Abstract
Morphological specialization for a specific role has, until now, been assumed to be restricted to social invertebrates. Herein we show that complete physical dimorphism has evolved between reproductives and helpers in the eusocial naked mole-rat. Dimorphism is a consequence of the lumbar vertebrae lengthening after the onset of reproduction in females. This is the only known example of morphological castes in a vertebrate and is distinct from continuous size variation between breeders and helpers in other species of cooperatively breeding vertebrates. The evolution of castes in a mammal and insects represents a striking example of convergent evolution for enhanced fecundity in societies characterized by high reproductive skew. Similarities in the selective environment between naked mole-rats and eusocial insect species highlight the selective conditions under which queen/worker castes are predicted to evolve in animal societies.
Both vertebrate and invertebrate societies exhibit a reproductive division of labor between breeders and helpers (1–3). In social insects, this functional specialization is frequently associated with morphologically distinct and irreversible castes (4–6). Natural selection can shape the specific form of each endpoint (caste) and the combination of endpoints that make up societies. So, a combination of morphological differentiation and relatively simple differences in behavioral repertoire produce complex organizations in insects.
Morphological specialization in insects is most commonly achieved through differential growth of both the exoskeleton and internal organs. When the different forms produced by this heterogonic growth are distinct forms without intermediates, they are generally termed polyphenisms and, in social insects, castes (6). In societies of mammals with a reproductive division of labor, the breeding female is typically among the largest (i.e., heaviest) of the females in the group [e.g., naked mole-rats (7), meerkats (8), and dwarf mongooses (9)]. Large body size, however, correlates with improved reproductive success in a wide variety of nonsocial vertebrates (10) and is thus not a phenomenon restricted to cooperatively breeding species. More importantly, body mass is a continuous variable and morphological differences among colony members are small and quantitative compared with substantial and qualitative in social insects with discrete castes.
The fundamental question is thus whether the evolution of morphological castes is phylogenetically constrained to invertebrates. If it is, then the implications that this has on our understanding of the elaboration of social systems should be considered. Recent reviews on the origin and definition of eusociality (11–13) concurred that caste differentiation effectively separated vertebrate from invertebrate social systems. Crespi and Yanega (12) suggest that one of the main tasks of studies of social behavior is to elucidate the distinct selective conditions under which castes evolved.
To date, no empirical evidence exists for morphological castes in social vertebrates. Herein, we test the hypothesis that social vertebrates lack castes. We have measured key anatomical features of a cooperatively breeding mammal, the naked mole-rat (Heterocephalus glaber), and applied criteria used to describe and define physical castes in social insects (6).
Naked mole-rats provide an ideal vertebrate test subject because they live in a similar selective environment to most social insects with a secure expandable nest that can accommodate future generations (14). They have the largest colony size of any social vertebrate (average = 75 individuals per colony; maximum = 285 individuals) (15), and large group size may be linked to the evolution of castes (11). In a preliminary unpublished study, Jarvis and Buffenstein reported that breeding female naked mole-rats had significantly longer vertebrae than nonbreeding females (16). Moreover, numerous researchers (7, 17, 18) working on the naked mole-rat have referred to the distinctly elongated shape of breeding females relative to other colony members. However, quantitative data on this phenomenon have apparently never been published.
The aim of this study is to apply the morphometric methods used by social insect biologists to determine whether physical castes do indeed exist in naked mole-rat colonies and to classify the allometric relationship according to accepted criteria.
Materials and Methods
Study Animals.
We used 106 animals from 16 laboratory-based colonies to explore the relationship between reproductive status and morphology. Long-term behavioral and life history records from 1981 through 1998 provided data on the reproductive history, sex, and social status of all individuals. All animals were captive and housed in artificial burrow systems and fed daily with a mixture of vegetables, fruit, and a balanced cereal, PRONUTRO (Bokomo, Malmesbury, South Africa).
Radiographing and Skeletal Measurements.
