Abstract
The World Health Organization grossly classifies the various types of astrocytomas using a grade system with grade IV gliomas having the worst prognosis. Oncolytic virus therapy is a novel treatment option for GBM patients. Several patents describe various oncolytic viruses used in preclinical and clinical trials to evaluate safety and efficacy. These viruses are natural or genetically engineered from different viruses such as HSV-1, Adenovirus, Reovirus, and New Castle Disease Virus. While several anecdotal studies have indicated therapeutic advantage, recent clinical trials have revealed the safety of their usage, but demonstration of significant efficacy remains to be established. Oncolytic viruses are being redesigned with an interest in combating the tumor microenvironment in addition to defeating the cancerous cells. Several patents describe the inclusion of tumor microenvironment modulating genes within the viral backbone and in particular those which attack the tumor angiotome. The very innovative approaches being used to improve therapeutic efficacy include: design of viruses which can express cytokines to activate a systemic antitumor immune response, inclusion of angiostatic genes to combat tumor vasculature, and also enzymes capable of digesting tumor extra cellular matrix (ECM) to enhance viral spread through solid tumors. As increasingly more novel viruses are being tested and patented, the future battle against glioma looks promising.
Keywords: Patent, glioma, astrocytoma, oncolytic virus, HSV-1, adenovirus, reovirus, new castle disease virus, angiogenesis, immune system, extra cellular matrix
INTRODUCTION
Astrocytomas are broadly classified as either diffusely infiltrating or localized (circumscribed). Localized astrocytomas are of lower grades, as classified by the World Health Organization (WHO), and have a potential of cure following surgical resection. These include pilocytic astrocytoma (WHO grade I), subependymal giant cell astrocytoma (WHO grade I) and pleomorphic xanthoastrocytoma (WHO grade I). The higher grade infiltrating astrocytomas (grades II, III and IV) are more biologically aggressive and resistant to therapy. Diffusely infiltrating astrocytomas are intrinsically invasive tumors with unfavorable prognosis and include diffuse astrocytoma (WHO grade II), characterized by the presence of cytologic atypia, anaplastic astrocytoma (WHO grade III), which have cytologic atypia and the presence of mitotic activity, and glioblastoma multiforme (GBM) (WHO grade IV), which show presence of tumor cell necrosis and/or micro vascular proliferation (angiogenesis), in addition to cytologic atypia and mitotic activity. Apart from the tumor grade, there are other prognostic factors affecting patient survival including age at diagnosis, tumor location, and extent of tumor resection. However, regardless of these variables, overall prognosis of astrocytomas remains dismal. While patients diagnosed with grade II tumors usually survive more than 5 years, those with grade III tumors do not survive beyond 2–3 years. And the outlook for patients suffering from GBM is the worst with a median survival of about 14 months with radiation and chemotherapy [1].
GBM can develop as a primary tumor (de novo) or by progression from a lower grade astrocytoma (secondary GBM). It is now known that these two broad subtypes of GBM are genetically unique and distinct disease processes make them different in their genesis. While the primary GBM is remarkable for both loss of heterozygosity (LOH) on chromosome arm 10q (70%) and EGFR amplification (36%), the secondary GBM most frequently demonstrates mutation of the TP53 tumor suppressor gene, which is already present in 60% of the precursor lower grade astrocytomas [2]. Given the poor prognosis of this disease, there is a desperate need for novel methods of intervention. The use of oncolytic viruses is one such biological therapy that is being investigated as a treatment option for malignant glioma. In this review, we will discuss some of the patents describing oncolytic viruses currently being investigated in clinical and preclinical studies.
Oncolytic virus (OV) therapy is based on the concept of using live viruses to selectively infect and replicate in cancer cells, with minimal destruction of non-neoplastic tissue. While the concept of using live viruses to infect and destroy tumors dates back to nearly over a century, advances in molecular biology and virology have accelerated the development of OVs in the last two decades [3]. Conditionally replication competent viruses are genetically engineered or selected to be avirulent in normal cells but can exploit the aberrant molecular/genetic pathways in tumors resulting in viral replication and cancer cell lytic destruction. This leads to their efficient replication within cancer cells and the lytic destruction of the infected malignant cell. Oncolytic viruses derived from Herpes Simplex Virus-1 (HSV-1), Adenovirus (Ad), New Castle Disease Virus(NDV), and Reovirus (RV) have been tested in several clinical trials for the treatment of malignant glioma. In all of these trials intratumoral administration of infectious oncolytic virus particles was found to be safe but significant evidence of efficacy remains to be established Table 1. Several innovative strategies to enhance intratumoral viral spread and antitumor efficacy without compromising its safety are currently under investigation. These studies have resulted in the development of several patents which may contribute towards the advancement in future therapeutics.
Table 1.
List of Clinical Trials Treating Glioma Patients with Oncolytic Viruses
| Oncolyti c virus | Virus | Virus Type | Patent Number | Genetic Alteration | Year Published | Patients M/F (Total) | Adverse events | Mode of delivery | Highest dose administered | Tumor type (n) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G207 | HSV-1 (Strain F) | Double stranded DNA virus | WO000007 | Deleted for both the copies of γ34.5 gene and disrupted ICP6/RR. | 2000 | 15/6(21) | None | I.T | 1 × 109 p.f.u. | GBM(16) AA(5) | [5] |
| G207 | HSV-1 (Strain F) | Double stranded DNA virus | WO000007 | Deleted for both the copies of γ34.5 gene and disrupted ICP6/RR. | (2008) | 6 | None± | I.T, and I.A.B: Post tumor resection (2 doses) | 1.15 × 109 | GBM | (Markert et al., In Press). |
| 1716 | HSV-1 (Glasgow strain 17) | Double stranded DNA virus | WO03068809 | Deleted for both the copies of γ34.5 gene | 2000 | 7/2(9) | None | I.T | 1 × 105 p.f.u. | GBM(8) AA(1) | [6] |
| 1716 | HSV-1 (Glasgow strain 17) | Double stranded DNA virus | WO03068809 | Deleted for both the copies of γ34.5 gene | 2002 | 7/5(12) | None | I.T: 4–9 days prior to resection | 1 × 105 p.f.u. | GBM(8) AA(1) | [7] |
| 1716 | HSV-1 (Glasgow strain 17) | Double stranded DNA virus | WO03068809 | Deleted for both the copies of γ34.5 gene | 2004 | 10/2(12) | None | I.A.B: Post tumor resection | 1 × 105 p.f.u. | GBM(10) AA(1) AO(1) | [8] |
| ONYX-015 | Adenovirus | Double stranded DNA virus | US5677178 | Deleted in its E1B gene | 2004 | 17/7(24) | None | I.A.B: Post tumor resection | 1 × 1010 p.f.u. | GBM(17) AA(5) Grade 2(2) | [9] |
| NDV-HuJ | New Castle Disease virus | Single stranded RNA virus | WO05113013 | None | 2005 | 9/5(14) | None* | I.V. | Upto 55 BIU | GBM | [10] |
| Reolysin | Reovirus | Double stranded RNA virus | WO00050051 | None | 2008 | 7/5(12) | None | I.T. | 1 × 109 p.f.u. | GBM(7) AA(3) AO(1) OA grade II (1) | [11] |
Legend: GBM: Glioblastoma Multiforme.AA: Anaplastic astrocytoma; I.A.B.: Injected into adjacent brain; I.T: Intra tumoral; I.V: intravenous; p.f.u: Plaque forming units; B.I.U: Billion infectious units; AO: Anaplastic oligodendroglioma; OA: Oligo astrocytoma.
One Patient had fever, headache vomiting and anorexia which was considered to be possibly related to administered virus
One Patient had transient fever, delirium, and hemiparesis after second dose of OV, which entirely resolved on high dose dexamethasone.
HERPES SIMPLEX VIRUS-1 (HSV-1) DERIVED ONCOLYTIC VIRUSES
HSV-1 is an enveloped, double-stranded DNA virus containing a large, well characterized, fully sequenced genome of about 152kb of DNA that encodes more than 80 genes. While the large size makes genetic manipulations cumbersome, it also provides ample opportunities to remove genes that are not essential for replication (estimated to be about 30kb) [4]. Removal of these genes allows for the insertion of therapeutic transgenes within the viral backbone. The ability of HSV-1 to remain as an episome avoids the possibility of any insertional mutagenesis of the infected cell, and the easy availability of antiherpetic drugs in order to keep viral replication in check makes it a very desirable vector for therapeutic applications. Taken together, these qualities of HSV-1 have led to the development of several oncolytic HSV-1 patents for the treatment of CNS tumors.
