Abstract
Concepts relating to the natural history of chronic obstructive pulmonary disease (COPD) arise most importantly from the classic study of Fletcher and colleagues (The Natural History of Chronic Bronchitis and Emphysema, Oxford University Press, New York, 1976). This study, which evaluated working English men over 8 years, was used to construct a proposed life-long natural history. Although this is a classic study that has greatly advanced understanding of COPD, it has a number of limitations. Its duration is relatively short compared with the duration of COPD, so it is more cross-sectional than longitudinal. It was unable to distinguish among varied “natural histories.” It assessed primarily the FEV1, and the natural history of other features of COPD is largely undescribed. With advances in understanding the clinical features of COPD and with the development of evaluating new tools to assess patients with COPD, longitudinal studies evaluating COPD in novel ways and for longer durations are needed.
Keywords: lung function, severity, symptoms, biomarkers
The progressive nature of chronic obstructive pulmonary disease (COPD) is believed to be sufficiently fundamental that it is included in the definition adopted in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (1). COPD, however, progresses slowly and variably and is often diagnosed relatively late in its course. Moreover, COPD is heterogeneous. Studies of the natural history of COPD, therefore, are difficult, both because of the size of the study needed and the duration required to obtain truly longitudinal data. Such studies are crucially needed, because interventions designed to alter the natural history of COPD hold the promise of altering the generally poor long-term prognosis of this condition.
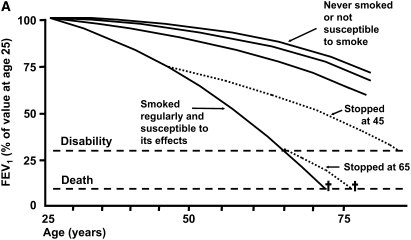
The classic monograph of Fletcher and colleagues, The Natural History of Chronic Bronchitis and Emphysema, published in 1976, remains the landmark reference for the natural history of COPD (2). This monograph presented conclusions based on an 8-year prospective study of working men in London. It was motivated, in large part, to test the hypothesis that mucus hypersecretion is related to progressive loss of lung function, the so-called British hypothesis. Conclusions relating to life-long natural history of COPD, which resulted in the much reproduced diagram, frequently termed the “Fletcher-Peto curve” (Figure 1A) (3), were not based on life-long longitudinal data, but were compiled from the set of 8-year data. This is nearly a cross-sectional dataset compared with the expected human lifespan. Nevertheless, the concepts proposed in this analysis remain the most common summary of COPD natural history and continue to form the basis for understanding the progression of this disorder.
