Abstract
Rationale: Acute airway response, measured as cross-shift change in FEV1, to cotton dust may lead to subsequent chronic loss of lung function in exposed workers.
Objectives: To explore the association between the magnitude and frequency of cross-shift change and chronic loss of FEV1.
Methods: Four hundred eight cotton workers and 417 silk workers from Shanghai textile mills were observed prospectively for 20 years, with cross-shift measurements at baseline and follow-up surveys at approximate 5-year intervals. To account for repeated measures of 5-year change, generalized estimating equations were used to estimate the relationship between the magnitude of cross-shift change in FEV1 (ΔFEV1) and subsequent 5-year annualized change. Linear regression models were used to examine the association between the number of drops in cross-shift FEV1 (ΔFEV1 < 0) and annualized change over the entire study period.
Measurements and Main Results: Exposure to cotton dust was associated with a 10 ml/year decrement in 5-year annualized FEV1 decline. In addition, every 10 ml in ΔFEV1 drop was associated with an additional 1.5 ml/year loss in annualized FEV1 decline. The association between the frequency of drops and annualized decline was stronger for cotton workers than for silk workers over the entire study period.
Conclusions: Cotton workers had larger and more frequent drops, as well as excessive chronic declines in FEV1, than did silk workers. The magnitude and frequency of cross-shift drops were associated with chronic loss in FEV1 over the entire 20-year period examined.
Keywords: cross-shift FEV1 change, chronic changes in lung function, cotton textile workers, cotton dust, occupational lung disease
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
There is a strong relationship between acute airway response and chronic loss in FEV1 in cotton textile workers. Chronic airway obstruction is most likely a consequence of both long-term exposure and repeated acute airway responses.
What This Study Adds to the Field
Cotton workers had larger and more frequent drops, as well as excessive chronic declines in FEV1, than did silk workers. The magnitude and frequency of cross-shift drops were associated with chronic loss in FEV1 over the entire 20-year period examined.
Occupational exposure to cotton dust is associated with acute airway responses and chronic airway obstruction. The acute airway response is expressed typically as a cross-shift drop in FEV1 (ΔFEV1), which may be or may not be accompanied by the byssinosis syndrome (1–3). It is generally believed that acute airway responses are reversible in the early stage or after a short-term exposure (4, 5). In contrast, chronic airway obstruction may result from continuous and prolonged exposure (6, 7).
Although the mechanism of acute bronchoconstriction and chronic airway limitation remains unclear, epidemiologic studies and animal experiments have suggested that airway inflammation and immune response are involved in the process and are triggered by gram-negative bacterial endotoxin contaminating the cotton dust (8). Some studies suggest that atopy may play a role, possibly in nonspecific allergic hypersensitivity, which indirectly influences the pathogenesis of byssinosis by increasing airway reactivity to cotton dust inhalation (9). The mechanism of chronic airway response in cotton textile workers remains unclear.
Previous studies have focused mostly on acute airway responses to cotton dust exposure in exposed workers. Although longitudinal cohort studies over the past two decades have documented accelerated loss in pulmonary function in cotton dust–exposed workers, important questions remain unanswered. For example, the relationship between acute airway responses, as measured by cross-shift drops in FEV1, and chronic airflow limitation is not well understood despite the reported relationship between cotton dust exposure and both cross-shift drops and chronic airway obstruction (10–12). A few cohort studies have assessed the possible link between acute and chronic lung function changes in cotton workers (7, 13). These studies, however, were limited by lack of exposure assessment, an unavailable control group, or a short follow-up time. Longitudinal studies with longer observation time are needed to address the acute–chronic relationship and ultimately to understand the natural history and underlying mechanism of chronic airway disease observed in workers exposed to cotton and other vegetable dusts.
The present study was conducted to determine whether repeated cross-shift drop in FEV1 is a significant predictor for the excessive annual declines in FEV1 observed in cotton textile workers. Biologically, it is plausible that repeated acute airway responses per se may lead to chronic airway changes. Alternatively, acute responses may lead to chronic airway remodeling as a direct result of cotton dust exposure. This epidemiologic study cannot distinguish between these two biologic hypotheses. However, we did attempt to distinguish the relevance of the magnitude of the acute responses from the frequency of such responses. In both instances, the relationship between the acute and chronic outcome measures was evaluated conditional on level of cotton dust exposure. Some of the results in this study were previously reported at the annual American Thoracic Society (ATS) meeting in 2006 (14).