Morphometric data were obtained from radiographs of live anesthetized (halothane inhalation) mole-rats. Individuals were radiographed from a dorsal and ventral perspective with a Shimadzu medical x-ray unit. X-ray dosage was kept to a minimum (40 kV and 25 mA for 2.5 s), and the entire procedure lasted <5 min for each animal. All animals recovered from the anesthetic with no apparent short- or long-term deleterious effects.
Six skeletal measurements were taken directly from the processed radiographs by using digital calipers, accurate to 0.01 mm. These included width across the zygomatic arch, length of the pelvis (including the ilium and ischium), length of the skull from the nasals to the foramen magnum, maximum width of the skull across the parietals, length of a randomly chosen lumbar vertebra (L5), and length of the femur. The measurements were chosen on the basis of the following two criteria: (i) that they provided a measure of body size and shape and (ii) that they could be measured accurately and reliably from the radiographs. Three readings were taken for each measurement and then averaged to provide a single value for each variable for all individuals.
Morphological analyses were carried out on two complete colonies (colony 100, n = 40 individuals; colony 1000, n = 31 individuals). Both colonies included a breeding pair and nonbreeders born in at least eight successive litters. Within each colony, each of the six skeletal variables was regressed against body mass to ascertain which of them provided the best overall estimate of body size. In addition, visual inspection of scatter plots for each set of variables, in addition to regression analysis, was used to explore whether morphological discontinuities were evident among members of a single colony.
To determine if the pattern observed in the two complete colonies was a general one, we x-rayed breeding females with similar reproductive histories from nine other colonies and compared them with a random selection of adult breeding males and nonbreeding females from their respective colonies. Breeding males were identified on the basis of behavioral interactions with the breeding female. Only those males observed directly mating with the breeding female in each colony were classified as a breeding male. Because mating is a rare event, we were not able to identify the breeding male in four of the colonies, limiting the sample size of breeding males to six individuals.
To determine the proximate cue for vertebral growth, we performed the following experiment. Seven nonbreeding females were removed from their natal colony and housed alone for a period of at least 6 months. Faulkes and Abbott (19) have shown that nonbreeding females that are housed singly become reproductively active. Indeed, all of these females had perforate vaginas and readily solicited males at the end of this study period. We x-rayed these females with the same methods described above and then compared two variables, the width across the zygomatic arch and the length of lumbar vertebra L5, with the same measurements obtained for 10 nonbreeding females and 7 breeding females that had produced at least five successive litters. statistica software was used for all statistics.
Results
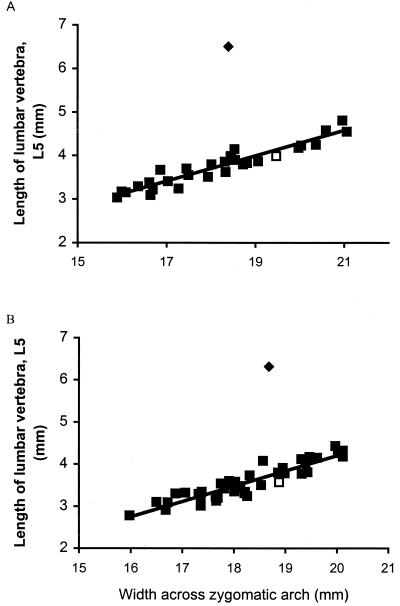
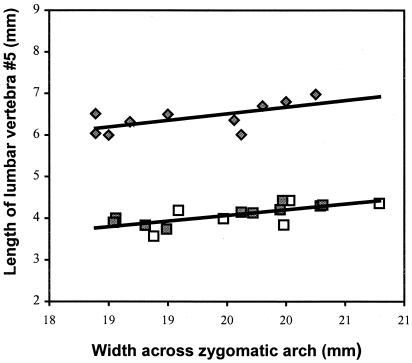