Figure 1.
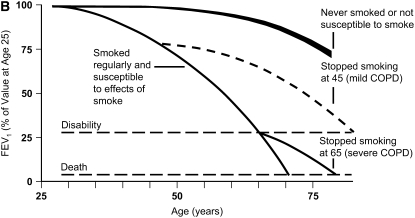
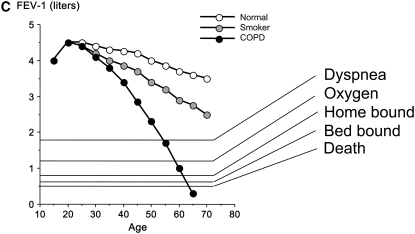
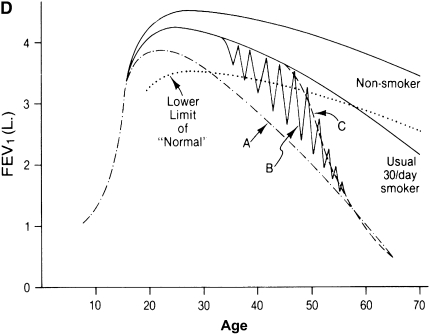
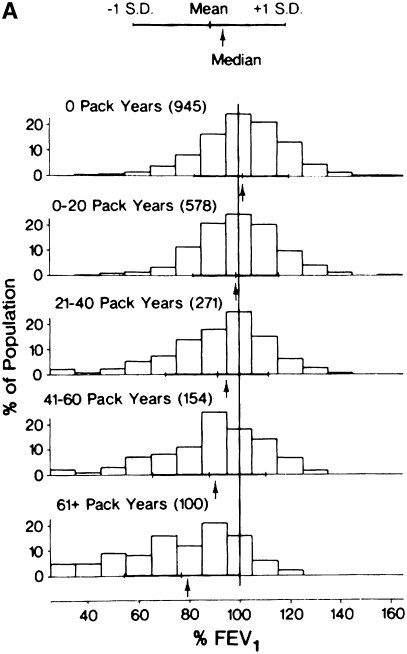
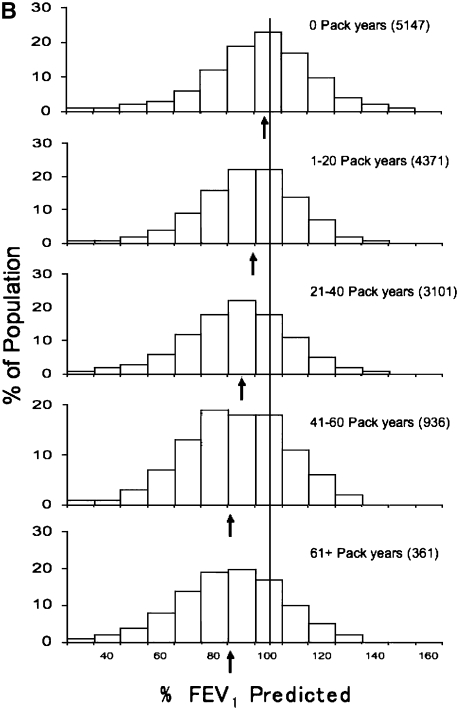
Natural history of chronic obstructive pulmonary disease (COPD). (A) Fletcher and Peto suggested a “natural history” for the airflow limitation in patients with COPD based on their 8-year study of working English men (3). Their exclusion of nonsmokers who develop COPD may have led to this group being relatively understudied. Reprinted by permission from Reference 3. (B) One of many derivative versions of the Fletcher-Peto curve, presented in the GOLD (Global Initiative for Chronic Obstructive Lung Disease) guidelines slide set, which has been rendered in black and white by the authors. Reprinted by permission from Reference 1. (C) A version prepared by the author using data from Reference 2. As airflow worsens, symptoms increase, but there is tremendous variability among individual patients in this regard. (D) Variable “natural histories” that can lead to severe COPD as described by Burrows (34). Although these may represent distinct pathophysiologic processes, by the time diagnosis is made, they may be impossible to distinguish using FEV1 since their natural histories will overlap. Reprinted by permission from Reference 34.
The major outcome variable on which Fletcher and colleagues focused was expiratory airflow limitation defined as FEV1 measured by spirometry (2). This was entirely reasonable because the major underlying question being evaluated in their study was the relationship between mucus hypersecretion and airflow limitation. Fletcher and colleagues were certainly aware of other relevant outcome measures. They present evidence and make a strong conclusion that “chest infections,” what would currently be termed “exacerbations,” are strongly related to mucus hypersecretion, for example (2, 4). Nevertheless, the focus on the FEV1 together with the compelling visual impact of the Fletcher-Peto curve has resulted in the FEV1 becoming the condicio sine qua non for COPD progression.
As pointed out by Fletcher and colleagues, in normal nonsmoking individuals, lung function declines with age through adulthood (2, 3). This rate of decline accelerates with increasing age. Aging is associated with structural changes in the alveolar wall including increase in the size and number of alveolar pores (5, 6), which may enlarge sufficiently to form fenestrae together with effacement of alveolar walls, and increase in alveolar size (7, 8). Whether these are related, however, remains unknown. At present, neither the pathogenesis of senile emphysema nor the anatomic basis for loss of expiratory airflow with “normal aging” is established.