METHODS
The cohort was established in 1981; it consisted of 447 cotton workers and 472 silk workers from Shanghai, China. Detailed information on subject selection and methodology is described elsewhere (10). Follow-up surveys were undertaken at approximate 5-year intervals—in 1986, 1992, 1996, and 2001. Thus, the study contained 5-point measurements of spirometry, environmental, and questionnaire data. The overall follow-up rates were 74% or above throughout the observation.
Cross-shift spirometric measurements were performed at baseline and in 1986 and 1992 for both cotton workers and silk workers; in 1996, they were performed for those cotton workers who remained at work. Preshift spirometric data were obtained from each survey for both groups. A total of 408 cotton workers and 417 silk workers who provided cross-shift measurements were included in this analysis, accounting for 91 and 88% of the original cotton and silk cohorts, respectively. Among them, 173 (42%) in the cotton group and 175 (42%) in the silk group had cross-shift FEV1 data from all of three surveys; an additional 143 (35%) in the cotton group and 141 (34%) in the silk group had data from two surveys.
A modified ATS standardized questionnaire was administered to collect detailed information with regard to work history, respiratory symptoms/diseases, and smoking history at each survey (10). Inhalable air samples for airborne cotton dust in various work areas were measured using a Vertical Elutriator (General Metalworks, Mequon, WI) and endotoxin assays were performed on the dust samples at all but the last survey (11). Estimated cumulative individual exposures to cotton dust and endotoxin were calculated based on workplace dust measurements and specific working period.
All spirometric maneuvers were performed in accordance with ATS criteria (15), with consistent methodology, conducted by the same technicians, using an 8-L water-sealed, filled spirometer (W.E. Collins, Braintree, MA), which was calibrated twice a day with a 3-L syringe. All values were corrected to conditions of body temperature and pressure saturated with water vapor.
Statistical Analysis
Cross-shift changes in FEV1 (ΔFEV1) were calculated by subtracting post-shift FEV1 from preshift FEV1, which represented acute FEV1 changes expressed in milliliters. Chronic changes in FEV1 were expressed as annualized changes in FEV1, which were based on differences in preshift measurements between each 5-year period over the entire 15 or 20 years of follow-up. To account for repeated measures in estimating the association between across-shift change in FEV1 on chronic 5-year changes, generalized estimating equations (GEEs) were used to fit linear regression models. The GEE model takes account of the repeated and correlated measurement design in its covariate structure and makes full use of available data (16).
Thus, there were four observations (at ∼5-yr intervals) per subject over the 20 years of follow-up. The outcome, change in FEV1 over each 5-year period, was annualized and expressed in milliliters per year. Using GEEs, we examined whether ΔFEV1 could predict subsequent changes in FEV1 over the next 5-year period, adjusting for age, height, sex, smoking, and exposure. Exposure was expressed either as a dichotomous variable (i.e., cotton vs. silk) or as cumulative exposures to cotton dust and endotoxin expressed as continuous variables in models restricted to cotton workers. Cumulative exposures to cotton dust and endotoxin were calculated based on geometric means of job-specific sampling data and years of work in specific work areas. Years since retirement were added to the model to assess the impact of cessation of exposure on chronic change in FEV1.
We also used linear regression to estimate the association between the frequency of cross-shift drops (representing repeatability) and annualized decline in FEV1 over the entire follow-up period (15 yr). Cross-shift drop was defined as a negative change in FEV1 over the work shift; annualized decline was computed by dividing the total change in FEV1 between 1981 and 1996 by 15, which represented an average annual rate of change. In linear regression models, drop was treated as a binary variable, which was defined as 1 if ΔFEV1 was less than 0, and as 0 otherwise. Furthermore, the number of cross-shift drops was defined as 0, 1, 2, or 3. Models were stratified for cotton workers and silk workers. All analyses were conducted using the SAS personal computer software (version 9.1, 2002; SAS Institute, Inc., Cary, NC), using PROC GENMOD or GLM. (Additional details about the methods and model fitting are provided in the online supplement.)