SINGLY ATTENUATED HSV-1 DERIVED ONCOLYTIC VIRUSES
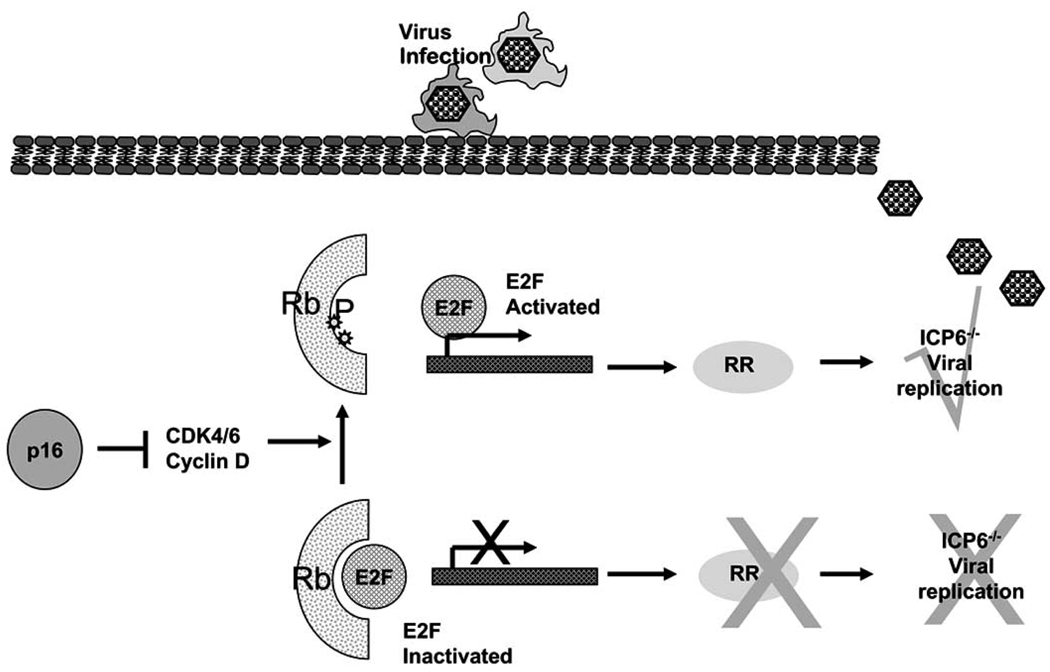
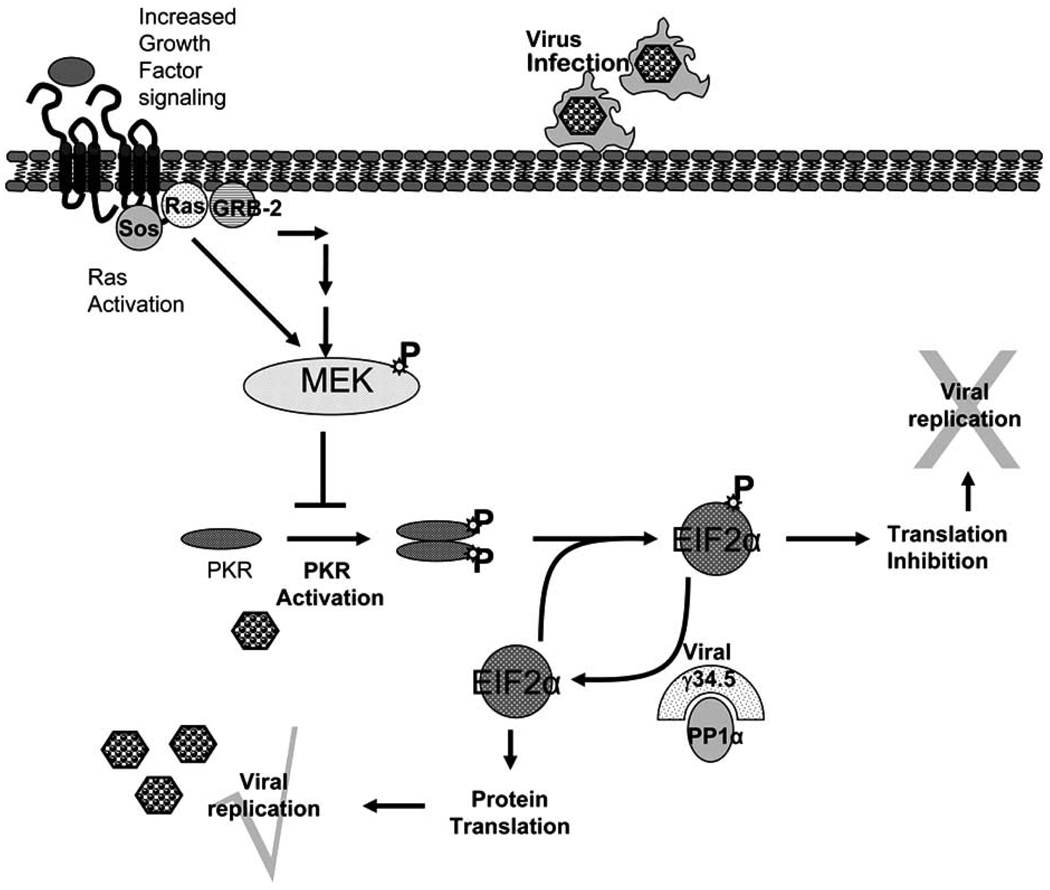
HSV-1 viruses mutated in the viral genes ICP6 and/or RL-1 have been tested in human patients Table 1. ICP6 encodes for the viral counterpart of mammalian ribonucleotide reductase (RR) which is required for the de novo synthesis of deoxyribonucleotides, essential for viral DNA synthesis and replication. A deficiency in ICP6 would make the virus replication competent only in mitotic cells which express the mammalian counterpart of the enzyme. This enzyme is normally not expressed in non-replicating cells, and hence viruses deficient in ICP6 gene would be replication incompetent in normal non cycling cells Fig. (1). To test this hypothesis, a mutant virus, deficient for the large subunit of RR (ICP6/RR), was created by the insertion of an Eschericia coli LacZ gene within the UL39 gene locus [5]. Cytopathic and drug sensitivity assays using this attenuated virus (hrR3) revealed not only efficient cancer cell killing in vitro and but also similarly effective tumor killing in animal models of tumorigenesis [13, 14]. More recent evidence also indicates that the RR mutant HSV-1 may be “molecularly targeted” to replicate more efficiently in cells with specific oncogenic mutations such as homozygous deletion of the p16 gene [15]. HSV-1 ICP34.5 (RL-1) was also identified to be a neurovirulence gene as attenuation of both copies of this gene led to a reduction in neurovirulence associated with HSV-1 in vitro and in vivo [16, 17]. Upon viral infection, PKR becomes rapidly activated and it phosphorylates the α-subunit of eIF-2α leading to a total shutoff of host protein synthesis. This response is overcome, however, by the HSV-1 gene RL-1/γ34.5. This gene encodes for the ICP34.5 protein which, when expressed, leads to the dephosphorylation of the α-subunit of eIF-2α and disinhibition of protein synthesis Fig. (2). Deletion of the viral RL-1 gene therefore makes HSV-1 unable to replicate in normal cells. However, proliferating cancer cells often have an activated Ras/Mitogen activated protein kinase kinase (MEK) pathway rendering the cells deficient in the antiviral PKR response [18]. Hence, an HSV-1 deficient in the viral γ34.5 gene can selectively replicate in Ras/MEK activated proliferating cancer cells. The concept of targeting such attenuated viruses for cellular proliferative disorders with an activated Ras pathways was patented in 2001 [19]. This mutant virus retained the ability to replicate in actively dividing mouse embryo cells but not in confluent mouse embryo cultures [17].
Fig. 1.
The p16 pathway blocks the CDK4-CDK6-cyclin-D complex which increases the phosphorylation state of RB, causing it to release the transcription factor E2F. Free E2F mediates the transcription of several cellular genes that are involved in G1/S progression, including ribonucleotide reductase (RR). In normal non cycling cells there is no cellular RR hence Herpes simplex virus (HSV-1) strains with mutations in viral ICP6 gene (encoding for viral counterpart of cellular RR), can not promote DNA synthesis and viral replication. Loss of p16 or RB function frequently found in neoplastic cells dys-regulates E2F activity, and leads to increased production of cellular proteins such as RR. This is exploited by HSV-1 defective in ICP6 to replicate in cancer cells.
Fig. 2.
Viral infection of cells initiates the homo-dimerization and subsequent phosphorylation and activation of interferon-induced, double-stranded RNA-activated protein kinase (PKR). Activated PKR phosphorylates its natural substrate, the alpha subunit of eukaryotic protein synthesis initiation factor-2 (EIF2-alpha), leading to the inhibition of cellular protein synthesis. HSV-1 encodes for γ34.5, a viral protein that mediated protein phosphatase 1α to dephosphorylate EIF2-alpha, releasing the translational block initiated in normal cells upon infection. In the absence of viral γ34.5 gene the viral replication is blocked by cellular responses in normal cells. Cancer cells with an activated RAS/MEK pathway suppress PKR autophosphorylation, hence allowing γ34.5−/− virus to replicate in them.
Consistent with these findings, an RL-1 null HSV-1 mutant (1716), which fails to produce ICP34.5 is able to efficiently replicate in and cause subsequent cell death of a majority of glioma cell lines and primary tumor derived cells [20]. WIPO patent WO2007087580 jointly issued to Kerrington, Mezhir, Weichselbaum, and Roizman discloses a γ34.5 deficient HSV-1 strain for the treatment of proliferative disorders [21]. Another neuron-attenuated HSV-1 ICP34.5 mutant (variant-1716) was found to have significant antitumor efficacy in vivo when administered to mice bearing experimental brain tumors [22, 23]. Pathological examination of mice injected intracranially with 1716, revealed a finite, self limiting host response to 1716, highlighting the potential of using it for therapeutic purposes. This virus (strain 1716) is covered by a worldwide patent applied by Crusade laboratories, Glasgow, UK [24].
