Abstract
Phoenix, Goy, Gerall, and Young first proposed in 1959 the organizational-activational hypothesis of hormone-driven sex differences in brain and behavior. The original hypothesis posited that exposure to steroid hormones early in development masculinizes and defeminizes neural circuits, programming behavioral responses to hormones in adulthood. This hypothesis has inspired a multitude of experiments demonstrating that the perinatal period is a time of maximal sensitivity to gonadal steroid hormones. However, recent work from our laboratory and others demonstrates that steroid-dependent organization of behavior also occurs during adolescence, prompting a reassessment of the developmental time-frame within which organizational effects are possible. In addition, we present evidence that adolescence is part of a single protracted postnatal sensitive period for steroid-dependent organization of male mating behavior that begins perinatally and ends in late adolescence. These findings are consistent with the original formulation of the organizational/activational hypothesis, but extend our notions of what constitutes “early” development considerably. Finally, we present evidence that female behaviors also undergo steroid-dependent organization during adolescence, and that social experience modulates steroid-dependent adolescent brain and behavioral development. The implications for human adolescent development are also discussed, especially with respect to how animal models can help to elucidate the factors underlying the association between pubertal timing and adult psychopathology in humans.
The 1959 landmark paper by Phoenix, Goy, Gerall, and Young first posited what became the organizational-activational hypothesis of hormone-driven sex differences in brain and behavior (Phoenix et al., 1959). In this hypothetical framework, a transient rise in testosterone during prenatal or early postnatal development masculinizes and defeminizes neural circuits in males, while the absence of testosterone in females results in development of a feminine neural phenotype. Upon gonadal maturation during puberty, testicular and ovarian hormones act on previously sexually differentiated circuits to facilitate expression of sex-typical behaviors in particular social contexts. Research in the 1960–70s identified a maximally sensitive period for hormone-dependent sexual differentiation that occurs during late prenatal and early neonatal development (reviewed in Baum, 1979; Wallen and Baum, 2002). Thus, the original conception was that steroid hormones organize brain structure during an early developmental sensitive period, and activate behavior during puberty and into adulthood.
Conceptualization of the dichotomy between organizational and activational effects of hormones has evolved over the past 50 years. In the 1970s, Scott and colleagues provided a theoretical basis for the existence of multiple sensitive periods for progressive organization of the nervous system, a framework derived from their developmental studies on sensitive periods and social attachment in dogs (Scott et al., 1974). Arnold and Breedlove argued in the 1980s that the distinction between organizational and activational effects is not always clear-cut, and that the potential for organizational effects is not restricted to early neural development (Arnold and Breedlove, 1985). Scott (1974) had noted that sensitive periods for behavioral development are most likely to occur during periods of rapid developmental change, and went on to speculate that “another obvious critical period for human development is that of adolescence, in which sexual behavior is organized either successfully or unsuccessfully”, even though there was little empirical basis for this assertion at the time. In this paper we will review work from our laboratory and others that calls for further refinement of the organizational-activational hypothesis, specifically as it relates to remodeling of the brain that occurs during adolescence. First, we summarize the behavioral data indicating that the adolescent brain, undergoing remodeling, is organized a second time by gonadal steroid hormones secreted during puberty. This second wave of brain organization builds on and refines circuits that were sexually differentiated during early neural development. Second, we will address the question of whether or not adolescence is a discrete sensitive period of development for organization of the brain by steroid hormones, separate from the perinatal period of sexual differentiation. Third, we review the evidence that ovarian hormones play an active role in female adolescent brain organization. Although the majority of this review focuses on adolescent testicular hormone influences on adult male behavior, this is primarily a reflection of the research disparity between males and females in the area of steroid-dependent behavioral organization. Finally, we discuss some of the structural mechanisms by which pubertal hormones organize neural circuitry during adolescence, and compare mechanisms of hormone-dependent and experience-dependent organization of neural circuits.
Hormone-dependent organization of behavior during puberty and adolescence
Social behaviors
Using the Syrian hamster as an animal model, we have established that a number of male social behaviors are organized by pubertal hormones. To assess the contribution of testicular hormones to the expression of social behaviors in adulthood, we’ve employed an experimental paradigm in which the testes are removed from weanling hamsters, i.e., after the perinatal period of sexual differentiation and before the onset of puberty. In adulthood, testosterone is replaced, and 1–2 weeks later, social interactions with a receptive female or an intruder male are studied. The behavior of these subjects is compared to others that are similarly treated, except that castration and hormone replacement occurs in adulthood, several weeks after the pubertal elevation in testicular hormones. In these experiments, we found that levels of sexual behavior, aggressive behavior, and flank-marking are all reduced in males in which testicular hormones are absent during adolescent brain development (i.e., males castrated before puberty), as compared to the behavior of males in which endogenous testicular hormones were present during adolescent brain development (i.e., males castrated after puberty, (Schulz et al., 2006a; Schulz et al., 2004a; Schulz and Sisk, 2006). Even a prolonged period of testosterone replacement in adulthood fails to normalize behavior in males castrated before puberty (Schulz et al., 2004a). Furthermore, male hamsters castrated before puberty, treated with estradiol and progesterone in adulthood, and tested with a male, show a shorter latency to assume lordosis than do males castrated in adulthood (Schulz et al., 2004a). Thus, testicular hormones during puberty enhance subsequent hormone activation of male social behaviors and both masculinize and defeminize behavioral responses in adulthood, outcomes similar to those of exposure to testosterone during perinatal brain development. Reports from other laboratories provide similar types of evidence for organizational effects of gonadal hormones during puberty on territorial scent marking male in tree shrews (Eichmann and Holst, 1999), aggression in male mice (Shrenker et al., 1985) and gerbils (Lumia et al., 1977), and play fighting in male rats (Pellis, 2002).
Anxiety-related behaviors
The amount of locomotor activity in an open field is used as an index of anxiety, xenophobia, or depression in rodents. Adult male rats ambulate less than female rats when tested in an open field arena, indicating that the open field is more anxiogenic to males than to females. Pubertal testicular hormones organize this sex difference in open field ambulation, because castration at the onset of puberty results in increased ambulation in males in adulthood (Brand and Slob, 1988; but see also Stewart and Cygan, 1980). Similarly, male-male social interactions are reduced in a novel environment compared with a familiar environment, indicating an anxiogenic effect of the novel environment in males. This effect of the novel environment develops during puberty (Primus and Kellogg, 1989). Prepubertal castration prevents the pubertal emergence of the novel environment effect, while testosterone replacement during the time of puberty (but not in adulthood) reinstates the novel environment effect in adulthood (Primus and Kellogg, 1990). Gonadal hormones appear to organize this anxiety-related behavior by altering the benzodiazepine-GABA receptor complex responses to environmental challenge (Primus and Kellogg, 1991).
