Abstract
Rationale: Higher rates of sepsis have been reported in minorities.
Objectives: To explore racial differences in the incidence and associated case fatality of severe sepsis, accounting for clinical, social, health care service delivery, and geographic characteristics.
Methods: Retrospective population-based cohort study using hospital discharge and U.S. Census data for all persons (n = 71,102,655) living in 68 hospital referral regions in six states.
Measurements and Main Results: Age-, sex- and race-standardized severe sepsis incidence and inpatient case fatality rates, adjusted incidence rate ratios, and adjusted intensive care unit (ICU) admission and case fatality rate differences. Of 8,938,111 nonfederal hospitalizations, 282,292 had severe sepsis. Overall, blacks had the highest age- and sex-standardized population-based incidence (6.08/1,000 vs. 4.06/1,000 for Hispanics and 3.58/1,000 for whites) and ICU case fatality (32.1 vs. 30.4% for Hispanics and 29.3% for whites, P < 0.0001). Adjusting for differences in poverty in their region of residence, blacks still had a higher population-based incidence of severe sepsis (adjusted rate ratio, 1.44 [95% CI, 1.42–1.46]) than whites, but Hispanics had a lower incidence (adjusted rate ratio, 0.91 [0.90–0.92]). Among patients with severe sepsis admitted to the ICU, adjustments for clinical characteristics and the treating hospital fully explained blacks' higher ICU case fatality.
Conclusions: Higher adjusted black incidence and the lower Hispanic incidence may reflect residual confounding, or it could signal biologic differences in susceptibility. Focused interventions to improve processes and outcomes of care at the hospitals that disproportionately treat blacks could narrow disparities in overall mortality from severe sepsis.
Keywords: severe sepsis, epidemiology, race, clinical practice variations
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
It has been reported that wide racial variation exists in the incidence of septicemia, with rates among nonwhites almost double those of whites. These observations persisted after adjusting for preexisting chronic illness and source of infection.
What This Study Adds to the Field
Higher adjusted black incidence and the lower Hispanic incidence in severe sepsis may reflect residual confounding, or could signal biologic differences in susceptibility.
Previous studies have reported wide racial variation in the incidence of septicemia (1, 2), with rates among nonwhites almost double those of whites. These observations persist after adjusting for preexisting chronic illness and source of infection (3). However, these racial differences in incidence could potentially be attributable to differences in socioeconomic status, and treatment and outcomes could be affected by region of residence and attendant provider access (4). Black and Hispanic patients live in geographically segregated regions and use systematically different hospitals than whites (5–7).
Severe sepsis, defined by international consensus conference criteria as an infection plus acute organ dysfunction, is a considerable public health problem that afflicts over 750,000 Americans each year, consumes considerable health care resources, and is associated with a hospital case fatality of 30 to 50% (8, 9). We extend prior work by using a larger, population-based sample, applying the diagnostic criteria for severe sepsis, and exploring the confounding effects of poverty, urbanicity, and region of residence on disease incidence and of case mix and treating hospital on intensive care unit (ICU) case fatality.
METHODS
Design and Data Sources
We conducted a retrospective population-based analysis of race-specific incidence and ICU case fatality rates for hospital-based infection and severe sepsis in six U.S. states (Florida, Massachusetts, New Jersey, New York, Virginia, and Texas). We obtained demographic and socioeconomic data from the 2000 U.S. Census and clinical data for hospitalized severe sepsis cases from the corresponding hospital discharge datasets (Calendar Year 2001). All data were obtained directly from the respective state and federal agencies. These six states were chosen because they were large and diverse, had a significant proportion of the U.S. population, and maintained high-quality hospital discharge data, including information on ICU use. The data were linked at the ZIP code (U.S. postal code) level, the smallest unit common to both population and hospitalization data.