Based on its safety profile in animals, the mutated HSV1716 was tested in the UK for use in human patients with malignant brain tumors [6]. This clinical study represented the first foray of inoculating mutated oncolytic HSV vectors into the brains of human patients with malignant brain tumors, and for concerns of safety, only a very moderate dosage of the virus was permitted to be tested. In this study, nine patients with malignant glioma were treated with escalating doses of HSV1716 to a maximum of 105 p.f.u. by direct intra-tumoral injection. Patients were closely monitored for any signs of treatment induced toxicity. No adverse clinical events which could be attributed to the administration of 1716 were observed. These was no evidence of encephalitis, viral shedding, or reactivation of endogenous latent virus in any patient [6]. Five of these nine patients underwent a subsequent tumor progression and biopsy samples from these patients showed no signs of 1716 or wild type HSV by PCR analysis. In a subsequent report by the investigators, it was noted that two of these nine patients were alive and stable at four years and four months and three years and seven months, respectively, after treatment [25]. Two more clinical trials were initiated to test the therapeutic efficacy of treating patients with malignant glioma by HSV mutant 1716. In one of the clinical trials, evidence of viral replication in human high grade glioma was examined in twelve patients with high grade malignant glioma after receiving 105 p.f.u. of HSV mutant 1716 by direct intratumoral stereotactic injection 4 to 9 days prior to tumor resection. The tissue was examined for presence of HSV DNA by PCR, southern blotting, and immunohistochemistry and for the presence of infectious viral particles by plaque assay. The results confirmed the presence of HSV in tumor tissue 4 to 9 days after treatment without any obvious signs of toxicity [7].
In the third study to test the therapeutic efficacy of the HSV mutant 1716 in human glioma patients, a total of twelve patients diagnosed with high grade recurrent (6 patients) or newly diagnosed (6 patients) glioma underwent gross tumor resection [8]. After the initial tumor debulking, 105 p.f.u. of HSV mutant 1716 was injected into 8–10 sites within the tissue surrounding tumor cavity. Despite the lack of toxicity seen in the previous trials, due to paramount safety concerns, the therapeutic dose given to the patients was not increased as would have been done for clinical trials with a chemotherapeutic agent. However, the lack of any virus-related toxicity in this study considerably strengthened the safety profile of this approach as the virus had been injected into tissue involving the functioning brain. The expected median survival for patients with GBM is one year from the time of initial diagnosis and only 3–6 months following diagnosis of a recurrent tumor. In this study, one patient with a recurrent tumor and two patients diagnosed with primary GBM survived for more than 22, 18, and 15 months, respectively. A causal relationship, however, between response and viral treatment could not be established.
This study demonstrated that HSV1716 can be injected into the normal brain of individuals without any ensuing toxicity. However since the maximum tolerated doses were not achieved in this trial, the safety of this virus at these doses may simply result the inadequacy of current technology to reach high enough doses.
HSV1716 is currently being pursued by Crusade Laboratories, Glasgow, UK. The company has been granted orphan drug status in Europe for “the use of HSV mutant 1716 in the treatment of glioma” and has received regulatory approvals to initiate a Phase III trial for glioma patients [22]. For this trial, glioma patients with a first recurrence after surgery will be randomized into one of two treatment arms: HSV1716 or conventional chemotherapy. Satisfactory results in this trial could lead to a license and marketing authorization in glioma research.
DOUBLY ATTENUATED HSV-1 DERIVED ONCOLYTIC VIRUSES
In order to minimize the chances of an oncolytic HSV-1 reverting back to a wild type virus G207, a doubly attenuated oncolytic HSV-1 that has deletions at both γ34.5 (RL1) loci as well as an in-frame, gene disrupting insertion of the E. coli β-galactosidase gene within the ICP6 gene, was created. Intraneoplastic administration of the virus resulted in slower tumor growth and/or prolonged survival [27, 28]. Martuza, Rabkin and Mineta jointly hold a patent describing G207 for the treatment of neoplastic disease [28]. Furthermore, G207 maintained the attenuated neurovirulence, temperature sensitivity, as well as ganciclovir hypersensitivity [27], and the insertion of β-galactosidase permitted easy detection of cells harboring the virus in infected tissue. Preclinical testing of this virus was performed in nude mice harboring subcutaneous or intracerebral gliomas. Preclinical toxicity studies of G207 were performed by direct intracerebral inoculation of mice and HSV-sensitive non-human primates revealing it to be safe [29]. Even after two years of inoculation of G207 in new world owl monkeys (Aotus nancymae), no infectious viral particles were recovered and viral DNA was found to be restricted to the brain. Absence of the viral DNA in tears, saliva, and vaginal secretions indicated a lack of viral shedding suggesting that strict biohazard management of G207 patients may not be required.
The safety in intracerebral inoculation of G207 in humans was determined by a dose escalation study with patients suffering from recurrent malignant glioma [5]. The protocol was approved by the Recombinant DNA Advisory Committee of the National Institute of Health and Food and Drug Administration. The trial was initiated with the administration of 106 p.f.u. of G207, into the brains of three patients post tumor resection. After 28 days of observation to confirm lack of acute toxicity three patients were then recruited into the next six cohorts and were treated with the next higher dose of G207 (doses of each cohort were 107, 3 × 107, 108, 3 × 108, 109, and 3 × 109 p.f.u. respectively). The three patients in the last cohort were treated with G207 inoculated into five sites after surgical resection of the brain tumor, while all other patients received the virus at a single locus post resection [5]. No toxicity which could be directly attributed to the infectious virus was noted in this study, suggesting that G207 can be safely inoculated into human brain tumors up to doses of 109 p.f.u. without any adverse events. This agent is currently being investigated for efficacy in clinical trials.
CONDITIONALLY REPLICATION COMPETENT ADENOVIRUS
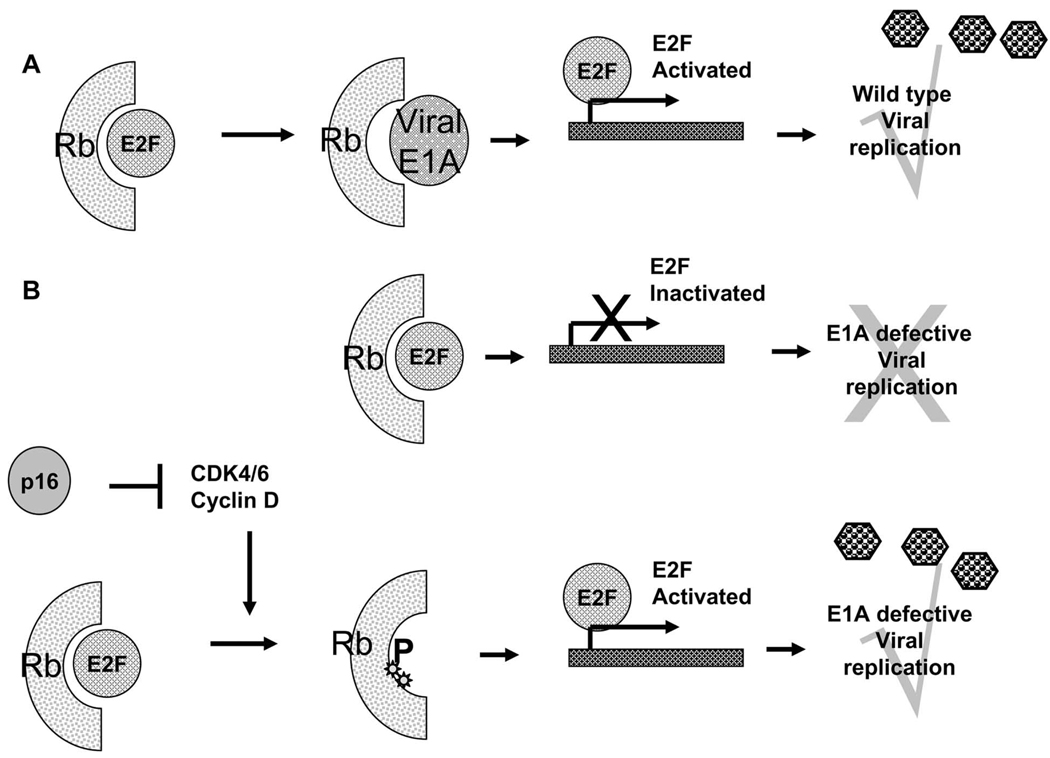
Adenovirus (Ad) is a non-enveloped virus containing a 36 kb double stranded linear genome. Based on temporal pattern of expression, its genome is divided into early functions and late functions. Early function genes encode for E1A, E1B, E2, E3, and E4 and are expressed early during viral replication whereas late function (L1-5) genes are expressed after the commencement of viral replication and encode for capsid proteins. The early viral genes encode for proteins that antagonize host anti viral defense mechanisms. The viral E1A protein provokes host cell induction of S phase by binding and inhibiting retinoblastoma protein (Rb). Rb binds to cellular E2F transcriptional factor and inhibits E2F dependent transcription arresting cell growth. Expression of viral E1A overcomes this arrest and permits entry into S phase, which is conducive for viral DNA replication Fig. (3). However E1A induced entry into S phase can also trigger cellular apoptosis by activation of tumor suppressor p53. Adeno-viral proteins E1B 55K, binds to and blocks p53 preventing premature host cell “suicide” and ensuring efficient viral replication [30]. Based on these observations ONYX-015 (dl1520) a conditionally replication competent adenovirus with a deletion in its 55kDa E1B gene was created. This virus was found to be replication competent only in neoplastic cells, and the reason for this was initially attributed to defective p53 pathways in most cancer cells [31]. However later studies have shown that E1B can also interfere with shut-off of host mRNA nuclear export and protein synthesis as well as induce nuclear localization of transcription factor YB to facilitate E2 late gene activation [32].