Cognition
On average, men perform better than women in tests of spatial cognition. Sex differences in spatial cognition in humans may be organized by pubertal hormones. Evidence for this comes from a study in which spatial cognition was compared in men with idiopathic hypogonadotropic hypogonadism (IHH) that began before puberty and men with IHH acquired in adulthood (Hier and Crowley, 1982). The former group had low or undetectable levels of circulating gonadal steroids during the normal time of puberty and adolescence, whereas the latter group experienced normal levels of pubertal gonadal hormones during adolescence. Spatial cognition was impaired in men not exposed to pubertal steroids, both in comparison to healthy control subjects and to men with acquired IHH in adulthood (Hier and Crowley, 1982), suggesting that the presence of testicular hormones during puberty organizes circuits underlying spatial cognition. Another study of human spatial cognition included women subjects with a variation of congenital adrenal hyperplasia that leads to slightly but chronically elevated levels of adrenal androgens during childhood and early puberty. These women performed better in a virtual Morris Water Maze (a test of spatial memory) compared with healthy subjects, again suggesting a pubertal organizational influence of adrenal androgens on spatial ability (Mueller et al., 2008). Spatial memory is hippocampus-dependent, and synaptic plasticity in the hippocampus appears to be organized by pubertal androgens. Specifically, activation of androgen receptor during puberty results in long term depression in CA1 in response to a tetanizing stimulus in adulthood, whereas if androgen receptor activation is blocked during puberty, long term potentiation occurs in response to a tetanizing stimulus in adulthood (Hebbard et al., 2003). These results provide a potential mechanism by which pubertal testosterone could organize hippocampus-dependent learning and memory, including spatial cognition.
Two stage model of steroid-dependent maturation of social behaviors
The preponderance of evidence that pubertal testicular hormones organize a wide range of male-typical behaviors prompted us to propose a two-stage model of behavioral development in which the perinatal period of steroid-dependent sexual differentiation is followed by a second wave of steroid-dependent neural organization during puberty and adolescence (Schulz and Sisk, 2006; Sisk et al., 2003; Sisk and Zehr, 2005, Figure 1). During the second wave, pubertal hormones first organize neural circuits in the developing adolescent brain, and then facilitate the expression of adult sex-typical behaviors in specific social contexts by activating those circuits. We view hormone-driven adolescent organization as a refinement of the sexual differentiation that occurred during perinatal neural development. That is, what occurs during perinatal brain organization determines the substrate upon which pubertal hormones act during adolescent organization. During the adolescent phase of organization, steroid-dependent refinement of neural circuits results in long-lasting structural changes that determine adult behavioral responses to hormones and socially-relevant sensory stimuli, outcomes again similar to those of the perinatal phase of organization.
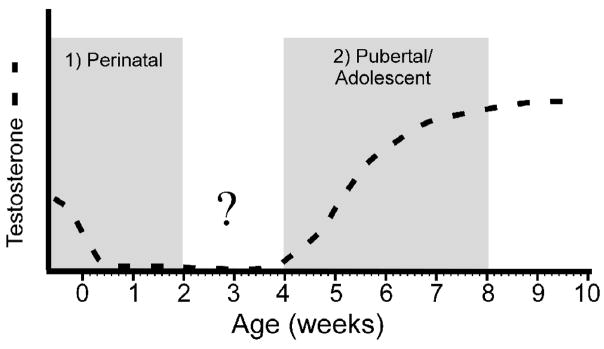
Fig 1.
Two stage model for steroid-dependent organization of behavior. Testosterone secretions during the perinatal and adolescent periods organize adult mating behavior. The dashed line approximates testosterone secretions across development, and the shading denotes the approximate timing of perinatal and adolescent development in the Syrian hamster. The question mark highlights that less is known about T-dependent behavioral organization in the time between the perinatal and adolescent periods.
Do these two periods of hormone-dependent organization involve two discrete periods of enhanced sensitivity to hormones? We know that a window of sensitivity opens in rodents during late embryonic development to permit the initial sexual differentiation of the brain organized by testicular hormones (Wallen and Baum, 2002). In addition, it is apparent from our work that a window of developmental sensitivity to hormone-dependent organization closes (or nearly so) by the end of adolescence, because a prolonged duration of testosterone replacement does not reverse or ameliorate the adverse consequences of the absence of testosterone during puberty on adult social behaviors (Schulz et al., 2004a). What is not clear is the extent to which the perinatal and peripubertal windows overlap (Figure 1). Does the perinatal window close before the peripubertal window opens? Alternatively, do the perinatal and peripubertal windows overlap enough to be considered a single period of sensitivity to hormone-dependent organization of the nervous system? What is the relationship between the timing of the pubertal rise in testosterone secretion and the opening of the putative adolescent window? The next section will review our work that addresses these questions and that provides insight into how the timing of the pubertal rise in gonadal hormone secretion relative to adolescent brain development contributes to the development of individual differences in adult social behavior.
Windows of sensitivity to testicular hormones
The timing of increases in postnatal testosterone secretion is determined by the endogenous activity of the hypothalamic-pituitary-gonadal (HPG) axis, and testosterone secretion during the neonatal and adolescent periods drives the masculinization and defeminization of adult male behavior, as discussed above. Less clear, however, is what happens if the timing of HPG axis activity is perturbed and testicular secretions are initiated outside the age-range during which adolescence normally occurs. In such cases, would testicular secretions have a similar organizing effect or any effect at all? If there are specific postnatal windows of sensitivity to the organizing actions of testosterone, how many windows exist? Previous research demonstrates that the potential for testosterone to organize behavior decreases across the early postnatal period, suggesting that a window of sensitivity may close around postnatal day 10 (Wallen and Baum, 2002). However, the appropriate test of whether adolescence marks the opening of a second window of sensitivity distinct from the neonatal period of sexual differentiation has only recently been conducted. Early, on-time, and late pubertal increases in testosterone secretion were simulated by castrating male hamsters on postnatal day 10, and implanting testosterone- or blank-filled silastic capsules for 19 days before (P10–29), during (P29–48) or after (P63–82) the normal time of puberty and adolescence. In adulthood, twenty-eight days after the removal of testosterone or blank pellets, all males were implanted with testosterone pellets to stimulate mating behavior during tests with a sexually receptive female 7 days later.