RESULTS
There were no statistically significant differences in age, height, and sex between the cotton and silk groups, although smoking was more common in the former (Table 1). Despite slightly higher baseline FEV1 in cotton workers compared with silk workers, significantly greater annualized declines were seen in cotton workers over either 15 or 20 years. Cross-shift changes in FEV1, on average, were below zero at each survey in both groups, implying drops after shift work at textile workshops (Table 2). Cotton workers, however, exhibited significantly greater drops than their silk counterparts at each survey.
TABLE 1.
DEMOGRAPHIC DATA AT BASELINE AND LAST SURVEY IN COTTON AND SILK WORKERS
| Cotton Workers (n = 408) | Silk Workers (n = 417) | |
|---|---|---|
| Age, yr | 37.1 (10.4) | 36.1 (10.5) |
| Height, cm | 163.9 (7.5) | 162.5 (7.2) |
| Sex, male, n (%) | 190 (46.6) | 178 (42.7) |
| Smoking, n (%) | 148 (36.3)‡ | 111 (26.6) |
| Pack-years* | 7.9 (9.3) | 9.2 (10.0) |
| Endotoxin exposure, EU/m3/yr† | 48,479.5 (43,780.2) | — |
| Dust exposure, mg/m3/yr† | 19.3 (13.3) | — |
| Years worked† | 27.0 (7.9) | 28.2 (7.3) |
| Baseline FEV1, ml | 2,915.3 (720.9) | 2,885.1 (662.2) |
| Annualized changes in FEV1 over 15 yr, ml/yr | −32.9 (20.1)§ | −28.9 (18.9) |
| Annualized changes in FEV1 over 20 yr, ml/yr | −29.2 (22.5)§ | −25.0 (21.1) |
Unless otherwise stated, data are presented as means (SD).
Calculated among ever-smokers only.
Data from the last survey.
P = 0.03 when compared with silk workers.
P = 0.01 when compared with silk workers.
TABLE 2.
MEANS (SD) OF ΔFEV1 IN THREE SURVEYS AMONG COTTON AND SILK GROUPS
| Cotton
|
Silk
|
|||||
|---|---|---|---|---|---|---|
| N | ΔFEV1 (ml) | ΔFEV1 (%) | n | ΔFEV1 (ml) | ΔFEV1 (%) | |
| 1981 | 391 | −57.9 (154.9)* | −1.98 (5.49)* | 376 | −5.6 (131.9) | −0.02 (4.82) |
| 1986 | 284 | −47.8 (135.3)† | −1.77 (6.62)† | 307 | −26.1 (115.9) | −0.87 (4.22) |
| 1992 | 222 | −54.2 (120.9)‡ | −1.87 (4.42)§ | 225 | −20.2 (98.9) | −0.69 (3.78) |
| 1996 | 119 | −66.6 (141.5) | −2.15 (5.51) | 0 | — | — |
Definition of abbreviation: ΔFEV1 = cross-shift change in FEV1.
Calculations are based on all available data at each survey.
P < 0.0001.
P = 0.04.
P = 0.001.
P = 0.003 in comparison with silk workers.
The relationship between drops and long-term declines in FEV1 over 15 years was assessed with all available data from both groups. ΔFEV1 was significantly associated with subsequently annualized 5-year changes in FEV1, with an excess of 1.4 ml/year for every 10-ml drop of ΔFEV1, after adjustment for exposure status and potential confounding factors (Table 3). Exposure to cotton dust added an additional decline of 10 ml/year for the cotton workers, in contrast to those working at silk mills. When the analysis was restricted to cotton workers only, ΔFEV1 data collected in 1996 were also included; the annualized 5-year decline in FEV1 was slightly greater (i.e., 1.5 ml /yr for every 10 ml in drop) over the 20-year period, after accounting for cumulative exposure and confounders (Table 4). Exposure to 1 EU/m3 of endotoxin resulted in an annualized decline in FEV1 by 0.04 ml, whereas the association with cotton dust was in the positive direction. Smoking contributed to an additional annualized decline of 7 ml. (Pack-year was associated with an annualized decline of 0.36 ml [−0.78 to 0.06] when smoking status was removed.) No significant interaction was found between exposure and smoking.