Sample Selection and Definitions
Veterans' Administration (VA) and military hospitalizations are not included in state hospital discharge data. We restricted our analysis to the hospital referral regions (HRRs) whose central city was located inside our sample states. We used a previously validated approach to the identification of severe sepsis involving the cooccurrence of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for a bacterial or fungal infectious process and acute organ dysfunction (9). All hospitalizations for patients with HIV/AIDS were excluded due to missing age data in one state, a privacy-based reporting limitation. We categorized race into four groups: non-Hispanic black (black), non-Hispanic white (white), Hispanic, and “other or missing.” We excluded the “other or missing” group from our analyses because of its relatively small size, wide racial diversity, and inconsistent coding. Patients were defined as having ICU care if ICU length of stay was greater than 0 days or ICU charges were greater than $0. We used ICD-9 diagnosis codes to identify the presence of the Charlson-Deyo conditions (10) in primary and secondary diagnosis fields. We categorized all admissions as medical or surgical based on the diagnosis-related group. We categorized site of infection and classified organisms as gram positive or negative. ICU case fatality was determined at hospital discharge. We modeled differences in ICU case fatality, rather than hospital case fatality, based on the assumption that some non-ICU deaths may have been among patients treated palliatively in anticipation of death. For each ZIP code, we defined its urbanicity using the U.S. Census assignment (urban, suburban, or rural) and poverty level using the proportion of white residents below the poverty line (0–9%, 10–19%, 20–29%, 30–39%, and 40+%).
The majority of blacks and Hispanics in our sample were cared for in a subset of the hospitals in our sample. For descriptive purposes, we report the characteristics of the set of hospitals used by blacks and by Hispanics, and the larger set used by whites, according to size, urbanicity, teaching status, and measures of severe sepsis processes of care and outcomes.
Data Analyses
To compare severe sepsis incidence, ICU admission, and ICU case fatality among races, we generated rates standardized directly by gender and age (in 5-yr increments) and tested for differences by χ2. We also tested for case mix differences that may have affected severe sepsis rates, including underlying diseases, medical versus surgical diagnosis, site of infection, gram-positive status, and acute organ dysfunction.
Because racial groups are segregated geographically by region of residence in the United States, we further used Poisson regression analysis clustered on the patient's zip code of residence to adjust for differences in urbanicity (urban, suburban, and rural) and poverty in our multivariable analysis of racial differences in population-based rates of severe sepsis cases. We used the proportion of whites below poverty as a measure of ZIP code–level economic privation, not a proxy for individual socioeconomic status (11, 12). Results were similar using the proportion of all persons below poverty, and using negative binomial regression.
To address potential differences in processes and outcomes of care between hospitals frequented by these geographically segregated minority groups, we used ordinary least squares linear probability models (corrected for heteroscedasticity of unknown form) for computation of the adjusted marginal probability of ICU admission and of ICU case fatality if a patient was black (or Hispanic) instead of white. (13) These models included patient demographics, site of infection, gram positivity, number of Charlson-Deyo conditions, and the presence of metastatic cancer. The hospital was treated as a fixed effect.
All multivariable regressions used the Huber/White/sandwich estimator of variance to adjust standard errors for clustering (persons within ZIP codes and patients within hospitals). Database management and calculation of descriptive statistics were performed using Visual FoxPro and Excel (Microsoft Corporation, Redmond, WA) and regression analyses were performed using Stata version 9.0 (Stata Corp., College Station, TX).
The study was reviewed and approved by the University of Pittsburgh Institutional Review Board.
RESULTS
Population Characteristics
In 2001, the six states comprised 77,707,427 million persons, or 27% of the total U.S. population, living in 89 HRRs and 6,627 ZIP codes. After excluding the 21 HRRs whose central hospitals lay outside the six states, our population sample comprised 75,512,925 persons, 4,410,270 of whom were excluded for “other” race or race not provided, leaving 71,102,655 persons (91.6% of the six-state population) in 68 HRRs (76%) and 5,957 ZIP codes (90%). The mean age of the population was 36.1 years, 54.4% were female, 72.7% lived in urban ZIP codes, and 59% lived in nonimpoverished ZIP codes (<10% of white population below poverty level). The largest racial group was white (66.2%), followed by Hispanic (19.7%) and black (14.1%). The mean age was much lower in blacks and Hispanics compared with whites (mean age: 31.8 and 29.4 vs. 39.5 yr, P < 0.0001). Blacks and Hispanics were more likely to live in urban ZIP codes (81.1 and 78.5% vs. 61.2%, P < 0.0001) and less likely to live in nonimpoverished ZIP codes (39.0 and 30.4% vs. 71.7%, P < 0.0001) than whites.