Fig. 3.
A: Wild type Adenovirus encodes for viral E1A protein that can bind to and inactivate cellular pRb protein. Inactivation of Rb releases E2F transcriptional factor driving the host cell into a proliferative phase, facilitating viral replication. B; Adenoviruses with a mutant E1A, can not inactivate cellular Rb, and can hence replicate only in cells with a mutant p16/pRb pathway.
ONYX Pharmaceuticals holds the patent for an E1B deleted oncolytic adenovirus which covers methods for treating cancer using such a cancer specific replicating adenovirus [33]. This virus was tested for safety in a dose escalation study by the New Approaches to Brain Tumor Therapy (NABTT) consortium with patients suffering from malignant glioma. Cohorts of six patients were treated with ONYX-015 from 107 p.f.u. to 1010 p.f.u. into a total of 10 sites within the resected tumor cavity. No adverse events during the patient follow up could be attributed to the virus. Among the 24 patients treated with ONYX-015, one died of a non-tumor related event and three were alive at the time of the published report [9]. An almost identical virus, H101, has been tested for efficacy in China in patients with head and neck cancer. China’s Sunway Biotech Company repor-ted a 79% response rate for H101 treatment in conjunction with chemotherapy, compared to 40% for chemotherapy alone in a phase III clinical trial. Based on these results H101 is currently the world’s only approved oncolytic virus (approved by China’s State Food and Drug Administration (SFDA)) and is marketed in China. Sunway Biotech plans to apply for testing H101 in Europe and the US.
An oncolytic adenovirus with a 24 base pair deletion in its E1A gene has been created and tested for its oncolytic efficacy. Deletion of these 24 bp within the E1A, renders the virus unable to bind to Rb, and block antiviral host defense reaction Fig. (3). This virus could efficiently replicate in glioma cells with a mutant Rb pathway, but not in normal or cancer cells with a restored Rb activity [34]. Further treatment of mice bearing subcutaneous glioma resulted in a significant reduction in tumor growth. Glioma specific tropism of this virus was further enhanced by the incorporation of an integrin targeting RGD-4C peptide motif into the adenoviral fiber [35]. This modification significantly increased the anti-glioma efficacy of Ad-Δ-24 RGD against human glioma cell lines and against brain tumor stem cells [36]. This virus is currently being tested for safety, and biological activity when administered intraperitoneally to patients with recurrent ovarian adenocarcinoma. A Phase I clinical trial to test the safety of this virus in human glioma patients is currently being planned. The accumulation of high levels of E1A in infected human cells results in an abnormal shift into S phase. Despite the enhanced infectivity and antiglioma efficacy there have been concerns about toxicity related to high levels of E1A in normal cells infected with this oncolytic virus. Transcriptional targeting of the mutant E1A by exploiting tumor specific transcriptional factors has resulted in the generation of ICOVIR-5, an oncolytic adenovirus with integrin targeted tropism and delta 24E1A under the control of E2F promotor. This virus has shown significant antiglioma efficacy in mouse xenograft models and future studies will reveal the safety of this virus in human patients [37, 38].
ONCOLYTIC VIRAL THERAPY USING REOVIRUS
Reovirus is a double-stranded RNA virus, which can efficiently infect most mammalian cells. However, despite its ability to infect human cells efficiently, cellular antiviral defense responses effectively control viral replication so that in most cases the infection is asymptomatic, and without any associated pathology [39]. Activated Ras signaling in neoplastic cells counters the cellular antiviral defense permitting reovirus to efficiently replicate in proliferating cells with an activated Ras pathway Fig. (2) [40]. Based on these in vitro observations, its administration as an antineoplastic agent was tested in several animal models of brain tumors, and the results revealed its antineoplastic efficacy. The inventors Lee, Strong, and Coffey, jointly hold a patent describing the use of Reovirus as a therapeutic agent for the treatment of cancer [41].
Safety studies with Reovirus include its intracerebral administration into the brains of non-human primates and the safety profile of intracerebral administration in human patients suffering from malignant glioma was recently completed in a dose-escalating phase I trial in Canada [41]. Twelve patients (7 men/5 women) with recurrent malignant glioma were treated with escalating doses of Reovirus up to 109 tissue culture infectious dose 50 (TCID50). No adverse events that could be attributed directly to the administered virus were observed. Bodily fluids from the patients were carefully monitored for evidence of viral shedding. Evidence of viral DNA/RNA was found in the saliva of one patient and in the feces of two patients after Reovirus administration. None of the patients demonstrated clinical evidence of encephalitis and there was no dose limiting toxicity observed. This study demonstrated the feasibility of using a live replication competent genetically modified Reovirus to treat malignant glioma. A second study using Reovirus delivered by intratumoral infusion over a period of 72 hours has been initiated in USA. This study will be able to administer 10 times more virus to the tumor and may also enhance viral dissemination through the tumor.
NEWCASTLE DISEASE VIRUS DERIVED ONCOLYTIC VIRUS
New Castle Disease Virus (NDV) is a single-stranded RNA virus that is contagious to birds causing fatal sickness in most avian species. In humans it is associated with minimal pathology comprised of mild conjunctivitis and laryngitis. The ability of NDV to directly replicate in and kill a variety of cultured human and rat neuroblastoma cells without effect in normal fibroblasts has been previously described [42]. This selective killing of human neuroblastoma (IMR-32) cells was maintained during the in vivo treatment of established tumors in athymic nude mice [43]. The strains HuJ and MTH-68 of NDV have been patented for their use as antineoplastic agents [44, 45]. Testing of the NDV strain MTH68 in patients with high grade glioma commenced in 1996 [46–48]. Csatary et al reported in 2004 that a total of 14 patients with high grade glioma had been treated [46]. This study enrolled both pediatric and adult patients suffering from high grade glioma, and anecdotal evidence of efficacy in some patients was noted. Elsewhere a case report of a fourteen year old patient suffering from recurrent GBM was treated by intravenous administration of NDV in 1996. The last published report of this patient indicated that he was taking only NDV vaccine treatment and was living a relatively normal life [46]. The NDV strain HuJ was tested for safety and efficacy in a phase I/II trial [10]. Fourteen patients were enrolled in this dose-escalation study. Intravenous administration of NDV was well tolerated and MTD was not reached in this study. A complete response was noted in one patient.
All of the clinical trials that have tested efficacy of the various oncolytic viruses in human patients with malignant glioma have revealed the safety of this approach, however it must be noted that these clinical trials have been unable to establish a maximum tolerated dose (MTD) and hence are in a sense incomplete. Future clinical trials testing preparations of more concentrated/potent viruses will lead to the definition of MTD in human patients. The use of doses closer to the MTD in human patients will provide a more clear view of any toxicity which may be attributed to the delivery of the virus.
ONCOLYTIC MEASLES VIRUS
Measles virus is a negative strand RNA paramyxovirus. Case reports of measles virus infection in African children suffering from hematologic malignancies indicated towards an association between measles virus infection and spontaneous tumor regression [49]. The oncolytic effect of measles virus infection is a result of cell-cell fusion mediated by viral F protein leading to the formation of synctia or multinucleated giant cell aggregates which undergo apoptotic cell death [50]. Glioma specific cell killing achieved by this virus is attributed in part to the over expression of CD46 in tumor cells compared to normal brain.
An attenuated strain of the Edmonston’s (MV-Edm) measles virus that has been used in vaccination studies has been the choice vector for these studies. This MV-Edm strain was engineered to express secreted carcinoembryonic antigen (CEA), a secreted protein, whose presence in serum could be used to evaluate the presence of replicating virus in tumor tissue [51]. The anti-tumor efficacy of the MV-Edm expressing CEA (MV-CEA) was evaluated in multiple glioma cell lines in vitro and against subcutaneous and intracranial glioma tumors in mice. There was a statistically significant reduction in tumor growth in subcutaneous tumors and a statistically significant prolongation of survival of mice treated with MV-CEA compared to untreated mice or mice treated with UV inactivated virus [51]. Furthermore, no clinical neurotoxicity and no significant pathology was observed after intracranial administration of MV-CEA in transgenic mice susceptible to MV infection [51]. A detailed toxicology study of repeat intracerebral administration of MV-CEA in rhesus macaques was carried out to test the safety of intracranial delivery of this virus prior to testing in human glioma patients [52]. Two different doses of MV-CEA (2 × 105 or 2 × 106) were injected into the frontal lobes of rhesus macaques (n=2/group), and the animals were closely followed for any changes in body weight, temperature changes, complete blood count, CEA levels, clinical chemistries, coagulation, complement levels, immunoglobulin, measles antibody titers, viremia and shedding (buccal swabs). No evidence of clinical or biochemical toxicity, including lack of neurological symptoms was observed up to 36 months after treatment [52]. On the basis of its anti tumor efficacy observed in animal models combined with the safety profile established in primates a Phase I clinical trial was initiated in 2006. The study is a dose escalation study, wherein cohorts of 1–6 patients will receive escalating doses of MV-CEA until a maximum tolerated dose (MTD) has been established. Once the MTD in group 1 has been determined, subsequent, patients will be assigned to a group 2, wherein they will undergo stereotactic biopsy (to confirm the diagnosis), followed by MV-CEA administration into the tumor. Five days post treatment patients undergo en block resection of their tumor followed by MV-CEA administered around the tumor bed. The study is currently ongoing, and will investigate safety of this approach as a therapeutic strategy for glioma patients.