TABLE 3.
DETERMINANTS OF ANNUALIZED 5-YEAR CHANGES IN FEV1 (ml/yr) OVER 15 YEARS OF FOLLOW-UP FOR COTTON AND SILK WORKERS
| Estimate (95% CI) | P Value* | |
|---|---|---|
| Age, yr | −0.11 (−0.41 to 0.62) | 0.69 |
| Height, cm | −0.07 (−0.78 to 0.65) | 0.86 |
| Male sex | −10.88 (−18.06 to −3.70) | 0.03 |
| Smoking | −0.01 (−0.02 to 0.01) | 0.74 |
| Cotton vs. silk | −9.70 (−16.77 to −2.63) | 0.005 |
| ΔFEV1 drop, 10 ml | −1.43 (−1.78 to −1.07) | <0.0001 |
Definition of abbreviations: CI = confidence interval; ΔFEV1 = cross-shift change in FEV1.
A generalized estimating equation model was used, in which all variables but sex and exposure status are time dependent.
Tests for whether coefficients are different from zero.
TABLE 4.
DETERMINANTS OF ANNUALIZED 5-YEAR CHANGES IN FEV1 (ml/yr) OVER 20 YEARS OF FOLLOW-UP FOR COTTON WORKERS
| Estimate (95% CI) | P Value* | |
|---|---|---|
| Age, yr | −0.48 (−1.34 to 0.38) | 0.27 |
| Height, cm | −0.53 (−1.24 to 0.17) | 0.13 |
| Male sex | −2.11 (−12.48 to 16.69) | 0.78 |
| Smoking | −6.79 (−4.29 to 17.87) | 0.23 |
| Years of retirement | 0.05 (−1.42 to 1.51) | 0.94 |
| Exposure to endotoxin, EU/m3 | −0.04 (−0.02 to 0.01) | 0.58 |
| Exposure to dust, mg/m3 | 2.92 (−6.91 to 12.76) | 0.56 |
| ΔFEV1 drop, 10 ml | −1.52 (−2.01 to −1.04) | <0.0001 |
Definition of abbreviations: CI = confidence interval; ΔFEV1: cross-shift change in FEV1.
A generalized estimating equation model was used, in which all variables but sex and exposure status are time dependent.
Tests for whether coefficients are different from zero.
The frequency of cross-shift drops varied across subjects over the multiple surveys. Eighty-eight percent (88%) of cotton workers experienced cross-shift drops (ΔFEV1 < 0) at least once out of the three surveys (baseline, 1986, and 1992), in contrast to 78% of silk workers, whose magnitude of drops was significantly smaller at each survey. In addition, more cotton workers than silk workers had multiple cross-shift drops (46 vs. 34%). When the calculation was restricted to the 173 cotton workers and 175 silk workers who had complete cross-shift measurement data, repeated cross-shift drops occurred in 70% of cotton workers and in 55% of silk workers. The difference was statistically significant (P < 0.01) by trend test, using either the data from all workers or the restricted data.
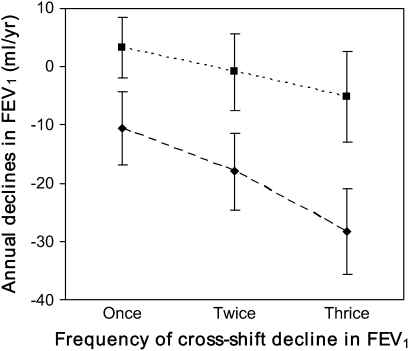
The association between multiple drops and annualized declines in FEV1 over 15 years was estimated by group, while adjusting for age, height, sex, and smoking. Using all the available data, for 408 cotton workers and 417 silk workers, the analysis showed greater annualized declines with multiple drops in both the silk and the cotton groups (Figure 1). However, the magnitude of long-term declines was greater for cotton workers than for the corresponding silk workers. In comparison with the respective reference group (no ΔFEV1 drop at all), cotton workers who had two and three negative ΔFEV1 had significantly larger declines in FEV1 (P = 0.007 and P = 0.0001, respectively), whereas no similar significant differences were seen in silk workers (P > 0.05). Furthermore, the analysis was restricted to the 173 cotton workers and 175 silk workers for whom there were complete ΔFEV1 measurements in all three surveys. A steeper gradient and greater magnitude of the declines were displayed, in which cotton workers with one, two, and three cross-shift drops had declines of 23.6, 30.4, and 41.9 ml/year, respectively. The corresponding rates of declines in silk workers were 15.4, 17.9, and 24.0 ml/year, respectively.
