183–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Ding L, Hikosaka O. Comparison of reward modulation in the frontal eye field and caudate of the macaque. J Neurosci. 2006;26(25):6695–6703. doi: 10.1523/JNEUROSCI.0836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J. Neurosci. 1998;18:7015–7026. doi: 10.1523/JNEUROSCI.18-17-07015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field: Effects on visual perception and attention. Journal of Cognitive Neuroscience. 2002;14(7):1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Hamker FH. The reentry hypothesis: the putative interaction of the frontal eye field, ventrolateral prefrontal cortex, and areas V4, IT for attention and eye movement. Cereb Cortex. 2005;15(4):431–447. doi: 10.1093/cercor/bhh146. [DOI] [PubMed] [Google Scholar]
- Hasegawa RP, Peterson BW, Goldberg ME. Prefrontal neurons coding suppression of specific saccades. Neuron. 2004;43(3):415–425. doi: 10.1016/j.neuron.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265(3):332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci U S A. 2004;101(43):15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25(18):4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaka Y, Mikami A, Kubota K. Neuronal activity in the frontal eye field of the monkey is modulated while attention is focused on to a stimulus in the peripheral visual field, irrespective of eye movement. Neurosci Res. 1997;28(4):291–298. doi: 10.1016/s0168-0102(97)00055-2. [DOI] [PubMed] [Google Scholar]
- Lawrence BM, White RL, 3rd, Snyder LH. Delay-period activity in visual, visuomovement, and movement neurons in the frontal eye field. J Neurophysiol. 2005;94(2):1498–1508. doi: 10.1152/jn.00214.2005. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J. Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Maunsell JH. Neuronal representations of cognitive state: reward or attention? Trends Cogn Sci. 2004;8(6):261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JHR. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J. Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BT, D'Esposito M. Searching for "the Top" in Top-Down Control. Neuron. 2005;48(4):535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Stoner GR, Reynolds JH. Object-based attention determines dominance in binocular rivalry. Nature. 2004;429(6990):410–413. doi: 10.1038/nature02584. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421(6921):370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40(4):671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci U S A. 2001;98(3):1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: From modulation of sensory processing to top-down control. Journal of Neuroscience. 2003;23(10):3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q. J. Exp. Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26(3):703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Rao SM, Caffarra P, Scaglioni A, Bobholz JA, Woodley SJ, Hammeke TA, Cunningham JM, Prieto TE, Binder JR. Neural basis of endogenous and exogenous spatial orienting. A functional MRI study. J Cogn Neurosci. 1999;11(2):135–152. doi: 10.1162/089892999563283. [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Moore T. Attention governs action in the primate frontal eye field. Neuron. 2007;56(3):541–551. doi: 10.1016/j.neuron.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields. J. Neurophysiol. 1991;66:559–579. doi: 10.1152/jn.1991.66.2.559. [DOI] [PubMed] [Google Scholar]
- Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Research. 2004;44(12):1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995;15(6):4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Ann. Rev. Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10(1):38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Hikosaka O. Reward-dependent spatial selectivity of anticipatory activity in monkey caudate neurons. Journal of Neurophysiology. 2002;87(1):508–515. doi: 10.1152/jn.00288.2001. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25(41):9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76(6):4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- Virsu V, Rovamo J. Visual resolution, contrast sensitivity, and the cortical magnification factor. Exp Brain Res. 1979;37(3):475–494. doi: 10.1007/BF00236818. [DOI] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26(16):4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Grunert U, Rohrenbeck J, Boycott BB. Retinal ganglion cell density and cortical magnification factor in the primate. Vision Res. 1990;30(11):1897–1911. doi: 10.1016/0042-6989(90)90166-i. [DOI] [PubMed] [Google Scholar]
- Zhou HH, Thompson KG. Society for Neuroscience Annual Meeting. San Diego, CA: 2004. Covert attention and saccade planning in the frontal eye field preceding spatially predictable visual stimuli. 313.12. [Google Scholar]