ONCOLYTIC VESICULAR STOMATITIS VIRUS
Vesicular Stomatitis virus (VSV) is a negative stranded RNA virus. VSV replication is severely attenuated in normal cells, but in malignant cells the virus is able to selectively replicate resulting in efficient oncolysis [53]. VSV has been found to replicate efficiently in rat C6 glioma cells in vitro and could efficiently inhibit tumor growth in vivo [54]. The cancer cell specificity of VSV has been attributed to cancer cell defects in the functionality of the IFN/PKR pathway. VSV mutants with defects in their ability to shut down innate immunity have been investigated as potential anti-cancer therapeutics [55, 56]. VSV viral M protein blocks the nuclear to cytoplasmic transport of IFN beta mRNA and helps circumvent cellular antiviral responses. VSV mutant with a deletion of a single amino acid (methionine 51) in M protein (VSVΔM51) has been shown to be able to replicate only in cancer cells defective in antiviral IFN response and its replications is severely attenuated in normal cells. This virus shows potent anti glioma effect against multifocal invasive glioma even when administered systemically [57, 58]. While the antiglioma efficacy of such oncolytic VSV has been established in preclinical animal models of glioma future clinical trials will test the safety and efficacy of VSV derived oncolytic viruses in human patients.
ONCOLYTIC VACCINIA VIRUS
Vaccinia virus (VV) is a member of the poxviridae family of viruses, and has been studied in preclinical animal models as an anti-glioma therapy [59]. Recombinant VV expressing wild type p53 (rVV-p53) was found to efficiently infect and cause apoptosis in a panel of human glioma cell lines in vitro [60, 61]. Treatment of radiation resistant rat C6 glioma with rVV-p53 resulted in a much slower tumor growth than with either agent alone indicating the potential of combining it with radiation [62]. Vaccinia virus mediated cytokine gene delivery alone or in combination with rVV-p53 has also shown anti glioma efficacy against established gliomas in mice [63, 64]. Recombinant oncolytic VV with targeted deletions in multiple viral genes have been recently described. This virus shows significant anti-tumor activity against multiple tumor models [65] and future studies will uncover if it also has an anti-glioma effect.
As oncolytic viruses derived from different strains of viruses are being developed and tested in clinical trials, there is a considerable interest in understanding the impact of the tumor microenvironment on oncolytic ability of the virus. Recent studies have resulted in several patents which modulate the tumor microenvironment in conjunction with viral treatment to improve therapeutic outcome. We will describe some of the recent advances describing viruses that modulate angiogenesis, host immune responses and tumor extra cellular matrix to enhance viral spread.
PATENTS RELEVANT TO COMBINING ANGIOSTATIC STRATEGIES AND ONCOLYTIC THERAPY
The impact of OV treatment on tumoral vasculature has been the focus of several studies. The ability of OV to infect and replicate in proliferating endothelial cells, leading to their lytic destruction has been previously reported [66]. Consistent with these reports, treatment of human ovarian carcinoma and malignant peripheral nerve sheath tumors grown as xenografts in athymic nude mice with oncolytic HSV mutants (G207, hrR3, and 1716) resulted in infection of tumor vasculature, and an antiangiogenic effect [67, 68]. While these studies demonstrated the immediate direct antiangiogenic effect of OV, two recent studies have revealed increased angiogenesis of the residual tumor that grows after oncolysis. Aghi et al. found a significant decrease in the production of antiangiogenic proteins TSP-1 and TSP-2 in glioma xenografts treated with G207 which resulted in an increased micro vessel density in tumors [69]. Further treatment of glioma bearing mice with a combination therapy regimen of G207 and 3TSR (a peptide containing the TSP-1 region) significantly reduced tumor micro vessel density and enhanced antitumor efficacy compared to G207 alone. More recently, Kurozumi et al. have reported induction of the integrin activating and angiogenic protein Cyr61 in gliomas treated with oncolytic HSV mutant viruses [70]. The authors tested the impact of Cilengitide (cRGD), an antagonist of Cyr61-mediated integrin activation, on OV treatment. Angioreactors filled with glioma cells and then treated with the OV in the presence of Cilengitide had significantly reduced angiogenesis as compared to angioreactors filled with OV alone. Together, these studies indicate that while treatment with OV has an immediate antiangiogenic effect, OV induced changes in the tumor angiotome permit re-growth of tumor vasculature in the residual tumor after viral clearance. We have recently created a recombinant OV (HSVQvasculo) expressing Vasculostatin within the γ34.5 deleted and ICP6 disrupted backbone of HSV-1 (strain F). Vasculostatin contains five thrombospondin type 1 repeats and an integrin antagonizing RGD motif, and expression of this protein should antagonize the increased expression of integrin activating Cyr61 and decreased expression of TSP-1 protein accompanying oncolysis [71]. HSVQvasculo showed a significant increase in antiglioma therapeutic efficacy compared to the control HSVQ virus in both subcutaneous and intracranial tumors established in athymic nude mice (Hardcastle, Kaur, unpublished data). These studies underscore the potential therapeutic advantage of combining OV treatment with antiangiogenic agents in vivo. The apparent effect of oncolytic viral therapy on angiogenesis as well as the innate role angiogenesis plays in the tumor microenvironment has lent to the interest in combating both tumor cells and endothelial cells. The concept of combining at least one oncolytic virus and at least one antiangiogenic agent for use in tumor therapy has been patented [72].
Combination treatment of an oncolytic adenovirus with an antiangiogenic agent has been observed to be more effective at treatment of colon cancer than either agent alone [73]. Combination of vKH6 with RAD001 (an angiogenic inhibitor) resulted in enhanced anti-tumor efficacy possibly by substantially delaying the re-growth of the tumor while still allowing the virus to spread within it. Future studies will investigate if this will also extend itself for the treatment of glioma. In a more recent study, pretreatment of gliomas with a single dose of an antiangiogenic agent prior to treatment with an oncolytic HSV-1 derived virus also resulted in an enhanced anti-tumor effect of the OV [70].
While the concept of combining various antiangiogenic agents with OV remains very exciting, careful preclinical studies are needed to identify dosing schedules and mechanisms to achieve maximal synergy. The significance of drug interactions with OV and a careful study of dosing schedule has been made more significant by recent observations wherein the combination therapy including OV and Bevacizumab, an anti-vascular endothelial growth factor (VEGF) antibody, did not significantly increase survival compared to animals treated with OV alone [74]. Nevertheless the concept of choking a tumor by attacking its vasculature in conjunction with oncolytic cancer cell killing has been exploited in the design of several novel oncolytic viruses armed with genes encoding for angiostatic factors. This has led to the development of several patented “dually armed viruses” which can kill cancer cells by oncolysis as well as deliver a transgene with potent antiangiogenic effects. For example, the regulatory cytokine IL-12 has been inserted into an oncolytic HSV virus, NV1023, to create an OV expressing IL-12 (NV1042) [75, 76]. Similarly, Ye et al. patented the delivery of the E1A gene by a recombinant oncolytic adenovirus (rAD-E1A) [77]. Treatment of experimental subcutaneous tumors with rAD-E1A oncolytic virus resulted in a significant reduction of tumor growth compared to control. It was determined that the inhibition of tumor growth could be partly attributed to the reduction in vascular density by rAD-E1 A.
Another oncolytic virus exploiting the use of antiangiogenic gene therapy in conjunction with OV mediated tumor destruction integrates the expression of Platelet Factor 4 (PF4) within the backbone of the oncolytic HSV G47Δ [78]. The resulting OV, bG47Δ-PF4, was found to significantly delay glioma growth compared to control. Further investigation of the antiangiogenic effects of PF4 revealed a reduced number of vasculature structures in harvested tumor tissue compared to control [79].
The incorporation of angiostatic factors such as angiostatin and endostatin into oncolytic viruses has also been tested leading to several patents [80]. Inclusion of a secreted endostatin or fusion endostatin-angiostatin protein within an attenuated HSV-1 backbone was found to have potent antitumor and antiangiogenic effects in colon carcinoma and lung cancer tumors in mice [81, 82]. Future studies will test the efficacy of these recombinant viruses against gliomas in vivo. Yoo et al. sought to exploit the angiostatic effect of VEGF signaling interference by constructing an adenovirus expressing a short hairpin RNA expression system against VEGF [83]. The conditionally replication competent Ad-ΔB7-shVEGF and the replication incompetent Ad-ΔE1-shVEGF were constructed and compared for their antiangiogenic efficacy. The viruses were found to successfully inhibit VEGF expression thereby enhancing the anti glioma efficacy of oncolytic adenoviral therapy through an antiangiogenic mechanism. The idea to use small interfering RNAs specific for VEGF was patented in 2006 [84]. Preclinical testing of combination oncolytic viral therapy with angiostatic gene delivery to combat both tumor cells and endothelial cells by way of an antiangiogenic mechanism has shown much promise in the field of cancer research.