Figure 1.
Annualized changes in FEV1 (ml/yr) over 15 yr associated with repeated cross-shift drops at the first three surveys among 408 cotton and 417 silk workers, estimated by multiple linear regression models, in which age, height, smoking status, and sex were adjusted. ΔFEV1 drop was defined as ΔFEV1 < 0. P = 0.007 and P = 0.0001 for cotton workers with two and three drops, respectively, in comparison with the cotton reference group (without a drop); P > 0.05 for silk workers with two or three drops in comparison with the silk reference group. Solid squares, silk; solid diamonds, cotton.
DISCUSSION
It is plausible that chronic airway obstruction observed in cotton textile workers is a result of continuous exposure, repeated acute airway responses, or both (17). The current analysis, taking advantage of long-term follow-up data over 15 and 20 years, was undertaken to address the relationship among acute airway responses and chronic functional changes and exposures to cotton dust and endotoxin. The major strengths of this study lay in the long-time observation with repeated measurements, high follow-up rates, and available assessments of exposure to both cotton dust and endotoxin. The results suggest that exposure to cotton dust/endotoxin is a significant risk factor for cross-shift drops in FEV1. Moreover, the cross-shift drop in FEV1 is strongly associated with chronic decline in FEV1.
The magnitude of cross-shift drop in FEV1 was associated with annualized declines in FEV1 over the following 5-year interval. Specifically, every 10-ml drop predicted an additional 1.5 ml per annum or a total of 7.5 ml of decline in FEV1 over 5 years in cotton workers. In a 5-year follow-up study, Glindmeyer and coworkers (7) observed a group of yarn manufacturing workers in the United States with different shifts, and reported annual declines in FEV1 of 11.2, 34.6, and 35.4 ml/year for shifts 1, 2, and 3, respectively, associated with a 200-ml cross-shift drop. Our findings are consistent in magnitude with these results for the second- and third-shift workers. In a 6-year follow-up study of Canadian grain elevator workers, the degree of acute changes in FEV1 over one work shift was correlated with the subsequent decrease in lung function (18). These studies support our finding that cross-shift drop in FEV1 predicts longitudinal changes in lung function among workers exposed to organic dust. Cross-shift drop contributes 7 to 9 ml/year to the annual loss in FEV1, given that the average cross-shift drops in the cotton workers ranged from 48 to 67 ml (Table 2). This result means that, in cotton workers, most of the excess annual loss is attributed to the cross-shift drop. Therefore, the result highlights the role of acute airway changes in consequent chronic airway obstruction.
It is noteworthy that repeated cross-shift drops are also an important predictor for the magnitude of chronic airway changes. The models using either unrestricted or restricted data showed a gradient of the chronic declines in FEV1 with repeated drops over the follow-up observation. Silk workers displayed a similar trend, but to a lesser extent, which may be because silk workers had smaller cross-shift drops in comparison with cotton workers. To our knowledge, ours is the first study to report the association of repeated cross-shift airway responses with chronic airway obstruction in cotton workers. The results may be interpreted in two ways: first, a generic association between acute and chronic airway responses exists, with the former predicting the latter; second, exposure to cotton dust induces significant cross-shift drops in FEV1 that are, in turn, associated with long-term loss of lung function in exposed workers. It is likely that both exposure and the short-term (cross-shift) airway response play a part in the development of chronic airway obstruction in exposed populations.