If adolescence marks the opening of a unique sensitive period for testosterone-dependent behavioral organization, then only those males receiving testosterone implants during the normal time of puberty and adolescence should display adult-typical mating behavior. Contrary to this prediction, however, we found that early and on-time testosterone treatments, but not late treatments, facilitated mating behavior in adulthood (Schulz et al., under review, Figure 2). In addition, the early testosterone treatments most effectively facilitated adult mating behavior, a finding supported by previous studies of steroid-dependent behavioral organization prior to puberty (Coniglio and Clemens, 1976; Eaton, 1970). These findings extend our knowledge of neurobehavioral development by demonstrating: 1) adolescence is not a sensitive period for testosterone-dependent behavioral organization distinct from the neonatal period, but rather, adolescence is part of a protracted sensitive period that likely begins perinatally and ends in late adolescence; and 2) the timing of testosterone secretion within this postnatal window programs expression of adult mating behavior. Earlier exposure to testosterone has a greater impact on behavior than does later exposure. Thus, these data have prompted us to revise the 2-stage model to include a large postnatal window of decreasing sensitivity to the organizing actions of testosterone (Figure 3). Importantly, the original two stage model of hormone-dependent organization is still relevant, but the stages are not defined by distinct windows of sensitivity to steroid hormones, but instead by the two periods of elevated hormone secretion within a prolonged postnatal window of decreasing sensitivity to steroid hormones.
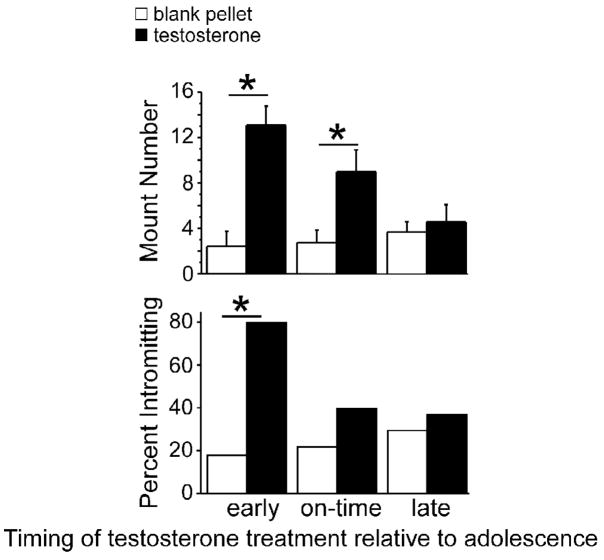
Fig 2.
Effects of early, on-time, and late adolescent treatments (blank or testosterone-filled capsules) on adult mating behavior. All animals were testosterone treated in adulthood prior to behavior testing. Early and on-time adolescent testosterone treatments, but not late-adolescent treatments facilitated mounting behavior relative to blank-treated controls (data expressed as mean +/− SEM). In contrast, only early adolescent testosterone-treatment facilitated intromissive behavior relative to blank-treated controls, suggesting that early adolescent treatments most effectively organize mating behavior (data expressed as percent). Asterisk indicates p < 0.05.
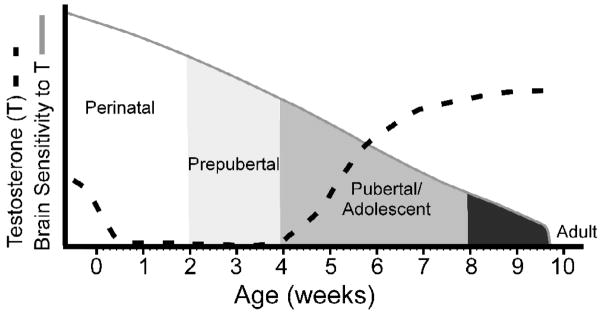
Fig 3.
Illustration depicting the overall findings of our study investigating the effects of early, on-time, and late adolescent testosterone treatments on adult mating behavior. Given that early adolescent testosterone treatment was initiated immediately following the period of sexual differentiation (postnatal day 10), our data suggest that adolescence is part of a protracted sensitive period for the organizing actions of testosterone (area under the solid gray curve). In addition, because early adolescent treatments most effectively organized adult mating behavior, we propose that sensitivity to the organizing actions of testosterone decreases across postnatal development. The dashed line approximates testosterone secretions across development, whereas the solid line depicts decreasing sensitivity to the organizing actions of testosterone across development. Shading approximates the timing of perinatal, prepubertal, adolescent periods in the Syrian hamster.
Behavioral consequences of variations in the timing of the pubertal rise in hormone secretion
Humans exhibit substantial variability in the timing of pubertal maturation (Dubas, 1991; Tanner, 1962), and deviations in normal pubertal timing are associated with various mental health problems (reviewed by Graber, 2003). Examples range from negative body self-image (McCabe and Ricciardelli, 2004) to increased incidence of depression (Ge et al., 2003; Graber et al., 2004; Michaud et al., 2006), anxiety (Kaltiala-Heino et al., 2003; Zehr et al., 2007b), symptoms of disordered eating (Striegel-Moore et al., 2001; Zehr et al., 2007a), conduct disorder (Burt et al., 2006; Celio et al., 2006) and increased alcohol and tobacco use (Biehl et al., 2007; Bratberg et al., 2007). Many of the psychological outcomes of pubertal timing have been demonstrated to persist into young adulthood (Graber et al., 2004; Zehr et al., 2007a).
The mechanism by which deviations in pubertal timing influence mental health outcomes in humans is likely a complex interplay between the psychosocial impact of physically maturing earlier or later than one’s peers, direct hormonal actions on the brain, and the individual’s unique environment. For example, social and environmental factors such as the adolescent peer group (Cavanagh, 2004; Ge et al., 2002), parenting styles (Ge et al., 2002), romantic partners (Halpern et al., 2007), and stressful life events (Ge et al., 2001) have all been found to moderate the effects of pubertal timing on mental health.
Figure 4 is a theoretical illustration based on our findings in hamsters depicting how the developing adolescent brain can be intercepted by pubertal hormone secretions at various time points, resulting in differences in brain and behavioral development. In the case of hamster mating behavior, gonadal hormones intercepting the developing brain early results in higher levels of behavior in adulthood. Given that gonadal hormones organize social and cognitive behaviors during adolescence, our finding that the timing of hormone exposure across adolescence determines adult sexual behaviors likely generalizes to other behaviors organized by adolescent gonadal hormones. However, for many of these behaviors organized by gonadal hormones during adolescence, we only know the behavioral effects of the complete absence of gonadal hormones. We do not yet know the duration or the nature of the sensitive period for each type of behavior (e.g. increasing, decreasing, or equal sensitivity across the sensitive period), making it is impossible to assess the specific effects of early or late pubertal timing for these behaviors. For example, if the sensitive period for a given behavior is of a very short duration during adolescence, late pubertal onset runs the risk of missing the sensitive period altogether. The nature of the sensitive period could also determine the effects of pubertal timing on behavioral outcomes. If sensitivity to gonadal hormones is constant across a sensitive period, then late pubertal onset may not be so damaging, so long as pubertal secretions do in fact overlap the window of sensitivity. Once the duration and nature of a sensitive period are determined, animal models have immense potential for investigating factors mediating the effects of pubertal timing on behavioral organization. Such studies will fill a fundamental gap in our understanding of how interactions between gonadal hormones, adolescent brain maturation, and the environment drive individual differences in adolescent and adult behaviors.