PATENTS RELEVANT TO HOST IMMUNE RESPONSES TO OV THERAPY
As the prospects of moving OV therapy into the clinic become increasingly tangible, additional attention has been directed towards the role of host immunity in the context of OV administration. The increasing understanding of the very complex interplay between antiviral immunity and antitumor immunity, has led to the development of several patents related to OV therapy and host immune responses. In this section, we will discuss some of the patents relevant to this research.
Host immunity can be simplified into two distinct components: the innate immune response and adaptive immunity. Mediators of the innate immune system function as the first responders to viral infection, clearing infected cells, and producing cytokines and chemokines leading to subsequent activation of the immune system. Consequently, innate immunity is critically important in limiting wild-type viral infections; however, in the context of OV therapy, this branch of the immune system appears to be a potent obstacle for achieving OV replication and tumor destruction [85–88]. Following activation of innate immunity, the adaptive component of the immune system is recruited to the site of infection and participates in both the killing of virally infected cells and the production of antibodies against foreign antigens. As a result, stimulation of adaptive immunity has a positive impact on therapy through its promotion of a cytotoxic T cell response, which creates an anti-tumor vaccination effect [79, 89].
Although the innate immune system has a variety of functional components to protect the host from infection, its critical objectives are to limit viral propagation, signal for maturation of antigen presenting cells, and activate the adaptive immune response through antigen-specific T cell and B cell maturation [90]. Owing to its significant contribution towards limiting viral infection, it has been described as one of the critical limitations to effective virotherapy. Treatment of rat glioma with an oncolytic HSV has been shown to result in a significant increase in IFNγ accompanied by a rapid recruitment of macrophages, microglia, and natural killer cells at the site of administration [91–93]. Both the antitumor efficacies as well as the intratumoral viral titers were found to be significantly increased with the concurrent depletion of mononuclear cells and the elimination of antiviral cytokines. Interestingly, Kurozumi et al. found that pretreatment of intracranial tumor bearing rats with an antiangiogenic agent prior to OV therapy limited the infiltration of antiviral host immune cells into the tumor. This resulted in reduced antiviral immune responses and increased viral propagation and efficacy [70]. With the elucidations of such pathways, the quest of combining novel pharmacological approaches with OV therapy to enhance viral infection, replication, and propagation is being pursued [94].
While antiviral immune responses are considered detrimental to therapy, activation of an adaptive anti-tumor immune response upon viral infection has been shown to be beneficial for cancer therapy. Cytotoxic T cell lymphocytes (CTL) have been implicated as the critical responders to viral antigens presented on the surface of tumor cells. CTLs are subsequently redirected to tumor cell antigens, thereby enhancing the efficacy of oncolytic HSV-1 by inducing antitumor immunity [95, 96].
The inclusion of genes encoding for various cytokines into viral vectors to enhance antitumor immune responses has been also tested and found to be beneficial in several preclinical models of cancer. For example, Post et al. found that the inclusion of IL-4 gene therapy in a hypoxia driven oncolytic adenovirus also had a rapid and maintained tumor regression in human glioma xenografts in mice [97]. Similarly the inclusion of IL-2 has been shown to result in the induction of immunotherapy, and subsequent tumor rejection in other tumor models [98]. Similar results were observed with the inclusion of IL-12 and GM-CSF which were intended to enhance OV therapy by ultimately facilitating an adaptive immune response in tumors [75, 99–102]. Based on these studies several patents have been issued which describe novel OVs encoding for T-cell co-stimulatory factors, pro-inflammatory cytokines, chemokines, and intercellular adhesion molecules designed to increase the autologous antitumor vaccination by OV [76, 95, 103].
While the significance of apoptotic bodies produced upon viral infection and their subsequent engulfment by antigen presenting cells (APCs) to potentiate a cytotoxic T lymphocyte activity and anti-tumor vaccines has been patented [104, 105], the potential to exploit this in conjunction with OV therapy to enhance anti-tumor immunity is only beginning to be realized. A better understanding of the mechanistic cues that happen during immunotherapy can then be exploited to harness the combined potential of antitumor immunity with OV therapy.
PATENTS RELEVANT TO ENHANCE VIRAL SPREAD THROUGH A SOLID TUMOR
Apart from efficient infection and host immune evasion, an OV needs to replicate and spread efficiently through the tumor interstitium to effectively infect and eradicate all cancer cells within the solid tumor. Physical barriers within the tumor microenvironment are problematic to efficient viral dissemination and hence limit antitumoral efficacy. The use of enzymes to selectively disrupt the extracellular matrix and surrounding tissue in order to enhance viral spread throughout the tumor has led to the development of several patents which will next be discussed.
One of the earlier research papers published with interest in breaking down the physical barriers impeding viral transduction was that by Maillard, et al. in 1998. It was observed that the endothelium and internal elastic lamina (IEL) were the main barriers to adenovirus-mediated gene transfer to medial smooth muscle cells (SMC). Treatment of rabbit iliac endothelium with elastase disrupted these barriers leading to increased adenoviral vector gene transfer [106]. This study provided evidence that the surrounding connective tissue and ECM may serve as potential barriers to virus transduction in tumor tissue, and these barriers may be the cause of uneven penetration and distribution of oncolytic viruses. A more recent study found that pretreatment of human glioma xenografts in mice with proteases such as trypsin resulted in a significant increase in virus-mediated gene transduction, possibly due to digestion of the tumor extracellular matrix [107]. Similarly, it has been observed that HSV virions injected into subcutaneous tumors grown in mice, did not spread efficiently in collagen-rich areas, and co-injecting HSV viral vectors with bacterial collagenase, increased viral distribution and antitumor efficacy of the oncolytic virus [108]. Recent reports have also shown enhanced efficacy of OV treatment of subcutaneous tumors in mice in conjunction with treatment with matrix modulating enzymes such as recombinant human hyaluronidase enzyme (rHuPH20), and matrix metalloproteinases (MMPs) such as MMP-1 and MMP-8 [109–111].
All of these results collectively indicate the significance of modulating the tumor ECM to enhance viral spread. The effect of Relaxin, (a peptide hormone that can induce the expression of MMPs) on viral spread in gliomas and other tumor types has been evaluated in two independent studies. First, Kim et al. engineered a replication incompetent and a replication competent oncolytic adenovirus that expressed relaxin Ad-ΔE1B-RLX [112]. Tumor spheroids transduced with Relaxin expressing virus permitted a better penetration of virus compared to control virus transduced spheres in vitro and in vivo. Treatment of mice with subcutaneous tumors revealed a potent anti-tumor effect of adenovirus Ad ΔE1B-RLX compared to control virus and histological examination of the tumor revealed an apparent lack of collagen and wide spread viral presence in tumors with Ad ΔE1B-RLX [113, 114]. Based on these results inventors Yun and Kim tested and patented an oncolytic adenovirus expressing relaxin [115].
These findings reveal the significant role that physical barriers, such as the ECM, play on the invasion and spread of oncolytic viruses. As increasing methods to effectively disrupt these barriers, while maintaining a degree of safety, come into view, the closer we get to enhancing overall OV therapeutic efficacy.