To identify whether repeated drops are related to the intensity of exposure, we compared the cumulative exposure levels of cotton dust and endotoxin over 15 years and over lifetime among the subgroups by frequency of the drops. No consistent trend was observed across the subgroups. The lowest exposure level of either cotton dust or endotoxin, however, was found in the subgroup that did not experience a cross-shift drop at all. This result implies that the intensity of exposure is associated with the occurrence of the cross-shift airway response but not necessarily with repeated acute airway responses. The response variation between individuals to what appears to be the same level of exposure is probably determined by susceptibility factors that have yet to be understood. Acute airway response is usually believed to be mediated through allergic and/or nonallergic mechanisms (8), in which inherited characteristics may play a role. If acute airway response is in the physiologic pathway leading to chronic airway obstruction, cross-shift changes can be used in identifying susceptible individuals among exposed workers and in evaluating the efficiency of environmental control.
It remains uncertain whether long-term exposure is a direct causal factor for chronic airway changes or whether it exerts adverse effects mostly through inducing repeated cross-shift airway responses. One of the reasons for the uncertainty is that no clear exposure–response relationship was observed with the data. There are two possible explanations for the lack of exposure–response relationship. One is that the estimated cumulative exposure did not reflect the actual level of individual exposures due to exposure misclassification; we estimated individual exposures based on workplace air-sampling data and not on personal sampling data. Another possible explanation is that the chronic airway obstruction may be influenced by an inherited predisposition, as suggested by a recent study from our group (19). Further study with more accurate exposure assessment is required to evaluate the exposure response relationships. Likewise, more research is needed to explore the precise role of genetic factors in cotton dust–related airway diseases, so as to understand better the underlying mechanism of cotton dust–related chronic airway obstruction.
Several limitations in our study should be pointed out. First, the data analysis was performed in 91% of the cotton and 88% of the silk workers out of the original cohort, based on the availability of cross-shift data. To examine whether respiratory health status in the excluded workers differed from those who were included, we compared their FEV1 at baseline and at each follow-up survey. The result showed that the excluded workers, in both the cotton and the silk groups, had lower FEV1 than the included workers. However, this difference was not statistically significant, implying an unsubstantial healthy worker survivor effect in both groups. Then, even within the included subjects, there were missing values of cross-shift FEV1 at a certain time point, which might have led to an underestimate of the association between repeated drop and chronic changes in lung function. Lack of personal air-sampling data of cotton dust and endotoxin is another limitation. Moreover, the air sampling from work areas was not performed throughout the entire period of follow-up but only during the months comprising the 5-year periodic surveys. This intermittent sampling might be another source of exposure misclassification.
In summary, the current data indicate that cotton workers experience greater and more repeatable drops in FEV1, and excess chronic loss in FEV1, than do their silk worker counterparts. On the basis of the results showing a relationship between cross-shift drops and chronic declines in FEV1, we conclude that the chronic airway obstruction observed in cotton textile workers is most likely a consequence of both long-term exposure and repeated cross-shift airway responses. The occurrence and repetition of cross-shift drops in FEV1 may be a potent predictor for the subsequent development and magnitude of chronic airway obstruction in cotton textile workers.
Supplementary Material
Acknowledgments
The authors thank the members of the Shanghai field team; professors Gu Xue-qi, Lu Pei-lian, and Ye Ting-ting of Shanghai Medical College of Fudan University; the First Hospital of the Shanghai Textile Bureau; workers and staff of the First and Second Textile Mills and the First Silk Mill; Dr. David Wegman and Dr. Steve Olenchock; Ms. Marcia Chertok and Ms. Janna Frelich for research assistance; and Mr. Michael Whitmer for assistance in endotoxin analysis.
Supported by NIOSH grants R01OH02421 and ES00002.