Among individuals who smoke, expiratory airflow is lost at an increased rate (2, 3). On average, this is approximately twice that associated with normal aging (Figures 1A–1C). However, there is considerable variation in the rate at which lung function is lost, a fact also recognized by Fletcher and colleagues. Modest degrees of physiologic compromise are often undiagnosed, perhaps because patients can avoid symptoms of dyspnea by gradually restricting activity and thus by avoiding the tachypnea-induced hyperinflation that is believed to be the most common mechanism for dyspnea (9). As a result, individuals are typically diagnosed with COPD when sufficient physiologic compromise results in undeniable symptoms. Often this occurs only after more than half of the initial lung function is lost, resulting in relatively late diagnosis. Fletcher and colleagues suggested, from their data, that approximately 13% of smokers would be diagnosed with COPD (see p. 83 of Reference 1). This has been rounded off and misquoted, stating that 15% of smokers will get COPD. In fact, the percentage of smokers who develop COPD, using current definitions, is more likely around 50% (10).
Fletcher and colleagues did not comment about accelerated loss of airflow among nonsmokers. Although less common than in smokers, a percentage of nonsmokers also develop COPD, a finding that has been observed consistently (Figure 2) (10, 11). The prevalence of nonsmokers with COPD will depend on the prevalence of smoking in a population. In the United States, 20% of those who have low lung function and 20% of those who die of COPD are nonsmokers (12, 13). This represents approximately 25,000 deaths annually in the United States, and is therefore a significant public health problem. Nevertheless, and perhaps as a consequence of nonsmokers who develop COPD being omitted from the Fletcher-Peto curve, few studies have been performed in this group, and little is known regarding their natural history and clinical course. In fact, this underlines one of the unspoken rules in epidemiology: the only risk factors that can be identified are those that are assessed. It is generally stated that 85–90% of COPD is caused by smoking in the Western world. This is likely wrong and Marsh and coworkers have suggested that only half of cases of COPD are caused by smoking (14).
Figure 2.
Distribution of FEV1 values in relationship to smoking history. Note that there is a reduction in FEV1 with increasing smoking history. For each smoking history, however, there is considerable heterogeneity in FEV1 response. Even among nonsmokers, there is a subset with severe lung function impairment. Note the striking similarity of the data presented by (A) Burrows (11) and that of the (B) Copenhagen City Study several decades later (10) Reprinted by permission from Reference 10. The time course for the progression of individuals with varying smoking histories is unknown and likely follows many “natural histories.”
FEV1, which was the single physiologic parameter used by Fletcher and colleagues to track the natural history of COPD, may be altered by several anatomic lesions (15). These include alveolar wall destruction, the defining feature of emphysema, which leads to both loss of lung elastic recoil and loss of anatomic tethering of small airways. Both can lead to collapse of distal airways, particularly with forced exhalation, resulting in decreased expiratory airflow. In addition, airway disease can also compromise airflow. Peribronchiolar fibrosis can develop, particularly in the small airways. Like all fibrotic lesions, these airways are contracted, and the resulting airway narrowing compromises airflow. In addition, airway secretions are associated with increased airflow limitation (16). Interestingly, Fletcher and colleagues noted the association of mucus hypersecretion with progressive airflow limitation. However, when they adjusted their data for cigarette smoking, the statistical significance for the effect of mucus hypersecretion on loss of FEV1 was lost (see chapter 5 of Reference 1). This led them to reject the British hypothesis. More recent data, however, confirm that mucus hypersecretion is associated not only with exacerbations but that mucus hypersecretion and exacerbations are also related to progressive loss of airflow (17, 18). It is likely that mucus hypersecretion is also related to clinical features of COPD other than FEV1 decline (19–21).
Although it is clear that there is progressive loss of expiratory airflow in patients with COPD, it is not clear that airway disease and emphysema have the same natural history. The two lesions are often present in the same individual, but their relative importance can vary greatly. In addition, emphysema and airway disease could progress at different rates at various times in the life of a given individual. In this context, studies using specimens obtained at surgery have suggested that the most important anatomic correlate of airflow limitation was the disease in small airways (22, 23). In contrast, autopsy studies have suggested that emphysema is the more important lesion (24, 25). Although this could be due to differences in patient sampling, both results could be correct if airway disease was the major determinant of airflow limitation in younger individuals with relatively milder disease, whereas emphysema was the major determinant in older individuals with more severe disease who are at risk for death from their COPD.