The 68 HRRs included 1,061 hospitals, of which 827 (78%) had one or more severe sepsis cases, 187 (18%) were teaching hospitals, and 67 (6%) had 450 beds or more. During 2001, there were 9,813,549 non-HIV/AIDS hospitalizations among individuals residing in the study ZIP codes, 873,271 of which were excluded for “other or missing” race, leaving 8,940,278 hospitalizations in the sample. Among these hospitalizations, 282,292 met criteria for severe sepsis, which represents 3.97 cases per 1,000 population. Severe sepsis incidence rose with age and was more common in men. The most common infections in our severe sepsis cohort were pneumonia, bloodstream, and genitourinary tract infections. Gram-positive infections accounted for 11.7% of cases. Three-quarters of all patients had only one coded organ dysfunction. Overall hospital case fatality among patients with severe sepsis was 24.6%; the case fatality rate among those admitted to an ICU (n = 142,656) was slightly higher at 29.9%.
Severe Sepsis Incidence Rates
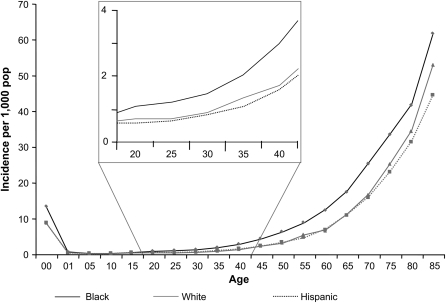
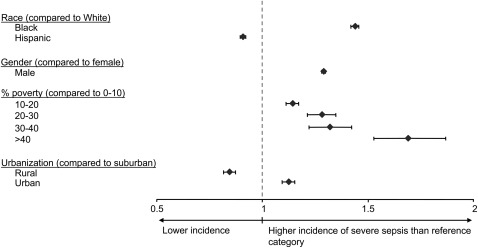
After direct age and sex standardization by ZIP code, blacks had the highest rate of severe sepsis (6.08 per 1,000), followed by Hispanics (4.06 per 1,000) and whites (3.58 per 1,000), corresponding to a rate ratio (RR) of 1.7 for blacks and 1.1 for Hispanics, compared with whites. The large difference in incidence among blacks was evident in those aged 20 and older (Figure 1). In a Poisson regression model of severe sepsis incidence rates at the ZIP code level, additional adjustment for ZIP code urbanicity and poverty mitigated the RR among blacks (adjusted RR [ARR],1.44 [1.42–1.46]) and reversed its direction among Hispanics (ARR, 0.91 [0.90–0.92]). Infancy (ARR, 12.42 [11.96–12.90]) and age older than 34 years increased risk of severe sepsis when compared with those aged 30 to 34 years (ARR, 1.43 [1.38–1.49] among those aged 35 to 39 years, and increasing to an ARR of 59.0 [57.0–61.0] among those ≥ 85 yr). Other predictors of higher severe sepsis incidence rates included male sex, poverty, and urbanicity (Figure 2).
Figure 1.
Population-based rates of severe sepsis by age and race in six U.S. states, 2001. Black rates are depicted as diamonds, non-Hispanic white rates as squares, and Hispanic rates as triangles. In addition to higher rates among newborns, blacks' higher incidence rate is evident among adults as young as 20 years. Incidence rates among non-Hispanic whites and Hispanics are comparable throughout the age range. pop = population.
Figure 2.
Adjusted rate ratios of severe sepsis in six U.S. states, 2001. Rate ratios reflect age, race, sex, ZIP code–level poverty, and urbanicity-adjusted estimates of relative risk for severe sepsis. Blacks, men, and persons residing in poor and urban ZIP codes are at higher risk compared with whites residing in affluent, suburban areas; Hispanics and those living in rural regions have lower risk.
