The shortage of organs for transplantation has been so severe and prolonged that many of us have become almost numb to the crisis facing patients and their families. In 2008, there are 98,992 patients on various organ transplantation waiting lists in the United States alone; during January and February alone, 4,471 transplants were performed but 1,044 patients died while on waiting lists[1]. This organ crisis shines an unwanted spotlight on the failure of tissue engineering to thus far deliver on its promises of the late 20th century.
Tissue engineering in the 21st century still has the same potential, in some ways rediscovering itself under the more reputable moniker of “Regenerative Medicine”. However, some of the problems that kept tissue engineering from delivering the goods in the 1990s remain important ones in this century. One of the critical challenges in building any new organ is the dependence of an implanted tissue construct on sufficient oxygen and nutrient transport for its cells to survive, both for access to substrate molecules and clearance of products of metabolism[2]. The principal mechanism for this transport, especially for small molecules, is passive diffusion along concentration gradients, and oxygen diffusion is of obvious importance. The transport of other nutrients is generally more favorable than that of oxygen since the diffusion of oxygen is relatively slow, consumption is high, and the tolerated time for any deficit is so short.
To provide sufficient oxygen tension to mitochondria inside the cell, the minimum distance from the cell to the closest capillary lumen in many metabolically active tissues is rarely greater than 40 to 200 µm[3]. With this proximity to flowing blood and its partial pressures of ~100mmHg, oxygen can maintain the concentration gradient required at the cell. The distance-to-oxygen problem is important not only in the body, but in the laboratory as well. Not surprisingly, then, the most successful tissue engineering constructs have employed thin tissue segments to allow oxygen and nutrient transport. Diffusion distances of >0.2 mm are generally poorly tolerated, and when the diffusion distance exceeds approximately 0.1 mm, necrosis may occur[4]. Notable exceptions include constructs of cartilage and skin epidermis, which can be engineered in the laboratory with greater thicknesses[2]. Since most tissue engineered constructs will have to be substantially thicker (>1 cm or more) and structurally and functionally more complex, they cannot be supported by simple diffusion and will require vascular networks.
Some tissues may become vascularized appropriately after implantation, but for many tissues like myocardium, substantial injury may occur before the vascularization process completes. Thus, generating a functional and stable microvasculature remains one of the major challenges in tissue engineering[5]. The development of a mature vasculature relies not only on endothelial proliferation and migration, as cooperation between endothelial cells and pericytes is fundamental for vascular development and maturation. Blood vessels develop early during embryogenesis and are derived from mesodermal precursors called angioblasts, which share a common precursor, hemangioblasts, with hematopoietic cell precursors[6]. Angioblasts divide and form dense syncytial masses which become networks of solid tubes that eventually canalize to form blood vessels[6]. In this process, termed vasculogenesis, precursor angioblasts differentiate into endothelial cells (EC) forming a vascular network[7]. This primordial plexus is refined into a functional network by a process termed angiogenesis where vessels undergo extensive elongation, inosculation, intussusception, and sprouting[8].
Pericytes, derived from mesenchymal stem cells (MSCs)[9], assume their positions surrounding a vessel, establishing contacts with numerous endothelial cells and transferring angiogenic signals by contact and in a paracrine manner. The bidirectional communication of pericytes and endothelial cells affect each other’s mitotic rate and can lead to endothelial differentiation and growth arrest[10]. Thus, both endothelial cells and pericytes are fundamental for vessel maturation and stability: this orchestration of cells interacting through a variety of growth factors such as vascular endothelial growth factor (VEGF) regulates vascular plasticity through control of growth, survival, remodeling, and regression of the endothelium[11].