Two variables, zygomatic arch width and length of the pelvis (including the ilium and ischium), consistently provided the highest R2 values for all animals in both colonies (R2 > 0.80), when regressed against body mass and all other variables. Visual inspection of scatter plots revealed that the relationship between all variables, with the exception of lumbar vertebra L5, was isometric (with a log-log curve slope of approximately 1.0) and characterized by limited size variability. Scatter plots (Fig. 1) of lumbar vertebra and width across the zygomatic arch (which provided the best estimate of body size), derived from the two complete colonies, revealed a distinct outlier in each colony, the breeding female. This outlier resulted in low R2 values (R2 ≤ 0.502) for lumbar vertebra L5 regressed against all other variables, including body mass. Nonbreeding colony members and the breeding male were clearly uniform in morphology, and by excluding the breeding females from statistical analyses, the relationship between vertebral length and, for example, zygomatic arch width was then highly statistically significant with high R2 values (regression analysis by the least squares method: R2 = 0.849, F1,38 = 214.1, and P < 0.0001, for colony 100; and R2 = 0.895, F1,29 = 247, and P < 0.0001, for colony 1000). It was not possible to accurately measure the length of individual thoracic and cervical vertebrae from the x-rays. However, a comparison of the length of the cervical, thoracic, and lumbar regions revealed that only the latter was disproportionately longer in breeding females. This morphological difference between the breeding female and all other colony members was confirmed statistically after regression analysis based on the morphometric data obtained from breeding and nonbreeding individuals in nine other colonies (Fig. 2). Regression lines were fitted separately for each category, and the slopes and intercepts of the two regression equations were compared statistically. Both regressions were statistically significant (breeding female: F = 6.03, P = 0.03, and n = 10; nonbreeders and breeding males: F = 14.88, P = 0.0017, and n = 16). There was no difference in the respective slopes (two-tailed t test: t = −0.32, P = 0.742, and n = 22), but the elevations were significantly different (two-tailed t test: t = 26.85, P < 0.0001, and n = 23).
Figure 1.
Zygomatic arch width plotted against vertebral length (lumbar vertebra 5) for all individuals from colony 100 (A) and colony 1000 (B). ▫, breeding males; ▪, all nonbreeders of both sexes; ⧫, breeding females.
Figure 2.
Plot of the regression line of vertebral length (lumbar vertebra L5)
against zygomatic arch width for breeding females
( ;
n = 10), adult nonbreeding females
(
;
n = 10), adult nonbreeding females
( ;
n = 10), and breeding males (□;
n = 6) from the same colonies.
;
n = 10), and breeding males (□;
n = 6) from the same colonies.
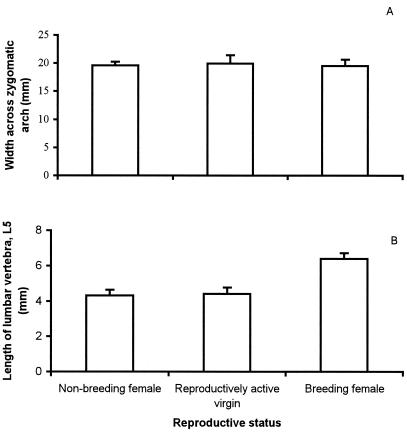
There was no significant difference (ANOVA, F2,0.20 = 0.336 and P = 0.72) in zygomatic arch width among nonbreeding females, reproductively active virgins, and breeding females (Fig. 3). In contrast, there was a marked significant difference in the length of vertebra L5 (ANOVA, F2.20 = 89.3 and P < 0.0001) among these three categories of females, with well-established breeding females (five litters or more) having significantly longer vertebrae than reproductively active virgins and nonbreeding females (Scheffé multiple comparisons test, P < 0.0001 for both categories; Fig. 3). There was no difference in vertebral length between nonbreeding females and reproductively active virgins (Scheffé multiple comparisons test, P = 0.898).
Figure 3.
Zygomatic arch width (A) and vertebral length (lumbar vertebra L5) (B) in nonbreeding females, reproductively active virgins, and well-established (five litters or more) breeding females. Data are the mean and standard deviation.