Severe Sepsis ICU Admission and Case Fatality Rates
Blacks had the highest age- and sex-standardized hospital case fatality compared with Hispanics or whites (26.1 vs. 24.6% and 24.2%, P < 0.0001). Blacks with severe sepsis were less likely to receive ICU care (47.5 vs. 55.3% and 49.6%, P < 0.0001), and more likely to die if they were admitted to the ICU (32.1 vs. 30.4% and 29.3%, P < 0.0001). As would be expected given chronic disease epidemiology in the United States, there were differences in comorbidities among black, Hispanic, and white patients with severe sepsis (Table 1). In addition, blacks and Hispanics had much lower rates of respiratory sources of infection and higher rates of primary bacteremia. Black patients, and to a lesser degree Hispanics, were more likely to receive treatment in large, urban teaching hospitals than whites (Table 2). In addition, the subset of hospitals in our sample treating the black and Hispanic patients had severe sepsis process and outcomes data among white patients suggestive of either greater illness severity or lower quality than the larger set of hospitals treating the white patients. Specifically, the hospitals had slightly higher severe sepsis rates (or more conscientious organ failure coding) among those with infections (22.1 and 21.2% vs. 20.4%, P < 0.0001), higher ICU admission rates (44.9 and 42.1% vs. 41.7%, P < 0.0001), and slightly higher ICU case fatality rates (31.6 and 31.2% vs. 30.3%, P < 0.0001).
TABLE 1.
CHARACTERISTICS OF PATIENTS WITH SEVERE SEPSIS IN SIX U.S. STATES
| Non-Hispanic Black | Hispanic | Non-Hispanic White | ||
|---|---|---|---|---|
| (n = 23,202) | (n = 16,434) | (n = 103,065) | P Value | |
| Underlying comorbidity | ||||
| Peptic ulcer disease, % | 8.0 | 9.6 | 15.1 | <0.0001 |
| Neoplasm, % | 9.4 | 8.8 | 11.5 | <0.0001 |
| Mild liver disease, % | 3.5 | 7.8 | 3.9 | <0.0001 |
| Severe liver disease, % | 2.3 | 4.4 | 2.2 | <0.0001 |
| Renal disease, % | 5.4 | 4.5 | 4.7 | <0.0001 |
| Metastatic neoplasm, % | 4.5 | 3.7 | 5.2 | <0.0001 |
| Diabetes, % | 18.7 | 19.7 | 15.1 | <0.0001 |
| Diabetes with complications, % | 5.4 | 5.5 | 4.0 | <0.0001 |
| Peripheral vascular disease, % | 3.3 | 2.8 | 3.8 | <0.0001 |
| Myocardial infarction, % | 1.3 | 1.2 | 2.6 | <0.0001 |
| Cerebrovascular disease, % | 3.6 | 2.3 | 2.3 | <0.0001 |
| Dementia, % | 1.6 | 1.2 | 1.7 | <0.0001 |
| Hemiplegia, % | 1.4 | 1.1 | 1.0 | <0.0001 |
| Rheumatologic diseases, % | 2.2 | 1.6 | 1.8 | <0.0001 |
| Comorbidity count | 0.75 | 0.76 | 0.76 | 0.0012 |
| Acute organ dysfunction | ||||
| Number of systems, % | ||||
| 1 | 75.4 | 77.6 | 77.0 | <0.0001 |
| 2 | 18.4 | 16.9 | 17.6 | <0.0001 |
| 3 | 5.2 | 4.5 | 4.5 | <0.0001 |
| 4+ | 1.1 | 1.0 | 1.0 | 0.3876 |
| Organ system, % | ||||
| Respiratory | 44.4 | 42.3 | 41.1 | <0.0001 |
| Cardiovascular | 21.8 | 22.2 | 25.6 | <0.0001 |
| Renal | 35.3 | 30.2 | 32.7 | <0.0001 |
| Hematologic | 20.3 | 24.4 | 19.4 | <0.0001 |
| CNS | 8.9 | 8.4 | 9.4 | <0.0001 |
| Hepatic | 1.3 | 1.4 | 1.3 | 0.3876 |
| Site of infection, % | ||||
| Respiratory | 38.0 | 38.6 | 42.6 | <0.0001 |
| Primary bacteremia | 36.3 | 34.2 | 30.6 | <0.0001 |
| Genitourinary | 32.4 | 31.6 | 32.8 | <0.0001 |
| Abdominal | 13.6 | 13.1 | 13.4 | <0.0001 |