Multiple factors are required for vascular maturation. Local delivery of VEGF is insufficient to create a mature and stable vasculature, generating the leaky and disorganized vessels seen in many tumors[12]. However, when multiple angiogenic and vascular maturation factors are delivered to a desired site with controlled release, generation of a functional new vascular network with smooth muscle cells thickly coating the new vessels is observed, demonstrating the importance of a spatial-temporally organized sequence of events in this process[13]. Premature vessel remodeling and regression within a tissue construct can occur if the vasculature is immature, and one approach to this problem is using in vivo vascularization in a surrogate host to prepare the tissue for implantation[14]. Most tissue engineered constructs will require not only their own functional vasculature, but anastamosis with the host circulation. This requires stimulation of an angiogenic response from the host, through recruitment of cells by angiogenic factors[15].
The importance of cell-cell interactions in the development of a stable vasculature is demonstrated by the numerous unsuccessful attempts using single cell types. Endothelial cells can be implanted in vivo, forming blood vessels; however, these vessels rapidly regress without the support of perivascular cells[16]. Endothelial cells derived from human embryonic stem cell (hES), when implanted alone in a fibronectin-collagen gel into cranial windows in immunodeficient mice can develop a vascular network, but this network fails to connect to the host vasculature, showing a regression of the vessels within few days. In contrast, when the hES cell–derived endothelial cells are co-implanted with mouse mesenchymal precursor cells, cells assemble to form luminal structures that connected to the mouse circulatory system[17]. Koike et al coimplanted human umbilical vein endothelial cells and perivascular cell precursors (10T1/2 cells) seeded in a three-dimensional fibronectin–type I collagen gel in immunodeficient mice, leading to endothelial cell proliferation with capillary-like vessels stabilized through pericyte recruitment. The network was stably anastomosed with the host system for one year. By contrast, constructs prepared from endothelial cells alone showed minimal perfusion and had generally disappeared after 60 days, stressing the importance of cell-cell interactions[18].
In this issue of Circulation Research, Melero-Martin et al provide a significant step forward in the vascularization process necessary for tissue engineering and regenerative medicine[19]. They demonstrate the ability of in vivo co-culture of human endothelial progenitor cells (EPCs), isolated from peripheral or cord blood, and mesenchymal progenitor cells (MPCs), isolated from bone marrow or cord blood, to produce an extensive and functional vascular network in Matrigel-based implants into mice. The importance of this study is based on the use of human postnatal progenitor cells that can be obtained by noninvasive procedures. Their results demonstrate the potential of MPCs in promoting vascular network development in engineered tissues, recapitulating the embryonic process of vasculogenesis (Figure 1). MPCs that are readily available may therefore be capable of the critical role of stabilizing the vascularization process.