Both airway disease and emphysema are strongly related to cigarette smoking. However, it is likely that these two anatomic lesions differ in the details of their pathophysiology (26). Consistent with this, it is also clear that there are genetic factors that account for the varying susceptibility of smokers to develop COPD (27, 28). However, it appears that different genetic determinants may impact airway disease and emphysema (29). This is consistent with differences in pathophysiology between airway disease and emphysema. Moreover, it is consistent not only with variable susceptibility to these two conditions but also with variable time courses for their progression.
Smoking cessation can clearly alter the natural history of COPD. The prospective Lung Health Study clearly demonstrated a reduction in the rate at which lung function was lost with successful smoking cessation among patients with relatively mild disease (30). However, it is unclear whether this was due to an effect on emphysema, airway disease, or both. Moreover, it is unknown if similar benefits would accrue in individuals with severe disease. In addition, cessation, at least in established COPD, does not appear to reverse the increased inflammation over the time frames studied (31, 32) and quitters with normal lung function are still at higher risk of COPD than never-smokers (33). Thus, whereas smoking cessation can alter COPD natural history, there are many questions about the natural history of COPD after cessation that remain unanswered.
The cross-sectional nature of the data used by Fletcher and colleagues to suggest a natural history has another major limitation. Specifically, it is unclear if patients with COPD all track along a similar course or if there are a number of alternatives. This was discussed by Benjamin Burrows, who pointed out that several “natural histories” are possible (Figure 1D) (34). For example, some may lose lung function at an accelerated rate throughout life. Others may have relatively normal lung function until some event in midlife results in an accelerated course. Yet others may have periods of exacerbation and remission with each successive episode that result in progressive loss of function. These three distinct natural histories would be extremely difficult to distinguish when COPD is most commonly diagnosed—that is, in a patient's mid-60s when, on average, FEV1 is approximately 45% of predicted. By this time, the course of lung function decline for the various natural histories will largely overlap. Support for part of Burrows' concept is provided by the observation of the relationship between exacerbations and lung function decline (17, 18).
To further complicate things, Burrows also pointed out that it is possible for individuals to be endowed with variable levels of lung function, a theme well illustrated by Kerstjens and colleagues (35). In this context, lung volumes increase with growth from infancy into adulthood, and airflows increase as well. In general, maximally attained lung function is achieved in young adulthood after a growth spurt in adolescence. A number of early life events can affect maximally attained lung function (36, 37). Maximally attained lung function, moreover, appears to be an independent risk factor for the subsequent development of COPD (38). Consistent with this, Barker and colleagues suggested that low birth weight was associated with increased risk for low lung function in the elderly and that this risk factor was independent of smoking status (39), although these results have not been uniformly replicated (40). Although the factors that determine maximally obtained lung function remain incompletely defined, these observations suggest that the natural history of COPD begins well before smoking starts. The physiologic and pathologic processes involved and their natural histories remain undefined.
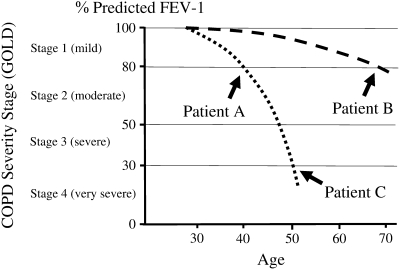
For historical reasons noted above, expiratory airflow limitation has been the primary measure used to track the natural history of COPD. It has also been used to grade COPD severity. However, because COPD progresses with age, severity and progression are not equivalent. Specifically, a young and old individual with the same severity stage of COPD will likely be progressing on different natural history trajectories (Figure 3). Frequently, individuals of a similar severity stage are grouped together for therapeutic or mechanistic studies. However, using severity stage to “phenotype” patients with COPD without considering age is likely to confound groups with different natural histories.
Figure 3.
Severity stage and natural history of chronic obstructive pulmonary disease (COPD). Two theoretical natural histories are shown. Grouping by severity would pool a 40-year-old (patient A) with a rapidly progressive natural history (dotted line) and a 70-year-old (patient B) with a slowly progressive natural history (dashed line). Conversely, to group individuals of many ages with the rapidly progressive natural history (dotted line), individuals with differing severities (patients A and C) would need to be grouped. GOLD = Global Initiative for Chronic Obstructive Lung Disease.