Discussion
Holldobler and Wilson (6) refer to complete dimorphism as the most advanced caste system to have evolved in social insects, the principal examples of which include queen/worker differences. Complete dimorphism is characterized by the existence of two very distinct morphological groups within a colony, separated by a gap in which no intermediates occur. Each class is either isometric or nonisometric, but the allometric regression curves are not aligned. This definition describes the results obtained for naked mole-rats. Breeding females represent a statistically discrete group of individuals from the breeding males and nonbreeders of both sexes, and there are no intermediates. Thus a modest increase in the relative size of the lumbar vertebrae in breeding females yields a new morphological type that is specialized for reproduction, the only empirical example of a vertebrate queen caste.
The principal difference between castes in naked mole-rats and those of social insects is the point at which morphological divergence takes place. In all of the ants and a proportion of the wasps and bees (including Apis), adult females exhibit two distinct phenotypes as a consequence of differential gene expression during larval development (20). In contrast dimorphic female naked mole-rats are produced by divergent growth involving reproductively mature individuals. Unlike the Hymenoptera where morphology is fixed after emergence from the cocoon, termite workers are generally immatures that retain the ability to molt and metamorphose into winged sexuals (5). This makes the dimorphic pattern in naked mole-rats more similar to that in termites. A further adaptation of insect queens is physogastry whereby the intersegmental membranes of the abdomen stretch to accommodate the enlarging ovaries (4). In army ants of the genus Eciton, physogastry allows the simultaneous activity of thousands of ovarioles (21). Similarly in Macrotermes termites, an established queen has an abdomen about 500 times larger than that of a young, newly fertile queen (22). This adaptation is clearly comparable to the phenomenon of lumbar vertebral lengthening that we document herein but differs in one important respect—irreversibility. Vertebral lengthening represents permanent change in morphology, and a breeding female that ceases to reproduce remains morphologically distinct from nonbreeders. Disperser morphs in naked mole-rats are also morphologically distinct (23) from other colony members but, after attaining reproductive status, are indistinguishable from nondispersers. This reversibility is similar to the phenomenon of physogastry in social insects in which the abdomen of queens having stopped egg-laying may shrink to a size similar to that of virgin queens.
The onset of vertebral lengthening in female naked mole-rats is dependent upon successful reproduction. Reproductively active females that had not yet had offspring are morphologically indistinguishable from nonbreeding females (Fig. 3). This distinction between reproductively active nonparous and parous females suggests that the high progesterone concentrations typical of the later stages of pregnancy and/or the hormones of parturition and lactation (e.g., oxytocin and prolactin) are most likely to initiate the lengthening of the vertebral column in the abdominal region. Indeed, progesterone stimulates bone formation through the activation of gene expression for various growth factors, most notably insulin-like growth factors (24, 25). Estrogen plays an important role in facilitating neuritic outgrowth and spinal chord formation (26) and thus also may be implicated in the elongation of the vertebrae and the spinal chord.
Regardless of the mechanism, the advantages of abdominal extension in naked mole-rats are considerable. (i) It allows for an enlarged reproductive tract and hence an increased pup carrying capacity. There was a statistically significant increase (t test, t = −5.69, P < 0.0001, n = 16) in the litter size of a subset of randomly chosen breeding females from their first (6.8 ± 2.1 pups [mean ± SD], n = 16) to their fourth litter (12.5 ± 3.3 pups, n = 16). Well-established breeding females are capable of producing litters of up to 28 pups in the wild (27) and 27 pups in the laboratory (28). In contrast, breeding females of other social species of mole-rats have a maximum litter size of only 6 pups. (ii) Considerable evidence suggests that finite gut capacity is the main limiting factor in the altered metabolic responses observed during reproduction in newly established naked mole-rat breeding females (29), and it is suggested that the observed gastrointestinal hypertrophy may enable fully elongated females to meet the increased nutrient requirements associated with this anabolic activity.