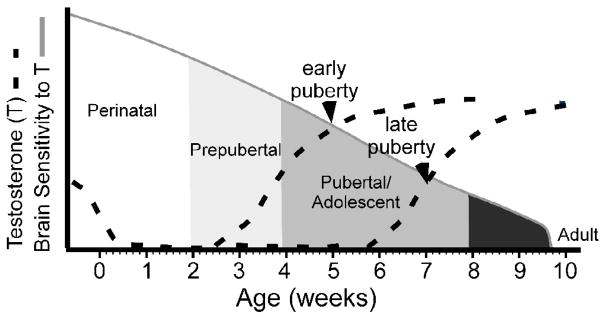
Fig 4.
Theoretical illustration depicting how early or late pubertal onset intersects the developing brain at different time points. Because sensitivity to the organizing actions of T decreases with time, differences in the timing of pubertal onset may result in differences in brain development and adult behavior. The dashed lines depict early or late testosterone secretions across development, whereas the solid line depicts decreasing sensitivity to the organizing actions of testosterone across development. Shading approximates the timing of perinatal, prepubertal, adolescent periods in the Syrian hamster.
What closes the window of sensitivity to organization by gonadal steroid hormones?
We do not yet know the mechanism by which the sensitive period for steroid-dependent organization of mating behavior closes, but if this sensitive period shares features of other well-known sensitive periods, it may end as a consequence of circuit reorganization or consolidation during adolescence (Bischof, 2007; Hensch, 2004; Knudsen, 2004). Specifically, particular experiences occurring during adolescence (e.g. the presence or absence of testosterone) may organize circuits and render them resistant to further modification or change. For example, patterns of synaptic connectivity change across adolescence within the hamster medial amygdala, concomitant with the pubertal rise in gonadal hormones (Zehr et al., 2006). These decreases in dendrites, spine densities, and spinophilin protein may reflect organizational changes induced by testosterone during the adolescent sensitive period, ultimately limiting the capacity for further steroid-dependent organization. Changes in dendritic morphology have been linked with the closing of the sensitive period for sexual imprinting and song learning in zebra finches (Bischof, 2003; Bischof, 2007; Bischof et al., 2002). Whether time-dependent reductions in MePD dendritic branches and spine densities reflect both the organizational influence of testosterone as well as the closing of the sensitive period remains to be determined.
Ovarian hormone exposure during adolescence organizes female behavior
Although our knowledge of behavioral organization induced by female ovarian hormones during adolescence lags behind our understanding of male testosterone-dependent behavioral organization, an interesting picture is emerging. Depending on the behavior, ovarian hormones during adolescence feminize (enhance female-typical attributes), masculinize (enhance male-typical attributes) or defeminize (suppress female-typical attributes) adult behavior. For example, food guarding is a sexually dimorphic behavior in rats, with males and females displaying different postural strategies for defending their food source (Field et al., 2004). Neonatal or pubertal ovariectomy significantly alters the defense strategy to be more “male-like”, whereas adult ovariectomy has no effect. Thus, these data suggest that ovarian hormones during the neonatal and/or adolescent periods actively feminize postural strategies for food defense. Another recent report demonstrates that pubertal estradiol feminizes ingestive responses to metabolic signals in rats (Swithers et al., 2008). Treatment with mercaptoacetate, a drug that interferes with fatty acid oxidation, causes an increase in food intake in male, but not female, rats. Ovariectomy in adulthood does not affect this sex difference. However, females that are ovariectomized prior to puberty show a male-like response to mercaptoacetate (i.e., increase food intake), and this effect of prepubertal ovariectomy can be prevented by treatment with estradiol during the time of puberty. These reports point to an active role for ovarian hormones in organization of the adolescent brain.
In contrast to the feminizing effects of ovarian hormones during adolescence on food guarding and ingestive behavior, ovarian hormones appear to drive species-specific defeminization and masculinization of mating behavior. In female Syrian hamsters, prepubertal, but not postpubertal, ovariectomy decreases lordosis latencies and increases overall lordosis durations in response to adult estradiol and progesterone treatment, suggesting that ovarian hormone exposure during adolescence defeminizes adult lordosis behavior (Schulz et al., 2004b). In addition, behavioral defeminization is also achieved by adolescent estradiol treatment following prepubertal ovariectomy, indicating that estradiol is the ovarian hormone responsible for behavioral defeminization during adolescence (Schulz et al., 2006b). Importantly, reduction of adult lordosis behavior following adolescent estradiol treatment does not appear to be the consequence of a general increase in locomotor activity. Instead, when females are not engaged in the lordosis posture, they are actively investigating their male partner, regardless of adolescent hormone treatment (Schulz et al., 2006b). Thus, while ovarian hormones during adolescence decrease the time spent engaged in the lordosis posture, this time is replaced with goal-directed rather than non-specific locomotor behavior.
While it may seem counterintuitive that ovarian hormones defeminize female lordosis behavior during adolescence, estrogen-receptor mediated behavioral defeminization also occurs during the perinatal period sensitive period (Clemens and Gladue, 1978; Coniglio et al., 1973; Paup et al., 1972; for review see Wallen and Baum, 2002). Whether estradiol-induced defeminization of lordosis behavior during development negatively impacts female reproductive success is not known. One possibility is that defeminization of lordosis behavior is the trade-off for estradiol-dependent organization of other behaviors that facilitate reproductive success. For example, adolescent ovarian hormone exposure may increase social dominance/aggression in female hamsters, as has been demonstrated for adolescent testicular hormone exposure in male hamsters (Schulz et al., 2006a; Schulz and Sisk, 2006). Female hamsters are well known for their aggressive behavior (e.g. Payne and Swanson, 1970), and previous work suggests that socially dominant females give birth to larger litters than socially subordinate females (Huck et al., 1988). Thus, although defeminization of lordosis behavior by estradiol during adolescence may reduce the duration of mating interactions with males, organization of other behavioral systems may ensure overall reproductive success.