| Device-related | 6.2 | 4.9 | 4.6 | <0.0001 |
| Wound/soft tissue | 7.4 | 9.4 | 9.9 | <0.0001 |
| CNS | 1.4 | 1.0 | 0.7 | <0.0001 |
| Endocarditis | 1.0 | 0.9 | 0.9 | 0.3469 |
| Other/undetermined | 23.5 | 20.6 | 21.7 | <0.0001 |
| Gram positive, % | 17.0 | 15.6 | 15.8 | <0.0001 |
| ICU admission, % | 54.3 | 52.0 | 53.6 | <0.0001 |
| Medical condition | 74.6 | 74.7 | 72.6 | <0.0001 |
| Surgical condition | 25.4 | 25.3 | 27.4 | <0.0001 |
Definition of abbreviations: CNS = central nervous system; ICU = intensive care unit.
All reported rates are crude, unadjusted rates.
TABLE 2.
DISTRIBUTION OF BLACK, HISPANIC, AND WHITE PATIENTS WITH SEVERE SEPSIS BY HOSPITAL CHARACTERISTIC
| Non-Hispanic Black | Hispanic | Non-Hispanic White | ||
|---|---|---|---|---|
| Hospital Characteristics | (n = 23,202) | (n = 16,434) | (n = 103,065) | P Value |
| Number of beds >449, % | 34.6 | 30.4 | 25.6 | <0.0001 |
| Urban, % | 69.2 | 61.8 | 60.8 | <0.0001 |
| Teaching, % | 53.8 | 37.0 | 33.7 | <0.0001 |
Ordinary least squares linear probability models indicated that the marginal probability of ICU admission if a patient was black instead of white (but otherwise identical on measured covariates and treated at the same hospital) was +0.7% (+0.2 to +1.1%) higher; if the patient was Hispanic instead of white, their probability of ICU admission was lower by 1 absolute percentage point (−1.0% [−1.5 to −0.4%]). The ICU case fatality was not statistically different if a patient was black (+0.7% [−0.02 to 1.3%]) or Hispanic (−0.8% [−1.8 to +0.1%]).
DISCUSSION
Consistent with Martin's findings (2), we found significant racial variation in the incidence and outcome of severe sepsis. Blacks do indeed have a higher rate of severe sepsis—almost double that of whites. The difference in incidence was evident by age 20 and continued throughout the adult lifespan. After accounting for differences in poverty and geography, black race remained independently associated with higher severe sepsis incidence. In contrast, Hispanic ethnicity appeared protective, conditional on similar regional urbanicity and poverty.
We were intrigued by these differences and considered several possible explanations. We considered whether blacks had a higher incidence due to susceptibility to particular infections or particular organ system dysfunctions. However, the severe sepsis syndrome characteristics were not markedly different between the groups with regard to site of infection, microbiologic etiology, and both the number and type of organ dysfunction. Another possibility was a difference in baseline population health. Although we could not evaluate the health status of the entire population, the burden of chronic conditions among severe sepsis cases did not differ substantially across racial groups. Furthermore, because differences in severe sepsis incidence occurred by age 20, differences in chronic disease are unlikely to explain differences in incidence, especially considering that patients with HIV/AIDS were excluded from the analysis. Blacks and Hispanics generally receive fewer elective procedures than whites, and the proportion of severe sepsis cases associated with operative procedures was much lower in these minorities than among whites (Table 3).
TABLE 3.