It is not known whether vertebral lengthening is accompanied by growth or stretching of the spinal cord. Consequently, this remains an important area for future research given the potential medical benefits. Similarly, the mechanisms triggering bone growth in adults, whose overall growth has reached its asymptote (30), may prove to be of considerable importance in our understanding of mammalian development and phenotypic plasticity.
The realization that physical castes are not a phenomenon restricted to social insects encourages the search for selective factors that are common to both groups. Given the large taxonomic difference between mammals and insects, these factors are unlikely to be intrinsic or genetic in origin but rather extrinsic and ecological.
Importantly, the selective environment of naked mole-rats has more in common with that of eusocial insects than with other cooperative breeding mammalian species (e.g., meerkat, wild dog, or dwarf mongoose). Like many termites and ant species, naked mole-rats live in permanent subterranean nests that are relatively safe from predators. The nest is expandable and thus able to accommodate individuals recruited in successive generations. Reproductives, in particular the breeding female, rarely engage in risky behaviors, such as colony defense (31), and their overall contribution to the colonies' task schedule is minimal (16, 17). All colony members are dependent on a communal nest for efficient thermoregulation (32), and thus the breeding female is able to closely monitor colony activities and the reproductive status of all colony members. This context has the following two clear advantages: (i) she is capable of parasitizing the foraging efforts of workers that typically return to the nest to consume food, and (ii) she can ensure regular contact with all colony members and thus maintain her monopoly on reproduction through overt physical aggression. Furthermore, she is the only colony member, with the exception of pups, that can solicit caecotrophs from workers when nutritionally stressed (e.g., during pregnancy).
Thus natural selection can favor breeding-female naked mole-rats that specialize more extremely in reproduction through permanent morphological changes. With an increase in the group size of colonies, possibly through continued adverse ecological conditions, the probability that a particular nonbreeding female would fill any within-group breeding vacancy in its lifetime decreases. Thus the evolutionary stage was set for individuals to follow one of two different life history trajectories, worker or breeder, and ultimately for the evolution of completely dimorphic physical castes. Where large group size is adaptive, selection will favor adaptations to enhance the fecundity and hence direct fitness of breeding females (1). Termite, ant, and naked mole-rat “queens” with their enlarged abdomens and long life-spans provide a striking example of convergent evolution in response to such selective pressure. The long potential life-spans (>20 years), with a safe defendable nest and morphological specialization for enhanced fecundity, represents a unique adaptation for maximizing their lifetime reproductive success.
Acknowledgments
We thank Joseph Booysen and Mary Vosloo for help with animal care, and D. Wheeler and three unknown referees for helpful comments on the manuscript. We thank the National Research Foundation and University of Cape Town for funding M.J.O. and J.U.M.J. and the Centre National de la Recherche Scientifique for funding M.J.O. and C.P. We also express our gratitude to the South African Museum for access to x-ray equipment.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wilson E O. The Insect Societies. Cambridge, MA: Harvard Univ. Press; 1971. [Google Scholar]