This article contains an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200702-318OC on November 1, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Castellan RM, Olenchock SA, Hankinson JL. Acute bronchoconstriction induced by cotton dust: dose-related responses to endotoxin and other dust factors. Ann Intern Med 1984;101:157–163. [DOI] [PubMed] [Google Scholar]
- 2.Rylander R, Haglind P, Lundholm M. Endotoxin in cotton dust and respiratory function decrement among cotton workers in an experimental cardroom. Am Rev Respir Dis 1985;131:209–213. [DOI] [PubMed] [Google Scholar]
- 3.Jennison E, Jacobs RR. Evaluation of the association of acute over shift change in pulmonary function and atopy using OSHA cotton dust surveillance data. Am J Ind Med 1994;25:737–747. [DOI] [PubMed] [Google Scholar]
- 4.Haglind P, Rylander R. Exposure to cotton dust in an experimental cardroom. Br J Ind Med 1984;41:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merchant JA, Halprin GM, Hudson AR, Kilburn KH, McKenzie WM Jr, Bermanzohn P, Hurst DJ, Hamilton JD, Germino VH Jr. Evaluation before and after exposure the pattern of physiological response to cotton dust. Ann N Y Acad Sci 1974;221:38–43. [DOI] [PubMed] [Google Scholar]
- 6.Beck GJ, Schachter EN, Maunder LR. The relationship of respiratory symptoms and lung function loss in cotton textile workers. Am Rev Respir Dis 1984;130:6–11. [DOI] [PubMed] [Google Scholar]
- 7.Glindmeyer GW, Lefante JJ, Jones RN, Rando RJ, Weill H. Cotton dust and across-shift change in FEV1 as predictors of annual change in FEV1. Am J Respir Crit Care Med 1994;149:584–590. [DOI] [PubMed] [Google Scholar]
- 8.Kunkel SL, Chensue SW, Standiford TI, Strieter RM. Endotoxin-dependent sytokine networks. In: Brigham KL, editor. Endotoxin and the lungs. New York; Marcel Dekker; 1994. pp. 305–320.
- 9.Mundie TG, Ainsworth SK. Etiopathogenic mechanisms of bronchoconstriction in byssinosis: a review. Am Rev Respir Dis 1986;133:1181–1185. [DOI] [PubMed] [Google Scholar]
- 10.Christiani DC, Ye TT, Wegman DH, Eisen EA, Dai HL, Lu PL. Cotton dust exposure, cross-shift drop in FEV1, and five-year change in lung function. Am J Respir Crit Care Med 1994;150:1250–1255. [DOI] [PubMed] [Google Scholar]
- 11.Christiani DC, Wang XR, Pan LD, Zhang HX, Sun BX, Dai HL, Eisen E, Wegman DH, Olenchock SA. Longitudinal changes in pulmonary function and respiratory symptoms in cotton textile workers: a fifteen-year follow-up study. Am J Respir Crit Care Med 2001;163:847–853. [DOI] [PubMed] [Google Scholar]
- 12.Wang XR, Eisen EA, Zhang ZH, Sun BX, Dai HL, Pan LD, Wegman DH, Olenchock SA, Christiani DC. Respiratory symptoms and cotton dust exposure: results of a 15-year follow-up observation. Occup Environ Med 2003;60:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuskin E, Ivankovic D, Schachter EN, Witek TJ. A ten-year follow-up study of cotton textile workers. Am Rev Respir Dis 1991;143:301–305. [DOI] [PubMed] [Google Scholar]
- 14.Wang XR, Zhang HX, Sun BX, Dai HL, Hang JQ, Eisen EA, Christiani DC. Acute airway response and chronic change in lung function over 20-years among cotton textile workers [abstract]. Proc Am Thorac Soc 2006;3:A20. [Google Scholar]
- 15.American Thoracic Society. Standardization of spirometry: 1987 update. Am Rev Respir Dis 1987;116:1285–1298. [DOI] [PubMed] [Google Scholar]
- 16.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22. [Google Scholar]
- 17.Becklake MR. Relationship of acute obstructive airway change to chronic (fixed) obstruction. Thorax 1995;50:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabona M, Chan-Yeung M, Enarson D, MacLean L, Dorken L, Schulzer M. Host factors affecting longitudinal decline in lung spirometry among grain elevator workers. Chest 1984;85:782–786. [DOI] [PubMed] [Google Scholar]
- 19.Hang JQ, Zhou W, Wang XR, Zhang HX, Sun BX, Dai HL, Su L, Christiani DC. Microsomal epoxide hydrolase, endotoxin, and lung function decline in cotton textile workers. Am J Respir Crit Care Med 2005;171:165–170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.