PROPORTION OF SEVERE SEPSIS CASES WITH THE MOST COMMON SURGICAL DIAGNOSIS RELATED GROUPS IN SIX U.S. STATES, BY RACE/ETHNICITY
| Proportion of Severe Sepsis Cases (%)
|
|||
|---|---|---|---|
| Non-Hispanic Black | Hispanic | Non-Hispanic White | |
| Diagnosis Related Group | (n = 23,202) | (n = 16,434) | (n = 103,065) |
| Major small and large bowel procedures | 3.3 | 2.4 | 21.7 |
| OR procedure for infectious and parasitic diseases | 4.1 | 2.3 | 14.0 |
| Extensive OR procedure unrelated to principal diagnosis | 3.4 | 2.2 | 13.5 |
| Other respiratory system OR procedures | 2.0 | 1.3 | 7.9 |
| Major cardiovascular procedures | 1.1 | 0.9 | 8.9 |
Definition of abbreviation: OR = operating room.
We noted that people living in areas with higher rates of poverty had higher severe sepsis incidence. This finding was consistent across all six states and is consistent with the class effect postulated by Isaacs and Schroeder, in which people in lower socioeconomic classes are hypothesized to engage in less healthy lifestyles (14), or the effect hypothesized by Marmot, in which those in lower classes experience higher stress due to lower social control (15). Nevertheless, the black disparity in incidence was independent of the disproportionate representation of blacks in ZIP codes from poorer areas. On the other hand, Hispanics had a lower adjusted incidence of severe sepsis than would have been predicted by their residence in poorer areas. This could occur if the exposure variable—proportion of whites below poverty line—overestimated Hispanic privation (i.e., that Hispanics in any given ZIP code were better off than whites), which is unlikely. Or it could indicate that the socioeconomic health gradient among Hispanics is less steep than among whites or blacks, a phenomenon observed among recent Mexican immigrants in particular (16).
Overall mortality for blacks is higher than for whites, due both to greater incidence and higher case fatality. We found that the higher ICU case fatality rates among blacks disappeared after accounting for differences in clinical characteristics and the specific treating hospital. This suggests that higher crude ICU case fatality among black patients is attributable in part to differences in measured illness severity, and also in part to the unmeasured illness severity among patients treated at hospitals to which blacks are disproportionately admitted or differences in the quality of these hospitals. Blacks were more likely to be admitted to urban teaching hospitals with slightly higher (white) case fatality. This phenomenon—blacks being treated at large, urban teaching hospitals and hospitals with poorer quality processes and outcomes of care—has been previously reported in the literature on acute myocardial infarction outcomes and is a significant contributor to populationwide disparities in treatment and outcome of acute myocardial infarction among blacks (5, 7, 17).
Of course, residual unmeasured differences in behavior (e.g., tobacco use), case mix (e.g., neutropenia) (18), pharmaceutical use (e.g., statins [19, 20]), health care resources, social factors such as poverty, and within-hospital variations in treatment by race (e.g., intensity [21, 22]) could still persist, despite our statistical analyses. Nevertheless, it also is possible that the greater adjusted incidence among blacks and the lower incidence among Hispanics could be due to differences in the underlying biologic response to infection and injury. Severe sepsis is often characterized as a syndrome of overly exuberant inflammation, and other inflammatory diseases, such as systemic lupus erythematosis, have also been reported to be more common and more severe in blacks (23). One possible explanation is different genetic susceptibility to sepsis between blacks and whites. Individuals of European and African ancestry likely faced different environmental selection pressures from infectious agents. For example, sickle cell disorders, which inhibit the malarial life cycle, are inherited predominantly among peoples originating from areas where malaria is endemic. Unlike sickle cell disorders, sepsis does not follow Mendelian inheritance, but a large number of studies have suggested both the incidence and outcome of severe sepsis are influenced by functional polymorphisms in many innate immunity genes (24). Recently, Ness and colleagues examined the allele frequency of several polymorphisms previously implicated in sepsis and other inflammatory disorders, reporting considerable racial differences in their distributions (25).