The fact that clinical features other than airflow limitation are relevant to COPD natural history, however, has been well understood for many decades. As noted above, Fletcher and colleagues commented about the relationship between mucus hypersecretion and exacerbations (see chapter 7 of Reference 1). More recently, efforts have been taken to develop tools that could measure features of COPD independent of FEV1. Among these is health status (41), sometimes termed “quality of life.” These measures, which are based on patient responses to standardized questions, have been validated and found to be tractable clinical measures (41). Importantly, they appear to measure something distinct from the FEV1. The St. George's Respiratory Questionnaire (SGRQ), for example, is correlated, but very weakly, with the FEV1 (42). The weak relationship suggests that other factors drive health status and implies that these other factors will have a natural history. The SGRQ, in this context, is more strongly related to exercise capacity, although this parameter is also, to some extent, independent (43).
The number of distinct variables required to define the COPD population remains undefined. Evaluating clinical databases with techniques such as principle components analysis, however, supports the concept that multiple parameters are required. Celli and colleagues reviewed a dataset from two clinical trials and concluded that at least six variables—FEV1, cough, health status, airflow reversibility, body mass index, and dyspnea—yielded independent information (44). Using a smaller dataset, Wegner and colleagues concluded that FEV1, dyspnea, and lung volumes yielded independent information (45). Similarly, Ries and associates derived four factors, which they believed reflected exercise/gas exchange, disease severity, lung volumes, and airflow (46). Wu and colleagues, reviewing a health claims dataset, suggested that 12 parameters were independent (47). Although the number of factors required to describe the COPD patient population remains undefined, each distinct factor may have a different natural history. Moreover, as with FEV1, each could also have multiple pathogenetic mechanisms and a heterogeneous time course. Defining the Fletcher-Peto curves for these variables, such as health status, is a major unmet clinical need. That such analyses can contribute to understanding the clinical condition, however, is well supported by existing data.
Clinical studies with therapeutic interventions have clearly demonstrated divergent natural histories for different COPD outcomes. Rehabilitation, for example, has been well demonstrated to have no effect on airflow limitation (48, 49). Despite this, rehabilitation has dramatic effects on health status. The impact of rehabilitation on airflow and health status is thus clearly distinct. Similarly, oxygen therapy clearly impacts COPD natural history because it improves survival in hypoxic patients with COPD (50, 51), but it has no apparent effect on airflow limitation. Interestingly, over the last several decades, COPD survival for hypoxic patients with COPD has further improved (52). Although there are many reasons for changes in prognosis over time, comparison with historical data suggests that some factors are altering COPD natural history.
Patients with COPD are affected not only by pulmonary problems but also by problems in other organ systems (53–57). Cardiovascular disease is the most common cause of death for patients with COPD (13), and the risk of a cardiac event is strongly related to the severity of the COPD (58). Importantly, the cardiac risk is present even with minor degrees of airflow limitation and appears to be independent of smoking (58, 59). Similarly, patients with COPD appear to be at increased risk for a variety of conditions, including osteoporosis, muscle weakness, anemia, depression, and thrombotic disease. Whether these conditions should be regarded as comorbidities or as nonpulmonary manifestations of COPD remains unsettled. However, the natural history of these nonpulmonary clinical problems in the patient with COPD and whether they differ in important ways from the natural history in the patient with COPD remains largely unstudied.
Because COPD progresses slowly, and because the disease is mechanistically and clinically heterogeneous, there is considerable interest in defining biomarkers that can be used to subcategorize the COPD population and to gauge disease severity and progression. A large number of biomarkers have been evaluated, to some degree, in these contexts. None have yet been validated as effective clinical tools (60–63). As the study of these makers advances, however, it will be important to define their “natural histories” in the context of COPD, both as they relate to “stable” disease and to acute exacerbations.