Organizational effects of gonadal steroid hormones during adolescence have also been found in rats. Exogenous testosterone administration in early adolescence defeminizes solicitation behavior (Bloch et al., 1995). In addition, female rats ovariectomized before adolescence display lower levels of testosterone-induced mounting and spend significantly less time with females than males during partner preference tests than do females ovariectomized after adolescence, suggesting that adolescent ovarian hormones masculinize mating behavior (de Jonge et al., 1988). Furthermore, it appears that the neonatal hormonal environment determines the extent to which adolescent ovarian hormones masculinize behavior: adolescent ovarian hormone exposure has little effect on the reproductive behavior of neonatally androgenized female rats (de Jonge et al., 1988). Thus, developmental processes occurring neonatally alter the neural substrate on which gonadal hormones act during the adolescent period, highlighting the complex and interactive nature of steroid-dependent periods of organization of behavior across development.
Ovarian hormones clearly organize some female behaviors during the adolescent period. What is less clear is whether the relative sensitivity to organizational effects of ovarian hormones is different over the course of postnatal development, because experiments comparing the effects of ovarian hormones across multiple development time points have not yet been conducted. Early (neonatal) ovariectomy necessarily removes any influence of ovarian hormones during adolescence, and unless the timing of hormone replacement is systematically varied, the unique contributions of neonatal and pubertal ovarian hormones to adult behavioral organization cannot be determined. Until such experiments are conducted we will not know whether adolescence is a particularly sensitive period for the organizing actions of ovarian hormones on adult behavior.
Neurobiological mechanisms of hormone-dependent organization of the adolescent brain
We have so far focused primarily on the compelling behavioral evidence for steroid hormone-dependent organization of neural circuits during adolescence. At the cellular level, what developmental events and processes are modulated by pubertal hormones to mold neural circuit structure? Perhaps not surprisingly, it appears that adolescent brain organization involves many of the same processes that underlie structural sexual differentiation during perinatal organization, and that pubertal hormones influence these processes, including cell number and cell group volume, cell death, and white matter volume.
Cell number and cell group volume
We recently discovered that gonadal hormone modulation of cell number and cell group volume is a potential mechanism for the active maintenance of sexual dimorphisms during adolescent development (Ahmed et al., 2008). This study focused on one female-biased cell group in the rat brain, the anteroventral periventricular nucleus of the hypothalamus (AVPV), and two male-biased cell groups, the sexually dimorphic nucleus of the preoptic area (SDN), and the medial amygdala. The cell birthdate marker bromodeoxyuridine (BrdU) was used to identify cells in the adult AVPV, SDN, and medial amygdala that were born during puberty. BrdU-immunoreactive cells were more numerous in the AVPV of females as compared to males, whereas they were more numerous in the male SDN and medial amygdala than females; these sex differences in BrdU cells paralleled sex differences in cell group volume. Prepubertal gonadectomy abolished sex differences in the number of BrdU-immunoreactive cells in adulthood, and resulted in corresponding changes in cell group volume. That is, prepubertal ovariectomy reduced AVPV BrdU cells and volume but did not affect SDN and amygdala BrdU cells, and prepubertal castration reduced BrdU cells and volume of the SDN and medial amygdala, but not AVPV. Some BrdU cells in both sexes also expressed either NeuN or GFAP, indicating that cells added to sexually dimorphic regions during puberty differentiate into functional neurons and glial cells. Thus, pubertal hormones modulate either cell proliferation or survival in sexually dimorphic cell groups in a sex- and brain region-dependent manner, and in this way contribute to the maintenance of structural and functional sex differences during remodeling of the adolescent brain.
Cell death
Hormone-modulated cell death appears to be responsible for the postnatal emergence of sex differences in volume of rat primary visual cortex (Nunez et al., 2001; Nunez et al., 2002). The number of cells in adult visual cortex is higher in male than in female rats, and this is due to greater rates of cell death during early puberty in females. Prepubertal ovariectomy abolishes the sex difference in neuron number in adulthood, indicating that ovarian hormones, acting during puberty promote cell death in visual cortex.
White matter volume
Testicular hormones may influence changes in white matter volume during adolescent brain development. Imaging studies of the human brain reveal linear increases in white matter volume during adolescence, with the rate of change and overall volume being greater in boys than in girls (Giedd et al., 1999; Lenroot and Giedd, 2006). The increase in white matter volume in males has recently been linked to testosterone androgen receptor (AR) activity (Perrin et al., 2008). White matter volume was measured in adolescent males, some of whom carried an AR gene variation with a higher number of CAG repeats, which decreases AR transcriptional activity. This study found a stronger positive correlation between testosterone levels and white matter volume in boys with the more efficient form of AR. Thus, individual variation in androgen receptor function may contribute to individual differences in neural transmission and conduction. Notably, testosterone appears to influence white matter volume by increasing axonal caliber and not myelination (Perrin et al., 2008).
Hormones and experience
It is clear from the examples above that pubertal steroid hormones can influence development of the adolescent brain by direct action within the nervous system. Hormones may also indirectly influence the adolescent brain by altering social interactions and experience. Experience is a powerful shaper of brain and behavioral development and operates via many of the same mechanisms that underlie hormonal influences on brain development. In this section, we compare mechanisms of hormone-dependent and experience-dependent organization of neural circuits.
Some experiences are common to all members of a species and provide sensory information about the environment in which the species has evolved. In “experience-expectant” brain development, experience is essential and must be encountered during a particular time in development for appropriate and adaptive nervous system development (Greenough et al., 1987). Examples of experience-expectant development include the requirement of exposure to light and visual patterns for proper development of visual cortex (Borges and Berry, 1978; Timney et al., 1978), and the requirement of exposure to language at particular a developmental time for normal language development (Fromkin et al., 1974; Kenneally et al., 1998). Many experience-expectant forms of neural patterning occur during critical periods of development, and arise through active elimination or pruning of overproduced synapses and strengthening of relevant neural circuits (Black and Greenough, 1997; Greenough et al., 1987). In contrast to experiences that are universal, some types of experience are particular to an individual and mediate “experience-dependent” brain development (Greenough et al., 1987). Experience-dependent development does not necessarily take place during a critical period, and occurs as new synapses are formed in response to significant events in the individual’s life. Synaptic connections that are activated and strengthened by experience are selectively integrated into a neural circuit.
Pubertal hormone effects on the adolescent brain, whether they are direct or indirect via altering social experience, are most likely a type of experience-dependent development, because the timing and amount of hormone exposure can vary greatly across individuals. Our work with the male Syrian hamster shows that the timing of the pubertal increase in gonadal hormone secretion is a variable that influences expression of social behaviors in adulthood. Furthermore, this influence of hormones must be via direct hormone action in the brain, because social experience was not manipulated in these experiments. During human adolescent development, however, changes in hormone secretion and changes in social experience are concomitant, and it is impossible to dissect out direct and indirect influences of hormones on behavioral development. To begin to address this question experimentally, we asked whether social experience during adolescence could ameliorate the adverse consequences of the absence of pubertal testosterone on sexual behavior in male hamsters.