CURRENT & FUTURE DEVELOPMENTS
Clinical trials in human patients have highlighted the relative safety associated with oncolytic viral therapy. Current preclinical research is now focusing on ways to better understand host responses, enhance viral spread, and combine tumor microenvironment modulation with oncolysis in order to design strategies to improve therapeutic efficacy. Recent advances in molecular biology have facilitated the cumbersome process of constructing novel recombinant viruses with additional features. As these viruses are being made and tested, it is of paramount importance to always keep in mind the safety of the recombinant virus being generated. Studies investigating the factors that limit viral infection, spread, and propagation in vivo, will lead to the development of newer generation OVs which will be able to replicate better and more specifically in cancer cells. Dosage studies investigating the impact of combining OV which with the current standard of care will need to be carefully evaluated to determine optimal dosage schedules to enhance therapeutic efficacy. There is also a significant effort being made to improve existing technology for producing clinical grade virus. Improvements in virus production will facilitate the ability to treat patients with larger doses than those currently feasible. Future clinical trials will reveal the safety and efficacy of the various preclinical treatment strategies being tested in translational laboratories across the world.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170(5):1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4(2):101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 4.Terada K, Wakimoto H, Tyminski E, Chiocca EA, Saeki Y. Development of a rapid method to generate multiple oncolytic HSV vectors and their in vivo evaluation using syngeneic mouse tumor models. Gene Ther. 2006;13(8):705–714. doi: 10.1038/sj.gt.3302717. [DOI] [PubMed] [Google Scholar]
- 5.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 6.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 7.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: A proof of principle study. Gene Ther. 2002;9(6):398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 8.Harrow S, Papanastassiou V, Harland J, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: Safety data and long-term survival. Gene Ther. 2004;11(22):1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 9.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13(1):221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16(3):627–632. doi: 10.1038/sj.mt.6300403. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: Isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62(1):196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasuya H, Nishiyama Y, Nomoto S, Hosono J, Takeda S, Nakao A. Intraperitoneal delivery of hrR3 and ganciclovir prolongs survival in mice with disseminated pancreatic cancer. J Surg Oncol. 1999;72(3):136–141. doi: 10.1002/(sici)1096-9098(199911)72:3<136::aid-jso5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Mineta T, Rabkin SD, Martuza RL. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994;54(15):3963–3966. [PubMed] [Google Scholar]
- 15.Aghi M, Visted T, Depinho RA, Chiocca EA. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene. 2008;27(30):4249–4254. doi: 10.1038/onc.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250(4985):1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 17.Bolovan CA, Sawtell NM, Thompson RL. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J Virol. 1994;68(1):48–55. doi: 10.1128/jvi.68.1.48-55.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith KD, Mezhir JJ, Bickenbach K, et al. Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Deltagamma(1)34.5 mutants of herpes simplex virus 1. J Virol. 2006;80(3):1110–1120. doi: 10.1128/JVI.80.3.1110-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coffey MC, Thompson BG: WO01035970. 2001 [Google Scholar]
- 20.McKie EA, MacLean AR, Lewis AD, et al. Selective in vitro replication of herpes simplex virus type 1 (HSV-1) ICP34.5 null mutants in primary human CNS tumours--evaluation of a potentially effective clinical therapy. Br J Cancer. 1996;74(5):745–752. doi: 10.1038/bjc.1996.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith KD, Mezhir JJ, Weichselbaum RR, Roizman B: WO2007087580. 2007 [Google Scholar]
- 22.Lasner TM, Kesari S, Brown SM, Lee VM, Fraser NW, Trojanowski JQ. Therapy of a murine model of pediatric brain tumors using a herpes simplex virus type-1 ICP34.5 mutant and demonstration of viral replication within the CNS. J Neuropathol Exp Neurol. 1996;55(12):1259–1269. doi: 10.1097/00005072-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Kesari S, Randazzo BP, Valyi-Nagy T, et al. Therapy of experimental human brain tumors using a neuroattenuated herpes simplex virus mutant. Lab Invest. 1995;73(5):636–648. [PubMed] [Google Scholar]
- 24.Brown SM, Conner J: WO03068809. 2003 [Google Scholar]
- 25.Harland J, Papanastassiou V, Brown SM. HSV1716 persistence in primary human glioma cells in vitro. Gene Ther. 2002;9(17):1194–1198. doi: 10.1038/sj.gt.3301782. [DOI] [PubMed] [Google Scholar]
- 27.Crusade-Laboratories. In. [Google Scholar]
- 27.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1(9):938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 28.Martuza RL, Rabkin SD, Mineta T. WO000007. 2000 [Google Scholar]
- 29.Todo T, Feigenbaum F, Rabkin SD, et al. Viral shedding and biodistribution of G207, a multimutated, conditionally replicating herpes simplex virus type 1, after intracerebral inoculation in aotus. Mol Ther. 2000;2(6):588–595. doi: 10.1006/mthe.2000.0200. [DOI] [PubMed] [Google Scholar]
- 30.Sonabend AM, Ulasov IV, Lesniak MS. Conditionally replicative adenoviral vectors for malignant glioma. Rev Med Virol. 2006;16(2):99–115. doi: 10.1002/rmv.490. [DOI] [PubMed] [Google Scholar]
- 31.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 32.Geoerger B, Grill J, Opolon P, et al. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62(3):764–772. [PubMed] [Google Scholar]
- 33.McCormick F. WO9301919. 1993 [Google Scholar]
- 34.Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19(1):2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 35.Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95(9):652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 36.Jiang H, Gomez-Manzano C, Aoki H, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: Role of autophagic cell death. J Natl Cancer Inst. 2007;99(18):1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 37.Alonso MM, Cascallo M, Gomez-Manzano C, et al. ICOVIR-5 shows E2F1 addiction and potent antiglioma effect in vivo. Cancer Res. 2007;67(17):8255–8263. doi: 10.1158/0008-5472.CAN-06-4675. [DOI] [PubMed] [Google Scholar]
- 38.Fueyo J, Lang FFJ, Gomez-Manzano C, Yung WK, Conrad CA. WO20086922. 2008 [Google Scholar]
- 39.Kim M, Chung YH, Johnston RN. Reovirus and tumor oncolysis. J Microbiol. 2007;45(3):187–192. [PubMed] [Google Scholar]
- 40.Strong JE, Tang D, Lee PW. Evidence that the epidermal growth factor receptor on host cells confers reovirus infection efficiency. Virology. 1993;197(1):405–411. doi: 10.1006/viro.1993.1602. [DOI] [PubMed] [Google Scholar]
- 41.Lee PW, Strong J, Coffey M. WO2005050051. 2005 [Google Scholar]
- 42.Reichard KW, Lorence RM, Cascino CJ, et al. Newcastle disease virus selectively kills human tumor cells. J Surg Res. 1992;52(5):448–453. doi: 10.1016/0022-4804(92)90310-v. [DOI] [PubMed] [Google Scholar]
- 43.Lorence RM, Reichard KW, Katubig BB, et al. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J Natl Cancer Inst. 1994;86(16):1228–1233. doi: 10.1093/jnci/86.16.1228. [DOI] [PubMed] [Google Scholar]
- 44.Csatary LK, Szeberenyi J, Fabian Z. WO2005051433. 2005 [Google Scholar]
- 45.Zakay-Rones Z, Panet A, Greenbaum E. WO2005113013. 2005 [Google Scholar]
- 46.Csatary LK, Gosztonyi G, Szeberenyi J, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol. 2004;67(1–2):83–93. doi: 10.1023/b:neon.0000021735.85511.05. [DOI] [PubMed] [Google Scholar]
- 47.Csatary LK, Bakacs T. Use of Newcastle disease virus vaccine (MTH-68/H) in a patient with high-grade glioblastoma. Jama. 1999;281(17):1588–1589. doi: 10.1001/jama.281.17.1588-a. [DOI] [PubMed] [Google Scholar]
- 48.Wagner S, Csatary CM, Gosztonyi G, et al. Combined treatment of pediatric high-grade glioma with the oncolytic viral strain MTH-68/H and oral valproic acid. Apmis. 2006;114(10):731–743. doi: 10.1111/j.1600-0463.2006.apm_516.x. [DOI] [PubMed] [Google Scholar]
- 49.Schattner A. Therapeutic role of measles vaccine in Hodgkin's disease. Lancet. 1984;1(8369):171. doi: 10.1016/s0140-6736(84)90112-0. [DOI] [PubMed] [Google Scholar]
- 50.Galanis E, Bateman A, Johnson K, et al. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum Gene Ther. 2001;12(7):811–821. doi: 10.1089/104303401750148766. [DOI] [PubMed] [Google Scholar]
- 51.Phuong LK, Allen C, Peng KW, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63(10):2462–2469. [PubMed] [Google Scholar]
- 52.Myers R, Harvey M, Kaufmann TJ, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther. 2008;19(7):690–698. doi: 10.1089/hum.2008.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50(2):135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 54.Balachandran S, Porosnicu M, Barber GN. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J Virol. 2001;75(7):3474–3479. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4(4):263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 56.Ozduman K, Wollmann G, Piepmeier JM, van den Pol AN. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J Neurosci. 2008;28(8):1882–1893. doi: 10.1523/JNEUROSCI.4905-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lun X, Senger DL, Alain T, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J Natl Cancer Inst. 2006;98(21):1546–1557. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 58.Bell JC, Sonenberg N, Stojdl DF, Brown EG, Atkins HL, Marius RM, Lichty BD, Knowles SB. WO01019380. 2001 [Google Scholar]
- 59.Cutter JL, Kurozumi K, Chiocca EA, Kaur B. Gene therapeutics: The future of brain tumor therapy? Expert Rev Anticancer Ther. 2006;6(7):1053–1064. doi: 10.1586/14737140.6.7.1053. [DOI] [PubMed] [Google Scholar]