There are, therefore, many crucial unaddressed questions relating to COPD natural history. First, the outcome measures that are both necessary and sufficient to track COPD natural history remain to be defined. Second, methods to evaluate these parameters must be applied in sufficiently large studies. Cross-sectional studies will provide some insight. However, as pointed out by Burrows, these studies will suffer from the same limitations as the initial study of Fletcher and colleagues. The heterogeneity of COPD natural history can only be determined by longitudinal studies. This is a particular problem for studies of stable disease that progresses over decades. Among the practical problems that these studies present is that investigators are unlikely to be attracted to such studies: It is a poor career choice to do lifetime studies of one's own species. Longitudinal studies of shorter duration are likely to provide insight into the natural history of acute exacerbations and may help define endpoints that can be used to assess interventions with the potential for altering the course of COPD. Full understanding of COPD natural history, however, will require investigators who are ready to initiate studies that others will bring to completion.
Supported by the Larson Endowment, University of Nebraska Medical Center, and by NIH R01 HL073739-01 and R21 ES013856-01 (S.J.R.).
Conflict of Interest Statement: S.I.R. has participated as a speaker at programs organized by AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline (GSK), Otsuka, and Pfizer; he serves on advisory boards for Altana, AstraZeneca, Dey, GSK, Novartis, Schering-Plough, and Talecris; he has conducted clinical trials for Almirall, Altana, Astellas, Centocor, GSK, Nabi, Novartis, and Pfizer; he has served as a consultant for Adams, Almirall, Altana, AstraZeneca, Bend, Biolipox, Centocor, Critical Therapeutics, GSK, ICOS, Johnson & Johnson, Novartis, Ono, Parengenix, Pfizer, Roche, Sankyo, Sanofi, and Schering-Plough; a patent is pending on a method for stem cell differentiation; S.I.R. is a coinventor of the patent owned by the University of Nebraska Medical Center. J.V. has been reimbursed by GSK for presenting at various meetings, receiving fees of $12,000 in 2005, $12,000 in 2006, and $10,000 in 2007; he received $4,000 from GSK for taking part in an advisory board; for similar activities, he received $2,000 in 2005, 2006, and 2007 from Boehringer Ingelheim; $2,000 in 2006 and $1,500 in 2007 from AstraZeneca; $2,000 in 2006 from Kamada; and $2,000 in 2006 from Hoffmann-La Roche; he received $450,000 in 2006 and expects to receive $400,000/year in 2007 and 2008 as research grants for participating in multi and single-center clinical studies. His wife is an employee of AstraZeneca, Denmark; neither he nor his wife own shares in any pharmaceutical company.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management and prevention of COPD, 2006. [Internet]. (Accessed 29 Aug 2008) Bethesda (MD): NHLBI.
- 2.Fletcher C, Peto R, Tinker C, Speizer F. The natural history of chronic bronchitis and emphysema. New York: Oxford University Press; 1976.
- 3.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ 1977;1:1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benowitz NL, Jacob P III. Individual differences in nicotine kinetics and metabolism in humans. NIDA Res Monogr 1997;173:48–64. [PubMed] [Google Scholar]
- 5.Mazzone RW, Kornblau S. Size of pores of Kohn: influence of transpulmonary and vascular pressures. J Appl Physiol 1981;51:739–745. [DOI] [PubMed] [Google Scholar]
- 6.Parra SC, Gaddy LR, Takaro T. Ultrastructural studies of canine interalveolar pores (of Kohn). Lab Invest 1978;38:8–13. [PubMed] [Google Scholar]
- 7.Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung: comparison with normal and emphysematous lungs. 2. Functional aspects. Chest 1992;101:800–809. [DOI] [PubMed] [Google Scholar]
- 8.Verbeken EK, Cauberghs M, Mertens I, Lauweryns JM, Van de Woestijne KP. Tissue and airway impedance of excised normal, senile, and emphysematous lungs. J Appl Physiol 1992;72:2343–2353. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:180–184. [DOI] [PubMed] [Google Scholar]
- 10.Rennard SI, Vestbo J. COPD: the dangerous underestimate of 15%. Lancet 2006;367:1216–1219. [DOI] [PubMed] [Google Scholar]
- 11.Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis 1977;115:195–205. [DOI] [PubMed] [Google Scholar]
- 12.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 2000;160:1683–1689. [DOI] [PubMed] [Google Scholar]
- 13.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 2003;58:388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsh S, Aldington S, Shirtcliffe P, Weatherall M, Beasley R. Smoking and COPD: what really are the risks? Eur Respir J 2006;28:883–884. [DOI] [PubMed] [Google Scholar]
- 15.Niewoehner D. Structure-function relationships: the pathophysiology of airflow obstruction. In: Stockley R, Rennard SI, Rabe K, Celli B, editors. Chronic obstructive pulmonary disease. Malden, MA: Blackwell; 2007. pp. 3–19.