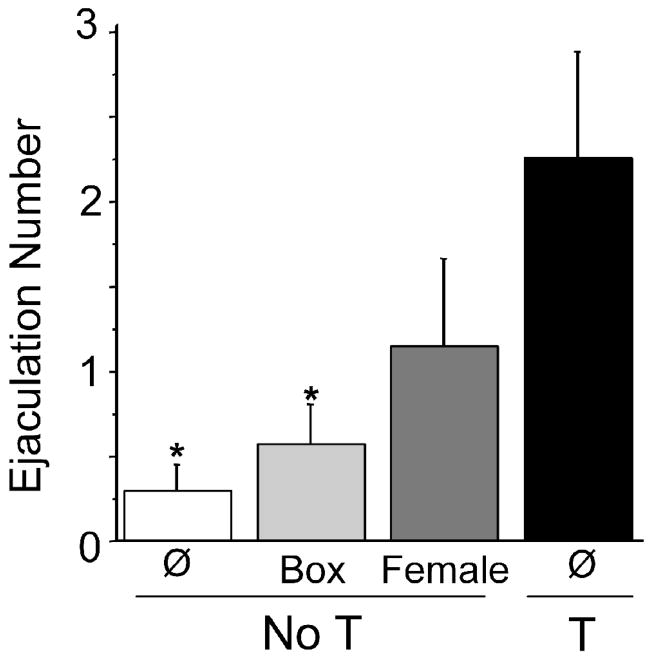
Hamsters were gonadectomized before puberty at three weeks of age. Between 4 and 8 weeks of age, during the normal time of puberty and adolescence, subjects were given either repeated (3x/week) 15-min non-contact exposure to estradiol- and progesterone-primed females enclosed in a mesh box (NoT+female), repeated exposures to an empty mesh box (NoT+mesh box), or no experience (NoT+Ø). Experience manipulations were discontinued at 8 weeks of age, and then at 11 weeks of age (7 weeks post-gonadectomy), all subjects received subcutaneous testosterone implants one week prior to behavior tests with a receptive female. Sexual behavior of these 3 groups of subjects was compared to that of a fourth group of males that experienced endogenous testosterone during puberty (T). Males in this group were gonadectomized as adults, received testosterone replacement seven weeks post-gonadectomy, and were tested with a receptive female one week later. Non-contact exposure of NoT males to estrous females during puberty partially restored several reproductive behaviors, including genital grooming and ejaculation. Ejaculation number (Figure 5) and ejaculation latency did not differ between NoT+female and T males. In contrast, the T group had more ejaculations than NoT+Ø and NoT+mesh box groups (Figure 5). In this experiment, plasma testosterone levels produced by the implants were unexpectedly low in the NoT+female animals (2.82 ± 0.36 ng/ml, p <0.038) compared to the NoT+mesh box (3.86 ± 0.17 ng/ml) and T groups (3.72 ± 0.19 ng/ml). Had T levels in NoT+female animals been similar to that of the T group, their behavior may have been even more like that of the T group, and different from the NoT+Ø and NoT+mesh box groups. Results from this experiment indicate that exposure to female sensory stimuli during adolescence can at least partially compensate for the absence of pubertal testosterone, and suggest that direct and indirect effects of hormones on behavior development may operate on the same neural substrates and via the same mechanisms. Pubertal hormones and experience may synergize to solidify the neural rewiring that takes place during adolescence.
Fig 5.
Adolescent social experience restores adult ejaculatory behavior in male Syrian hamsters lacking pubertal testosterone. In the absence of social experience, males gonodectomized (GDX) before puberty displayed significantly fewer ejaculations than positive control males GDX after puberty. However, adolescent social experience with a female partially restored ejaculation number in males GDX before puberty to the levels of the positive control group. Exposure to the mesh box alone did not restore ejaculations to the level of positive controls. Asterisk indicates p<0.05.
Summary and future directions
Here we reviewed the evidence that the adolescent brain is organized a second time by gonadal steroid hormones secreted during puberty, and described our recent experiment addressing whether the adolescent organization of mating behavior by pubertal hormones is regulated by a discrete sensitive period that opens and closes at the onset and offset of adolescence, respectively. We found that the sensitive period for testosterone-dependent organization of male mating behavior is likely not separable from the neonatal period, given that our “early-puberty” testosterone treatments were initiated immediately following the generally accepted endpoint of the perinatal period on postnatal day 10 and effectively organized adult behavior. These data indicate that the classical view of organizational and activational mechanisms of steroid action should be revised to incorporate an extended window of decreasing postnatal sensitivity to the organization of male mating behavior by steroid hormones that ends after puberty and adolescence.
Revision of the classical view of organizational and activational effects of steroid hormones necessarily leads to new questions. In the classical view, ovarian hormones play little or no role in brain and behavioral sexual differentiation, but evidence is accumulating that ovarian hormone exposure during adolescence permanently alters the adult behavior of females. What is not yet known is whether adult female behaviors are regulated by discrete adolescent sensitive periods, or whether behavioral organization is possible at earlier or later time points. Understanding sensitive period regulation of steroid-dependent organization will greatly enhance our understanding of the consequences of early or late pubertal onset in girls, which is a timely question considering that pubertal onset in girls has advanced substantially in recent years (Parent et al., 2003).
Another important area of investigation is to determine the mechanisms by which social and environmental factors mediate the effects of gonadal steroid hormones on behavior. We have demonstrated that social experiences ameliorate the behavioral deficits induced by the absence of gonadal hormones during adolescence, suggesting that social experiences may modulate the same neural circuits which are rewired and fine-tuned during adolescence to permit the appropriate display of adult behaviors. Future studies will focus on the neural mechanisms by which social experiences and pubertal hormones interact to organize the brain during adolescence. It will also be fruitful to determine how experience and pubertal hormones interact to influence other types of social behaviors such as aggression and parental behavior.
Finally, much remains to be learned about the similarities, differences, and relationship between the perinatal and peripubertal periods of organization. It seems clear that two-stage organization in males is a function of the two times in life that testosterone secretion becomes elevated. Furthermore, testosterone appears to masculinize and defeminize neural circuits during both times. In these respects, the perinatal and peripubertal periods are similar. However, it is not clear at this time whether both androgenic and estrogenic actions of testosterone are at play during peripubertal organization, as they are perinatally, and as already discussed, ovarian hormones appear to be a bigger player during female peripubertal organization than during perinatal organization. The potential role of elevated levels of adrenal steroids during adrenarche, which precedes puberty in humans, is also largely unexplored. Another notable difference between the perinatal and peripubertal periods, at least for the organization of male mating behavior, is the degree of organization that takes place. Sensitivity to steroid-dependent organization is less during adolescence than during early postnatal life, which is likely because the perinatal period of organization reduces the future malleability and plasticity of the circuits that are organized. Beyond this quantitative difference, whether there is a qualitative difference in the mechanisms through which perinatal and pubertal hormones sculpt structural sexual dimorphisms, e.g., modulation of cell proliferation, cell survival, cell phenotype, connectivity, is not clear. Although experimental study of the relationship between the perinatal and peripubertal periods of hormone-dependent organization is nascent, it seems safe to say that the perinatal period of organization, by determining the initial developmental trajectory of sexual differentiation, sets the parameters within which peripubertal hormones operate and foreshadows what is to come during adolescence.