A biologic basis for racial disparities in susceptibility and outcome of sepsis has potentially important implications for future therapeutic interventions. Understanding why these differences exist, including careful evaluation of genetic variance, could provide insight into the pathophysiology of sepsis. Furthermore, it is possible that different strategies will have different clinical efficacy in different racial groups, akin to recent findings in heart failure, with implications for future trial design in sepsis (26). Hypotheses regarding potential race-associated differences in innate susceptibility do not belie the reality that differences in incidence and outcomes among blacks in particular could be narrowed with the eradication of disparities in primary care access and in income, education, and social stressors that underlie differences in healthy lifestyles.
There are important limitations to our study. Statewide hospital discharge datasets are good but imperfect data sources for generating rates of diseases within hospitalized patients. The identification of severe sepsis using ICD-9 codes to identify infection and acute organ dysfunction may be insensitive (27), and misclassification of infections may affect case fatality estimates (28). However, we would not expect coding error to differ by race within the same hospital in our ICU admission and case fatality models. Also, because we are using hospital discharge data, we can explore inpatient case fatality only, and not longer term postdischarge outcomes.
The source of race data for the numerator (severe sepsis cases in hospital administrative data) and the denominator (census-based population studies) may not be strictly comparable since self-reporting of racial/ethnic group may be limited among critically ill patients. When we compare the distribution of whites, blacks, Hispanics, and “other” race/ethnic groups in our six-state database with the Medicare Provider Analysis and Review (MedPAR) files from the same six states (the race data for MedPAR comes from Social Security enrollment data files, which have 95% sensitivity for black race, but <60% sensitivity for Hispanic ethnicity [positive predictive value for black race and Hispanic ethnicity are both >96%] [29]), we find that black race may be slightly underreported in our data (8.6 vs. 9.2%) and Hispanic ethnicity is more frequently reported (7.3 vs. 3.2%). Thus, it appears that blacks in our discharge data may sometimes be misclassified as white, Hispanic, or other, or to have missing race data, which would bias our findings toward the null.
We estimated our incidence model at the ZIP code level, the smallest unit of geography for which we had both hospital discharge data and census data. Smaller units of geography, such as the block group or census track, are more sensitive to identification of the socioeconomic gradients (30). It is possible that our ZIP code–level analyses failed to detect gradients that would be found at smaller units of aggregation. It is less likely, but possible, that our ZIP code–level findings would be contrary to those that would be found using smaller units of aggregation.
The generalizability of our findings to patients with HIV/AIDS or patients of VA/military hospitals is unknown. Both these groups have a high percentage of minorities. Thus, their exclusion from the numerator counts in our analysis likely leads to an underestimate of the discrepancy in severe sepsis incidence rates. Our data were limited to six states in the northeast, southeast, and south. Generalizability to other areas may be limited, although our states do comprise a large proportion of the U.S. population and our results were generally consistent across states.
In summary, in this large-scale population-based study, we found that black race is associated with higher incidence and case fatality from severe sepsis, whereas Hispanic ethnicity is associated with lower incidence. Blacks' residence in regions with greater economic privation contributes to greater severe sepsis incidence. The remaining adjusted incidence differences may reflect residual confounding or could signal biologic differences in susceptibility. Blacks' clinical characteristics and differences in the hospitals to which they are admitted explained their greater ICU case fatality rate. The explanatory power of the particular treating hospitals may represent unmeasured illness severity among all patients in those hospitals and/or lower quality of care. Thus, it is possible that the overall mortality disparity among blacks could be partially ameliorated by focused interventions to improve processes and outcomes of care at the hospitals that disproportionately treat blacks.
Supported by a career development award from the NIA (K08 AG21921 to A.E.B.).
This study was presented at the Society for Critical Care Medicine Annual Meeting in Phoenix, Arizona, in January 2005.
Originally Published in Press as DOI: 10.1164/rccm.200703-480OC on November 1, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Crit Care Med 2007;35:763–768. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979. through 2000. N Engl J Med 2003;348:1546–1554. [DOI] [PubMed] [Google Scholar]
- 3.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med 2006;34:2576–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smedley B, Stith A, Nelson A, editors. Unequal treatment: confronting racial and ethnic disparities in health care. Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Board on Health Sciences Policy, Institute of Medicine. Washington, DC: National Academy Press; 2002.