- 60.Timiryasova TM, Chen B, Haghighat P, Fodor I. Vaccinia virus-mediated expression of wild-type p53 suppresses glioma cell growth and induces apoptosis. Int J Oncol. 1999;14(5):845–854. doi: 10.3892/ijo.14.5.845. [DOI] [PubMed] [Google Scholar]
- 61.Ellenhorn JD, Diamond DJ. WO2004058801. 2004 [Google Scholar]
- 62.Gridley DS, Andres ML, Li J, Timiryasova T, Chen B, Fodor I. Evaluation of radiation effects against C6 glioma in combination with vaccinia virus-p53 gene therapy. Int J Oncol. 1998;13(5):1093–1098. doi: 10.3892/ijo.13.5.1093. [DOI] [PubMed] [Google Scholar]
- 63.Chen B, Timiryasova TM, Haghighat P, et al. Low-dose vaccinia virus-mediated cytokine gene therapy of glioma. J Immunother. 2001;24(1):46–57. doi: 10.1097/00002371-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Chen B, Timiryasova TM, Andres ML, et al. Evaluation of combined vaccinia virus-mediated antitumor gene therapy with p53, IL-2, and IL-12 in a glioma model. Cancer Gene Ther. 2000;7(11):1437–1447. doi: 10.1038/sj.cgt.7700252. [DOI] [PubMed] [Google Scholar]
- 65.Yang S, Guo ZS, O'Malley ME, Yin X, Zeh HJ, Bartlett DL. A new recombinant vaccinia with targeted deletion of three viral genes: Its safety and efficacy as an oncolytic virus. Gene Ther. 2007;14(8):638–647. doi: 10.1038/sj.gt.3302914. [DOI] [PubMed] [Google Scholar]
- 66.Cinatl J, Jr, Michaelis M, Driever PH, et al. Multimutated herpes simplex virus g207 is a potent inhibitor of angiogenesis. Neoplasia. 2004;6(6):725–735. doi: 10.1593/neo.04265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benencia F, Courreges MC, Conejo-Garcia JR, et al. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum Gene Ther. 2005;16(6):765–778. doi: 10.1089/hum.2005.16.765. [DOI] [PubMed] [Google Scholar]
- 68.Mahller YY, Vaikunth SS, Currier MA, et al. Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model. Mol Ther. 2007;15(2):279–286. doi: 10.1038/sj.mt.6300038. [DOI] [PubMed] [Google Scholar]
- 69.Aghi M, Rabkin SD, Martuza RL. Angiogenic response caused by oncolytic herpes simplex virus-induced reduced thrombospondin expression can be prevented by specific viral mutations or by administering a thrombospondin-derived peptide. Cancer Res. 2007;67(2):440–444. doi: 10.1158/0008-5472.CAN-06-3145. [DOI] [PubMed] [Google Scholar]
- 70.Kurozumi K, Hardcastle J, Thakur R, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99(23):1768–1781. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 71.Kaur B, Brat DJ, Devi NS, Van Meir EG. Vasculostatin, a proteolytic fragment of brain angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene. 2005;24(22):3632–3642. doi: 10.1038/sj.onc.1208317. [DOI] [PubMed] [Google Scholar]
- 72.Iggo R, Lukashev A, Homicsko K, McLaughlin F, Fuerer C. WO2006075165. 2006 [Google Scholar]
- 73.Homicsko K, Lukashev A, Iggo RD. RAD001 (everolimus) improves the efficacy of replicating adenoviruses that target colon cancer. Cancer Res. 2005;65(15):6882–6890. doi: 10.1158/0008-5472.CAN-05-0309. [DOI] [PubMed] [Google Scholar]
- 74.Guse K, Ranki T, Ala-Opas M, et al. Treatment of metastatic renal cancer with capsid-modified oncolytic adenoviruses. Mol Cancer Ther. 2007;6(10):2728–2736. doi: 10.1158/1535-7163.MCT-07-0176. [DOI] [PubMed] [Google Scholar]
- 75.Wong RJ, Chan MK, Yu Z, et al. Angiogenesis inhibition by an oncolytic herpes virus expressing interleukin 12. Clin Cancer Res. 2004;10(13):4509–4516. doi: 10.1158/1078-0432.CCR-04-0081. [DOI] [PubMed] [Google Scholar]
- 76.Whitley R, Markert J, Gillespie Y, Parker N. WO00075292. 2000 [Google Scholar]
- 77.Frisch SM. WO96007322. 1996 [Google Scholar]
- 78.Liu TC, Zhang T, Fukuhara H, et al. Oncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacy. Mol Ther. 2006;14(6):789–797. doi: 10.1016/j.ymthe.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 79.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98(11):6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bolanowski MA, Caparon MH, Casperson GF, Gregory S, Klein BK, McKearn JP. WO9916889. 1999 [Google Scholar]
- 81.Yang CT, Lin YC, Lin CL, et al. Oncolytic herpesvirus with secretable angiostatic proteins in the treatment of human lung cancer cells. Anticancer Res. 2005;25(3B):2049–2054. [PubMed] [Google Scholar]
- 82.Mullen JT, Donahue JM, Chandrasekhar S, et al. Oncolysis by viral replication and inhibition of angiogenesis by a replication-conditional herpes simplex virus that expresses mouse endostatin. Cancer. 2004;101(4):869–877. doi: 10.1002/cncr.20434. [DOI] [PubMed] [Google Scholar]
- 83.Yoo JY, Kim JH, Kwon YG, et al. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol Ther. 2007;15(2):295–302. doi: 10.1038/sj.mt.6300023. [DOI] [PubMed] [Google Scholar]
- 84.Reich SJ. WO07146953. 2007 [Google Scholar]
- 85.Balachandran S, Thomas E, Barber GN. A FADD-dependent innate immune mechanism in mammalian cells. Nature. 2004;432(7015):401–405. doi: 10.1038/nature03124. [DOI] [PubMed] [Google Scholar]
- 86.Abordo-Adesida E, Follenzi A, Barcia C, et al. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther. 2005;16(6):741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balachandran S, Barber GN. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5(1):51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 88.Friedman A, Tian JP, Fulci G, Chiocca EA, Wang J. Glioma virotherapy: Effects of innate immune suppression and increased viral replication capacity. Cancer Res. 2006;66(4):2314–2319. doi: 10.1158/0008-5472.CAN-05-2661. [DOI] [PubMed] [Google Scholar]
- 89.Andreansky S, He B, van Cott J, et al. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Ther. 1998;5(1):121–130. doi: 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- 90.Wakimoto H, Johnson PR, Knipe DM, Chiocca EA. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Ther. 2003;10(11):983–990. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- 91.Otsuki A, Patel A, Kasai K, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16(9):1546–1555. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- 92.Fulci G, Dmitrieva N, Gianni D, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007;67(19):9398–9406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fulci G, Breymann L, Gianni D, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103(34):12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Molnar-Kimber K, Toyoizumi T, Kaiser L. WO00054795. 2000 [Google Scholar]
- 95.Todo T, Rabkin SD, Sundaresan P, et al. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther. 1999;10(17):2741–2755. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- 96.Thomas DL, Fraser NW. HSV-1 therapy of primary tumors reduces the number of metastases in an immune-competent model of metastatic breast cancer. Mol Ther. 2003;8(4):543–551. doi: 10.1016/s1525-0016(03)00236-3. [DOI] [PubMed] [Google Scholar]
- 97.Post DE, Sandberg EM, Kyle MM, et al. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res. 2007;67(14):6872–6881. doi: 10.1158/0008-5472.CAN-06-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mizuno H, Yanoma S, Nishimura G, et al. Therapeutic efficiency of IL-2 gene transduced tumor vaccine for head and neck carcinoma. Cancer Lett. 2000;152(2):175–185. doi: 10.1016/s0304-3835(00)00336-0. [DOI] [PubMed] [Google Scholar]
- 99.Stanford MM, Barrett JW, Gilbert PA, Bankert R, McFadden G. Myxoma virus expressing human interleukin-12 does not induce myxomatosis in European rabbits. J Virol. 2007;81(22):12704–12708. doi: 10.1128/JVI.01483-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Varghese S, Rabkin SD, Nielsen PG, Wang W, Martuza RL. Systemic oncolytic herpes virus therapy of poorly immunogenic prostate cancer metastatic to lung. Clin Cancer Res. 2006;12(9):2919–2927. doi: 10.1158/1078-0432.CCR-05-1187. [DOI] [PubMed] [Google Scholar]
- 101.Ramesh N, Ge Y, Ennist DL, et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor--armed oncolytic adenovirus for the treatment of bladder cancer. Clin Cancer Res. 2006;12(1):305–313. doi: 10.1158/1078-0432.CCR-05-1059. [DOI] [PubMed] [Google Scholar]
- 102.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 103.Fong Y, Federoff H, Rosenblatt J. WO98042855. 1998 [Google Scholar]
- 104.Gregoire M, Bartoleyns J. WO99058645. 1999 [Google Scholar]
- 105.Albert M, Bhardwaj N, Steinman RM, Inaba K. WO99042564. 1999 [Google Scholar]
- 106.Maillard L, Ziol M, Tahlil O, et al. Pre-treatment with elastase improves the efficiency of percutaneous adenovirus-mediated gene transfer to the arterial media. Gene Ther. 1998;5(8):1023–1030. doi: 10.1038/sj.gt.3300682. [DOI] [PubMed] [Google Scholar]
- 107.Kuriyama N, Kuriyama H, Julin CM, Lamborn K, Israel MA. Pretreatment with protease is a useful experimental strategy for enhancing adenovirus-mediated cancer gene therapy. Hum Gene Ther. 2000;11(16):2219–2230. doi: 10.1089/104303400750035744. [DOI] [PubMed] [Google Scholar]
- 108.McKee TD, Grandi P, Mok W, et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66(5):2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 109.Ganesh S, Gonzalez-Edick M, Gibbons D, Van Roey M, Jooss K. Intratumoral coadministration of hyaluronidase enzyme and oncolytic adenoviruses enhances virus potency in metastatic tumor models. Clin Cancer Res. 2008;14(12):3933–3941. doi: 10.1158/1078-0432.CCR-07-4732. [DOI] [PubMed] [Google Scholar]
- 110.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: A tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3(3):207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 111.Mok W, Boucher Y, Jain RK. Matrix metalloproteinases-1 and-8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 2007;67(22):10664–10668. doi: 10.1158/0008-5472.CAN-07-3107. [DOI] [PubMed] [Google Scholar]
- 112.Kim JH, Lee YS, Kim H, Huang JH, Yoon AR, Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006;98(20):1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- 113.Ganesh S, Gonzalez-Edick M, Idamakanti N, et al. Relaxin-expressing, fiber chimeric oncolytic adenovirus prolongs survival of tumor-bearing mice. Cancer Res. 2007;67(9):4399–4407. doi: 10.1158/0008-5472.CAN-06-4260. [DOI] [PubMed] [Google Scholar]
- 114.Wickham TJ, Roelvink PW, Kovesdi I. US5846782. 1998 [Google Scholar]
- 115.Yun CO, Kim J. WO2006075819. 2006 [Google Scholar]