- 2.Michener C D. The Social Behavior of Bees. Cambridge, MA: Harvard Univ. Press; 1974. [Google Scholar]
- 3.Jarvis J U M, O'Riain M J, Bennett N C, Sherman P W. Trends Ecol Evol. 1994;9:47–51. doi: 10.1016/0169-5347(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 4.Gotwald W H. Army Ants: The Biology of Social Predation. Ithaca: Cornell Univ. Press; 1995. [Google Scholar]
- 5.Noirot C. In: Social Insects: An Evolutionary Approach to Castes and Reproduction. Engels W, editor. Berlin: Springer; 1990. pp. 5–35. [DOI] [PubMed] [Google Scholar]
- 6.Holldobler B, Wilson E O. The Ants. Cambridge, MA: Harvard Univ. Press; 1990. [Google Scholar]
- 7.Jarvis J U M. Science. 1981;212:571–573. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- 8.Clutton-Brock T H, MacColl A, Chadwick P, Gaynor D, Kansky R, Skinner J D. Afr J Ecol. 1999;37:69–80. [Google Scholar]
- 9.Creel S R, Creel N M. Behav Ecol Sociobiol. 1991;28:263–270. [Google Scholar]
- 10.Clutton-Brock T H. Reproductive Success. Chicago: Chicago Univ. Press; 1988. [Google Scholar]
- 11.Sherman P W, Lacey E A, Reeve H K, Keller L. Behav Ecol. 1995;6:102–108. [Google Scholar]
- 12.Crespi B J, Yanega D. Behav Ecol. 1995;6:109–115. [Google Scholar]
- 13.Costa J T, Fitzgerald T D. Trends Ecol Evol. 1996;11:285–288. doi: 10.1016/s0169-5347(96)91656-0. [DOI] [PubMed] [Google Scholar]
- 14.Alexander R D. In: The Biology of the Naked Mole-Rat. Sherman P W, Jarvis J U M, Alexander R D, editors. Princeton: Princeton Univ. Press; 1991. pp. 3–44. [Google Scholar]
- 15.Brett R A. In: The Biology of the Naked Mole-Rat. Sherman P W, Jarvis J U M, Alexander R D, editors. Princeton: Princeton Univ. Press; 1991. pp. 97–136. [Google Scholar]
- 16.Jarvis J U M, O'Riain M J, McDaid E. In: The Biology of the Naked Mole-rat. Sherman P W, Jarvis J U M, Alexander R D, editors. Princeton: Princeton Univ. Press; 1991. pp. 258–383. [Google Scholar]
- 17.Lacey E A, Sherman P W. In: The Biology of the Naked Mole-rat. Sherman P W, Jarvis J U M, Alexander R D, editors. Princeton: Princeton Univ. Press; 1991. pp. 275–336. [Google Scholar]
- 18.Sherman P W, Braude S, Jarvis J U M. J Mammal. 1999;80:720–733. [Google Scholar]
- 19.Faulkes C G, Abbott D H. In: Cooperative Breeding in Mammals. Solomon N G, French J A, editors. New York: Cambridge Univ. Press; 1997. pp. 302–334. [Google Scholar]
- 20.Wheeler D E. Am Nat. 1986;128:13–34. [Google Scholar]
- 21.Hagan H R. American Museum Novitates. 1954;1663:1–8. [Google Scholar]
- 22.Whelden R M. J New York Ento Soc. 1963;71:158–164. [Google Scholar]
- 23.O'Riain M J, Jarvis J U M, Faulkes C G. Nature (London) 1996;380:619–621. doi: 10.1038/380619a0. [DOI] [PubMed] [Google Scholar]
- 24.Prior J C. Endocr Rev. 1990;11:86–94. doi: 10.1210/edrv-11-2-386. [DOI] [PubMed] [Google Scholar]
- 25.Barengolts E I. J Bone Miner Res. 1996;11:1406–1412. doi: 10.1002/jbmr.5650111006. [DOI] [PubMed] [Google Scholar]
- 26.Lustig R H, Hua P, Yu W, Ahmad F J, Baas P W. J Neurosci. 1994;14:3945–3949. doi: 10.1523/JNEUROSCI.14-06-03945.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherman P W, Jarvis J U M, Braude S D. New Sci. 1992;267:72–78. [Google Scholar]
- 28.Jarvis J U M, Bennett N C. Behav Ecol Sociobiol. 1993;33:253–260. [Google Scholar]
- 29.Urison N T, Buffenstein R H. J Therm Biol. 1994;19:365–371. [Google Scholar]
- 30.O'Riain M J, Jarvis J U M. J Zool (London) 1998;246:49–60. [Google Scholar]
- 31.O'Riain M J, Jarvis J U M. Anim Behav. 1997;53:487–498. [Google Scholar]
- 32.Withers P C, Jarvis J U M. Comp Biochem Physiol. 1980;66:215–219. [Google Scholar]