- 5.Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation 2005;112:2634–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jha AK, Orav EJ, Li Z, Epstein AM. Concentration and quality of hospitals that care for elderly black patients. Arch Intern Med 2007;167:1177–1182. [DOI] [PubMed] [Google Scholar]
- 7.Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital-level racial disparities in acute myocardial infarction treatment and outcomes. Med Care 2005;43:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA 1997;278:234–240. [PubMed] [Google Scholar]
- 9.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 11.Kwok RK, Yankaskas BC. The use of census data for determining race and education as SES indicators: a validation study. Ann Epidemiol 2001;11:171–177. [DOI] [PubMed] [Google Scholar]
- 12.Geronimus AT, Bound J. Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples. Am J Epidemiol 1998;148:475–486. [DOI] [PubMed] [Google Scholar]
- 13.Wooldridge JM. Econometric analysis of cross-section and panel data. Cambridge, MA: MIT Press; 2002.
- 14.Isaacs SL, Schroeder SA. Class: the ignored determinant of the nation's health. N Engl J Med 2004;351:1137–1142. [DOI] [PubMed] [Google Scholar]
- 15.Marmot M. Social determinants of health inequalities. Lancet 2005;365:1099–1104. [DOI] [PubMed] [Google Scholar]
- 16.Zsembik BA, Fennell D. Ethnic variation in health and the determinants of health among Latinos. Soc Sci Med 2005;61:53–63. [DOI] [PubMed] [Google Scholar]
- 17.Bradley EH, Herrin J, Wang Y, McNamara RL, Webster TR, Magid DJ, Blaney M, Peterson ED, Canto JG, Pollack CV Jr, et al. Racial and ethnic differences in time to acute reperfusion therapy for patients hospitalized with myocardial infarction. JAMA 2004;292:1563–1572. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med 2007;146:486–492. [DOI] [PubMed] [Google Scholar]
- 19.Gupta R, Plantinga LC, Fink NE, Melamed ML, Coresh J, Fox CS, Levin NW, Powe NR. Statin use and hospitalization for sepsis in patients with chronic kidney disease. JAMA 2007;297:1455–1464. [DOI] [PubMed] [Google Scholar]
- 20.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ 2006;333:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnato AE, Chang CC, Saynina O, Garber AM. Influence of race on inpatient treatment intensity at the end of life. J Gen Intern Med 2007;22:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips RS, Hamel MB, Teno JM, Bellamy P, Broste SK, Califf RM, Vidaillet H, Davis RB, Muhlbaier LH, Connors AF Jr. Race, resource use, and survival in seriously ill hospitalized adults. The SUPPORT Investigators. J Gen Intern Med 1996;11:387–396. [DOI] [PubMed] [Google Scholar]
- 23.Ward MM, Studenski S. Clinical manifestations of systemic lupus erythematosus: identification of racial and socioeconomic influences. Arch Intern Med 1990;150:849–853. [PubMed] [Google Scholar]
- 24.Yende S, Kammerer CM, Angus DC. Genetics and proteomics: deciphering gene association studies in critical illness. Crit Care 2006;10:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ness RB, Haggerty CL, Harger G, Ferrell R. Differential distribution of allelic variants in cytokine genes among African Americans and white Americans. Am J Epidemiol 2004;160:1033–1038. [DOI] [PubMed] [Google Scholar]
- 26.Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049–2057. [DOI] [PubMed] [Google Scholar]
- 27.Ollendorf DA, Fendrick AM, Massey K, Williams GR, Oster G. Is sepsis accurately coded on hospital bills? Value Health 2002;5:79–81. [DOI] [PubMed] [Google Scholar]
- 28.Gedeborg R, Furebring M, Michaelsson K. Diagnosis-dependent misclassification of infections using administrative data variably affected incidence and mortality estimates in ICU patients. J Clin Epidemiol 2007;60:155–162. [DOI] [PubMed] [Google Scholar]
- 29.Arday SL, Arday DR, Monroe S, Zhang J. HCFA's racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 30.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health 1997;18:341–378. [DOI] [PubMed] [Google Scholar]