Acknowledgments
The work reviewed from our laboratory was supported by R01-MH068764, T32-MH070343, NIH F31-MH070125, and NIH F32-MH068975.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed EI, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–97. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Differentiation of coital behavior in mammals: a comparative analysis. Neurosci Biobehav Rev. 1979;3:265–284. doi: 10.1016/0149-7634(79)90013-7. [DOI] [PubMed] [Google Scholar]
- Biehl MC, et al. The influence of pubertal timing on alcohol use and heavy drinking trajectories. Journal of Youth and Adolescence. 2007;36:153–167. [Google Scholar]
- Bischof HJ, et al. Limitations of the sensitive period for sexual imprinting: neuroanatomical and behavioral experiments in the zebra finch (Taeniopygia guttata) Behavioural Brain Research. 2002;133:317–322. doi: 10.1016/s0166-4328(02)00016-5. [DOI] [PubMed] [Google Scholar]
- Bischof HJ. Neural mechanisms of sexual imprinting. Animal Biology. 2003;53:89–112. [Google Scholar]
- Bischof HJ. Behavioral and neuronal aspects of developmental sensitive periods. Neuroreport. 2007;18:461–465. doi: 10.1097/WNR.0b013e328014204e. [DOI] [PubMed] [Google Scholar]
- Black JE, Greenough WT. How to build a brain: Multiple memory systems have evolved and only some of them are constructivist. Behavioral and Brain Sciences. 1997;20:558-+. [Google Scholar]
- Bloch GJ, et al. Prepubertal testosterone treatment of female rats: defeminization of behavioral and endocrine function in adulthood. Neurosci Biobehav Rev. 1995;19:177–86. doi: 10.1016/0149-7634(95)00065-m. [DOI] [PubMed] [Google Scholar]
- Borges S, Berry M. Effects of Dark Rearing on Development of Visual-Cortex of Rat. Journal of Comparative Neurology. 1978;180:277–300. doi: 10.1002/cne.901800207. [DOI] [PubMed] [Google Scholar]
- Brand T, Slob A. Peripubertal castration of male rats, adult open field ambulation and partner preference behavior. Behavioural Brain Research. 1988;30:111–117. doi: 10.1016/0166-4328(88)90141-6. [DOI] [PubMed] [Google Scholar]
- Bratberg GH, et al. Perceived pubertal timing, pubertal status and the prevalence of alcohol drinking and cigarette smoking in early and late adolescence: a population based study of 8950 Norwegian boys and girls. Acta Paediatrica. 2007;96:292–295. doi: 10.1111/j.1651-2227.2007.00102.x. [DOI] [PubMed] [Google Scholar]
- Burt SA, et al. Timing of menarche and the origins of conduct disorder. Archives of General Psychiatry. 2006;63:890–896. doi: 10.1001/archpsyc.63.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh SE. The sexual debut of girls in early adolescence: The intersection of race, pubertal timing, and friendship group characteristics. Journal of Research on Adolescence. 2004;14:285–312. [Google Scholar]
- Celio M, et al. Early maturation as a risk factor for aggression and delinquency in adolescent girls: a review. International Journal of Clinical Practice. 2006;60:1254–1262. doi: 10.1111/j.1742-1241.2006.00972.x. [DOI] [PubMed] [Google Scholar]
- Clemens LG, Gladue BA. Feminine sexual behavior in rats enhanced by prenatal inhibition of androgen aromatization. Horm Behav. 1978;11:190–201. doi: 10.1016/0018-506x(78)90048-x. [DOI] [PubMed] [Google Scholar]
- Coniglio LP, et al. Hormonal specificity in the suppression of sexual receptivity of the female golden hamster. J Endocrinol. 1973;57:55–61. doi: 10.1677/joe.0.0570055. [DOI] [PubMed] [Google Scholar]
- Coniglio LP, Clemens LG. Period of maximal susceptibility to behavioral modification by testosterone in the golden hamster. Horm Behav. 1976;7:267–282. doi: 10.1016/0018-506x(76)90033-7. [DOI] [PubMed] [Google Scholar]
- de Jonge FH, et al. Sexual behavior and sexual orientation of the female rat after hormonal treatment during various stages of development. Horm Behav. 1988;22:100–15. doi: 10.1016/0018-506x(88)90034-7. [DOI] [PubMed] [Google Scholar]
- Dubas JS. Cognitive abilities and physical maturation. In: Petersen AC, Brooks-Gunn J, editors. Encyclopedia of Adolescence. Garland Publishing; New York, NY: 1991. pp. 133–138. [Google Scholar]
- Eaton G. Effect of a single prepubertal injection of testosterone propionate on adult bisexual behavior of male hamsters castrated at birth. Endocrinology. 1970;87:934–40. doi: 10.1210/endo-87-5-934. [DOI] [PubMed] [Google Scholar]
- Eichmann F, Holst DV. Organization of territorial marking behavior by testosterone during puberty in male tree shrews. Physiol Behav. 1999;65:785–91. doi: 10.1016/s0031-9384(98)00230-3. [DOI] [PubMed] [Google Scholar]
- Field EF, et al. Neonatal and pubertal, but not adult, ovarian steroids are necessary for the development of female-typical patterns of dodging to protect a food item. Behavioral Neuroscience. 2004;118:1293–1304. doi: 10.1037/0735-7044.118.6.1293. [DOI] [PubMed] [Google Scholar]
- Fromkin V, et al. Development of Language in Genie - Case of Language Acquisition Beyond Critical Period. Brain and Language. 1974;1:81–107. [Google Scholar]
- Ge X, et al. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37:404–17. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge XJ, et al. Contextual amplification of pubertal transition effects on deviant peer affiliation and externalizing behavior among African American children. Developmental Psychology. 2002;38:42–54. doi: 10.1037//0012-1649.38.1.42. [DOI] [PubMed] [Google Scholar]
- Ge XJ, et al. It’s about timing and change: Pubertal transition effects on symptoms of major depression among African American youths. Developmental Psychology. 2003;39:430–439. doi: 10.1037/0012-1649.39.3.430. [DOI] [PubMed] [Google Scholar]
- Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Graber JA. Puberty in context. In: Hayward C, editor. Gender Differences at Puberty. Cambridge University Press; New York: 2003. [Google Scholar]
- Graber JA, et al. Is pubertal timing associated with psychopathology in young adulthood? Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Greenough WT, et al. Experience and brain development. Child Dev. 1987;58:539–59. [PubMed] [Google Scholar]
- Halpern CT, et al. Perceived physical maturity, age of romantic partner, and adolescent risk behavior. Prevention Science. 2007;8:1–10. doi: 10.1007/s11121-006-0046-1. [DOI] [PubMed] [Google Scholar]
- Hebbard PC, et al. Two organizational effects of pubertal testosterone in male rats: transient social memory and a shift away from long-term potentiation following a tetanus in hippocampal CA1. Exp Neurol. 2003;182:470–5. doi: 10.1016/s0014-4886(03)00119-5. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annual Review of Neuroscience. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hier DB, Crowley WF., Jr Spatial ability in androgen-deficient men. N Engl J Med. 1982;306:1202–5. doi: 10.1056/NEJM198205203062003. [DOI] [PubMed] [Google Scholar]