Figure 1. Coimplantation of human Mesenchymal Progenitor Cells and Endothelial Progenitor Cells induces in vivo vascularization of a tissue construct.
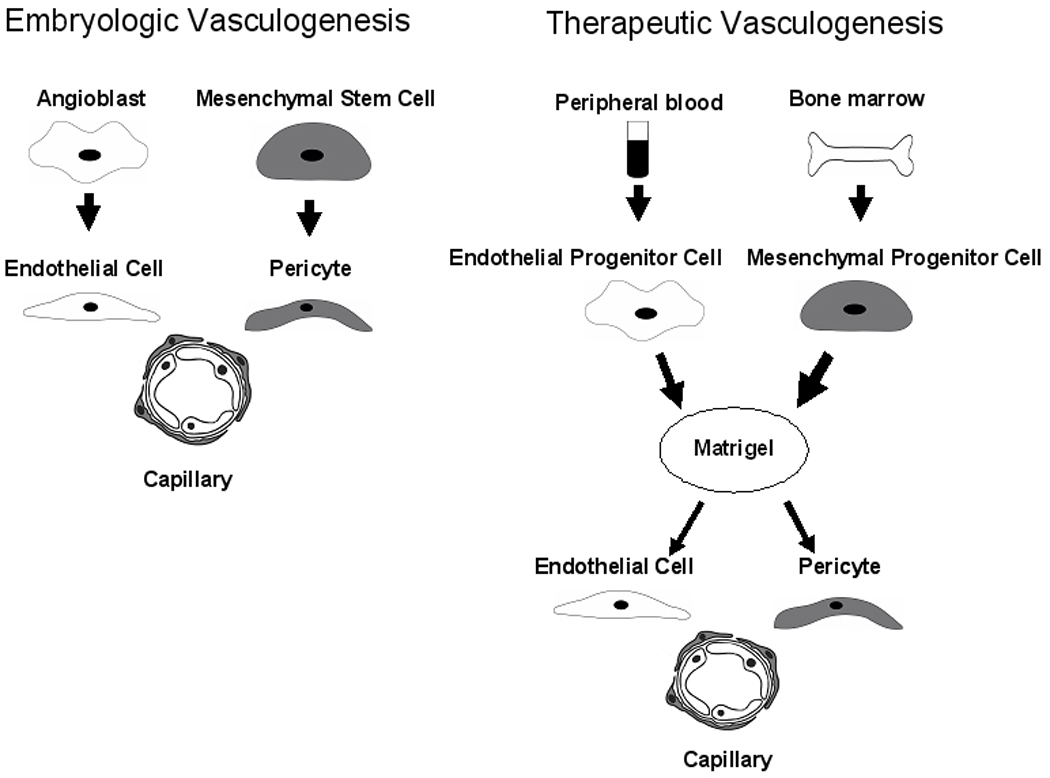
During embryogenesis, angioblasts differentiate into Endothelial Cells and organize to form the primordial capillary-like structures. Mesenchymal Stem Cells differentiate into pericytes assuming positions surrounding the endothelial cells in the newly formed vessels. Pericytes regulate maturation and stabilization of the vascular network. Melero-Martin and colleagues reproduced vasculogenesis in a tissue construct using Matrigel as the scaffold. Cord blood-derived Endothelial Progenitor Cells or adult blood Endothelial Progenitor Cells where co-implanted with either bone marrow-derived or cord blood derived Mesenchymal Progenitor Cells to produce a stable and mature vascular network when implanted in vivo. The vascular structures where composed of human Endothelial Progenitor Cells surrounded by α-smooth muscle actin positive mesenchymal cells anastomosed with host vessels.
Melero-Martin et al provide important insight into MPC-EPC crosstalk by carefully studying the importance of EPCs/MPC ratio in determining successful vascularization. Just as a successful orchestra requires a balance between the strings and the brass, an imbalance of MPCs or EPCs interfered with vascularization. This demonstrates that cell therapy or tissue engineering with only one cell type may, in many circumstances, never reach the potential achieved by using two different types of cells. Because EPCs and MPCs can be isolated simply from peripheral blood and bone marrow, their ability to generate a mature and stable vascular network make them suitable for further characterization in tissue engineering constructs where durable vascularization is required. Use of these cells could overcome ethical issues related to the use of human embryonic cells as well as immunological issues from allogeneic cells.
Melero-Martin et al show patency of their functional vascular networks for up to 4 weeks. Patency of the network is crucial, but this is only the first step in demonstrating the potential of this dual adult cell progenitor approach. The mature vasculature of any tissue requires physiologically appropriate vasomotor control. Furthermore, vascular networks must also interact with other cells of a given tissue to provide physiologically functional tissue[20]; for example, vascular networks may guide electrical connections between cardiomyocytes[21]. Thus, although this study shows that readily-available adult progenitor cells can provide vascularization, further investigation of the long-term application of this approach in specific tissue types is essential. Can these cells guide organization and differentiation of other adult progenitor cells? Will these cells require signals from a third type of progenitor cell or mature cell to establish appropriate vasomotor function?