- 16.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 17.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med 1996;153:1530–1535. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennard S, Decramer M, Calverley PM, Pride NB, Soriano JB, Vermeire PA, Vestbo J. Impact of COPD in North America and Europe in 2000: subjects' perspective of Confronting COPD International Survey. Eur Respir J 2002;20:799–805. [DOI] [PubMed] [Google Scholar]
- 20.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–1422. [DOI] [PubMed] [Google Scholar]
- 21.Islam SS, Schottenfeld D. Declining FEV1 and chronic productive cough in cigarette smokers: a 25-year prospective study of lung cancer incidence in Tecumseh, Michigan. Cancer Epidemiol Biomarkers Prev 1994;3:289–298. [PubMed] [Google Scholar]
- 22.Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary function tests. N Engl J Med 1978;298:1277–1281. [DOI] [PubMed] [Google Scholar]
- 23.Nagai A, West WW, Thurlbeck WM. The National Institutes of Health intermittent positive-pressure breathing trial: pathology studies II. Correlation between morphologic findings, clinical findings, and evidence of expiratory airflow obstruction. Am Rev Respir Dis 1985;132:946–953. [DOI] [PubMed] [Google Scholar]
- 24.Hale KA, Ewing SL, Gosnell BA, Niewoehner DE. Lung disease in long-term smokers with and without chronic airflow obstruction. Am Rev Respir Dis 1984;130:716–721. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell RS, Stanford RE, Johnson JM, Silvers GW, Dart G, George MS. The morphologic features of the bronchi, bronchioles and alveoli in chronic airway obstruction: a clinicopathologic study. Am Rev Respir Dis 1976;114:137–145. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro SD, Snider GL, Rennard SI. Chronic bronchitis and emphysema. In: Mason RJ, Broadus VC, Murray JF, Nadel JA, editor. Textbook of respiratory medicine, 4th ed. Philadelphia: Elsevier; 2005. pp. 1115–1167.
- 27.Silverman EK. Progress in chronic obstructive pulmonary disease genetics. Proc Am Thorac Soc 2006;3:405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandford AJ, Joos L, Pare PD. Genetic risk factors for chronic obstructive pulmonary disease. Curr Opin Pulm Med 2002;8:87–94. [DOI] [PubMed] [Google Scholar]
- 29.Patel B, Make B, Coxson HO, Muller NL, Pillai S, Anderson W, Silverman E, Lomas D. Airway and parenchymal disease in chronic obstructive pulmonary disease are distinct phenotypes. Proc Am Thorac Soc 2006;3:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002;166:675–679. [DOI] [PubMed] [Google Scholar]
- 31.Willemse BW, ten Hacken NH, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J 2005;26:835–845. [DOI] [PubMed] [Google Scholar]
- 32.Spurzem JR, Romberger DJ, Manouilova LS, Stoner J, Hepp L, Minton R, Vessey R, Horstman D, Rennard SI. Smoking cessation is followed by an increase in T lymphocytes recovered by bronchoalveolar lavage (BAL) in COPD [abstract]. Proc Am Thorac Soc 2006;3:A628. [Google Scholar]
- 33.Lokke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax 2006;61:935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrows B. An overview of obstructive lung diseases. Med Clin North Am 1981;65:455–471. [DOI] [PubMed] [Google Scholar]
- 35.Kerstjens HA, Rijcken B, Schouten JP, Postma DS. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax 1997;52:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet 2007;370:758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, Sears MR. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med 2002;165:1480–1488. [DOI] [PubMed] [Google Scholar]
- 38.Weiss ST, Ware JH. Overview of issues in the longitudinal analysis of respiratory data. Am J Respir Crit Care Med 1996;154:S208–S211. [DOI] [PubMed] [Google Scholar]