- Huck UW, et al. Social-Dominance and Reproductive Success in Pregnant and Lactating Golden-Hamsters (Mesocricetus-Auratus) under Seminatural Conditions. Physiology & Behavior. 1988;44:313–319. doi: 10.1016/0031-9384(88)90031-5. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R, et al. Early puberty is associated with mental health problems in middle adolescence. Social Science & Medicine. 2003;57:1055–1064. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- Kenneally SM, et al. Language intervention after thirty years of isolation: A case study of a feral child. Education and Training in Mental Retardation and Developmental Disabilities. 1998;33:13–23. [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lumia A, et al. Effects of androgen on marking and aggressive behavior of neonatally and prepubertally bulbectomized and castrated male gerbils. J Comp Physiol Psychol. 1977;91:1377–89. [Google Scholar]
- McCabe MP, Ricciardelli LA. Body image dissatisfaction among males across the lifespan - A review of past literature. Journal of Psychosomatic Research. 2004;56:675–685. doi: 10.1016/S0022-3999(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Michaud PA, et al. Gender-related psychological and behavioural correlates of pubertal timing in a national sample of Swiss adolescents. Molecular and Cellular Endocrinology. 2006;254:172–178. doi: 10.1016/j.mce.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Mueller SC, et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33:973–80. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, et al. Cell death in the development of the posterior cortex in male and female rats. J Comp Neurol. 2001;436:32–41. [PubMed] [Google Scholar]
- Nunez JL, et al. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52:312–21. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Parent AS, et al. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocrine Reviews. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- Paup DC, et al. Masculinization of the female golden hamster by neonatal treatment with androgen or estrogen. Horm Behav. 1972;3:123–31. doi: 10.1016/0018-506x(72)90014-1. [DOI] [PubMed] [Google Scholar]
- Payne AP, Swanson HH. Agonistic behaviour between pairs of hamsters of same and opposite sex in a neutral observation area. Behaviour. 1970;36:259. [PubMed] [Google Scholar]
- Pellis SM. Sex differences in play fighting revisited: traditional and nontraditional mechanisms of sexual differentiation in rats. Arch Sex Behav. 2002;31:17–26. doi: 10.1023/a:1014070916047. [DOI] [PubMed] [Google Scholar]
- Perrin JS, et al. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–24. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix C, et al. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–43. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Primus R, Kellogg C. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Hormones and Behavior. 1990;24:311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal status and pubertal age influence the responsiveness of the benzodiazepine/GABA receptor complex to environmental challenge in male rats. Brain Res. 1991;561:299–306. doi: 10.1016/0006-8993(91)91608-4. [DOI] [PubMed] [Google Scholar]
- Schulz KM, et al. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004a;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schulz KM, et al. Ovarian hormones partially defeminize female lordosis behavior during puberty. Society for Neuroscience, Vol. Program No. 538.20. Abstract Viewer/Itinerary Planner. Online; San Diego, CA. 2004b. [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006:254–255. 120–6. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Schulz KM, et al. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm Behav. 2006a;50:477–83. doi: 10.1016/j.yhbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Schulz KM, et al. Estradiol defeminizes lordosis behavior during adolescence in female Syrian hamsters. Society for Behavioral Neuroendocrinology; Pittsburg, PA. 2006b. [Google Scholar]
- Schulz KM, et al. Testosterone programs adult social behavior before and during, but not after, adolescence. doi: 10.1210/en.2008-1708. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JP, et al. Critical periods in the organization of systems. Dev Psychobiol. 1974;7:489–513. doi: 10.1002/dev.420070602. [DOI] [PubMed] [Google Scholar]
- Shrenker P, et al. The role of postnatal testosterone in the development of sexually dimorphic behaviors in DBA/1Bg mice. Physiol Behav. 1985;35:757–62. doi: 10.1016/0031-9384(85)90408-1. [DOI] [PubMed] [Google Scholar]
- Sisk CL, et al. Puberty: A finishing school for male social behavior. Annals of the New York Academy of Sciences. 2003;1007:189–198. doi: 10.1196/annals.1286.019. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Stewart J, Cygan D. Ovarian hormones act early in development to feminize adult open-field behavior in the rat. Hormones and Behavior. 1980;14:20–32. doi: 10.1016/0018-506x(80)90012-4. [DOI] [PubMed] [Google Scholar]
- Striegel-Moore RH, et al. Exploring the relationship between timing of menarche and eating disorder symptoms in black and white adolescent girls. International Journal of Eating Disorders. 2001;30:421–433. doi: 10.1002/eat.1103. [DOI] [PubMed] [Google Scholar]
- Swithers SE, et al. Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm Behav. 2008;54:471–7. doi: 10.1016/j.yhbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. Growth at Adolescence. Blakwell Scientific; Oxford: 1962. [Google Scholar]
- Timney B, et al. Development of Vision in Cats after Extended Periods of Dark-Rearing. Experimental Brain Research. 1978;31:547–560. doi: 10.1007/BF00239811. [DOI] [PubMed] [Google Scholar]
- Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff DW, et al., editors. Hormones, Brain and Behavior. Elsevier; 2002. pp. 385–423. [Google Scholar]
- Zehr JL, et al. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66:578–90. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]
- Zehr JL, et al. An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Hormones and Behavior. 2007a;52(4):427–35. doi: 10.1016/j.yhbeh.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, et al. An association of early puberty with disordered eating and anxiety in a population of undergraduate women and men. Hormones and Behavior. 2007b doi: 10.1016/j.yhbeh.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]