Solving the organ shortage crisis will be an iterative process, exploiting both the lessons learned from mechanistic studies and crucial technology development studies. Most investigators have resisted using two or more different cell types, for good reason; it is more challenging to carefully phenotype two different progenitor cells types. Furthermore, dose ranging with two or more different cells or compounds increases the complexity of phase I studies exponentially. However, the bottom line in many tissues may be that more than one type of cell may be necessary for therapeutic benefits, just as more than one type of cell is necessary in most circumstances in embryonic development. The combined use of readily-available endothelial progenitor cells and mesenchymal progenitor cells represents a technological advance based on solid mechanistic studies in developmental biology. It brings us one step closer to generating real organs for real people, but we still have many more steps to take.
Acknowledgments
Sources of Funding: This work was supported by grant HL081404 from the National Institutes of Health.
Footnotes
Disclosures: None
References
- 1.U.S. Department of Health and Human. U.S. Government web site for organ and tissue donation and transplantation. www.organdonor.gov.
- 2.Kellner K, Liebsch G, Klimant I, Wolfbeis OS, Blunk T, Schulz MB, Gopferich A. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol Bioeng. 2002;80:73–83. doi: 10.1002/bit.10352. [DOI] [PubMed] [Google Scholar]
- 3.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. I. Krogh's model. Biophys J. 2001;81:675–684. doi: 10.1016/S0006-3495(01)75732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avgoustiniatos ES, Colton CK. Effect of external oxygen mass transfer resistances on viability of immunoisolated tissue. Ann N Y Acad Sci. 1997;831:145–167. doi: 10.1111/j.1749-6632.1997.tb52192.x. [DOI] [PubMed] [Google Scholar]
- 5.Moldovan NI, Ferrari M. Prospects for microtechnology and nanotechnology in bioengineering of replacement microvessels. Arch Pathol Lab Med. 2002 Mar;126:320–324. doi: 10.5858/2002-126-0320-PFMANI. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P. Manipulating angiogenesis in medicine. J Intern Med. 2004 May;255:538–561. doi: 10.1111/j.1365-2796.2003.01297.x. [DOI] [PubMed] [Google Scholar]
- 7.Murohara T. Therapeutic vasculogenesis using human cord blood-derived endothelial progenitors. Trends Cardiovasc Med. 2001 Nov;11(8):303–307. doi: 10.1016/s1050-1738(01)00128-1. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen LL, D'Amore PA. Cellular interactions in vascular growth and differentiation. Int Rev Cytol. 2001;204:1–48. doi: 10.1016/s0074-7696(01)04002-5. [DOI] [PubMed] [Google Scholar]
- 9.Creazzo TL, Godt RE, Leatherbury L, Conway SJ, Kirby ML. Role of cardiac neural crest cells in cardiovascular development. Annu Rev Physiol. 1998;60:267–286. doi: 10.1146/annurev.physiol.60.1.267. [DOI] [PubMed] [Google Scholar]
- 10.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003 Oct;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 11.Bondjers C, Kalen M, Hellstrom M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003 Mar;162:721–729. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinnunen K, Korpisalo P, Rissanen TT, Heikura T, Viita H, Uusitalo H, Yla-Herttuala S. Overexpression of VEGF-A induces neovascularization and increased vascular leakage in rabbit eye after intravitreal adenoviral gene transfer. Acta Physiol (Oxf) 2006 Aug;187:447–457. doi: 10.1111/j.1748-1716.2006.01588.x. [DOI] [PubMed] [Google Scholar]
- 13.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001 Nov;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 14.Lokmic Z, Stillaert F, Morrison WA, Thompson EW, Mitchell GM. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. Faseb J. 2007 Feb;21:511–522. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- 15.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003 Jun;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 16.Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007 Oct;:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, Arzigian M, Fukumura D, Jain RK, Scadden DT. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007 Mar;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 18.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004 Mar 11;428:138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 19.Melero-Martin JM, De Obaldia ME, Kang S, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circulation Research. 2008;103:XXX–XXX. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh PC, Davis ME, Lisowski LK, Lee RT. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu Rev Physiol. 2006;68:51–66. doi: 10.1146/annurev.physiol.68.040104.124629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004 Aug 24;110:962–968. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]