- 39.Barker DJP, Godfrey KM, Fall CHD, Osmond C, Winter PD, Shaheen SO. Relation of birthweight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 1991;303:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaheen SO, Sterne JA, Florey CD. Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax 1998;53:549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax 2001;56:880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones PW. Issues concerning health-related quality of life in COPD. Chest 1995; 107(5, Suppl):187S–193S. [DOI] [PubMed] [Google Scholar]
- 43.Jones PW, Quirk FH, Baveystock CM. The St. George's Respiratory Questionnaire. Respir Med 1992;85(Suppl B):25–31. [Discussion, p. 33−37.] [DOI] [PubMed] [Google Scholar]
- 44.Celli BR, Calverley PM, Rennard SI, Wouters EF, Agusti A, Anthonisen N, Macnee W, Jones P, Pride N, Rodriguez-Roisin R, et al. Proposal for a multidimensional staging system for chronic obstructive pulmonary disease. Respir Med 2005;99:1546–1554. [DOI] [PubMed] [Google Scholar]
- 45.Wegner RE, Jorres RA, Kirsten DK, Magnussen H. Factor analysis of exercise capacity, dyspnoea ratings and lung function in patients with severe COPD. Eur Respir J 1994;7:725–729. [DOI] [PubMed] [Google Scholar]
- 46.Ries AL, Kaplan RM, Blumberg E. Use of factor analysis to consolidate multiple outcome measures in chronic obstructive pulmonary disease. J Clin Epidemiol 1991;44:497–503. [DOI] [PubMed] [Google Scholar]
- 47.Wu EQ, Birnbaum HG, Cifaldi M, Kang Y, Mallet D, Colice G. Development of a COPD severity score. Curr Med Res Opin 2006;22:1679–1687. [DOI] [PubMed] [Google Scholar]
- 48.Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation of physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823–832. [DOI] [PubMed] [Google Scholar]
- 49.Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, Make B, Rochester CL, Zuwallack R, Herrerias C. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest 2007; 131(5, Suppl):4S–42S. [DOI] [PubMed] [Google Scholar]
- 50.Council MR. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981;1:681–686. [PubMed] [Google Scholar]
- 51.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980;93:391–398. [DOI] [PubMed] [Google Scholar]
- 52.Rennard S, Carrera M, Agusti AG. Management of chronic obstructive pulmonary disease: are we going anywhere? Eur Respir J 2000;16:1035–1036. [DOI] [PubMed] [Google Scholar]
- 53.Agusti A. Systemic effects of chronic obstructive pulmonary disease: what we know and what we don't know (but should). Proc Am Thorac Soc 2007;4:522–525. [DOI] [PubMed] [Google Scholar]
- 54.Rennard SI. Chronic obstructive pulmonary disease: linking outcomes and pathobiology of disease modification. Proc Am Thorac Soc 2006;3:276–280. [DOI] [PubMed] [Google Scholar]
- 55.Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med 2005;26:142–153. [DOI] [PubMed] [Google Scholar]
- 56.Cazzola M, Matera MG, Rogliani P, Page C. Treating systemic effects of COPD. Trends Pharmacol Sci 2007;28:544–550. [DOI] [PubMed] [Google Scholar]
- 57.Fabbri LM, Luppi F, Beghe B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J 2008;31:204–212. [DOI] [PubMed] [Google Scholar]
- 58.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005;127:1952–1959. [DOI] [PubMed] [Google Scholar]
- 59.Ashley F, Kannel WB, Sorlie PD, Masson R. Pulmonary function: relation to aging, cigarette habit, and mortality. Ann Intern Med 1975;82:739–745. [DOI] [PubMed] [Google Scholar]
- 60.Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, Brusasco V, Burge PS, Calverley PM, Celli BR, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008;31:416–469. [DOI] [PubMed] [Google Scholar]
- 61.Tzortzaki EG, Lambiri I, Vlachaki E, Siafakas NM. Biomarkers in COPD. Curr Med Chem 2007;14:1037–1048. [DOI] [PubMed] [Google Scholar]
- 62.Franciosi LG, Page CP, Celli BR, Cazzola M, Walker MJ, Danhof M, Rabe KF, Della Pasqua OE. Markers of exacerbation severity in chronic obstructive pulmonary disease. Respir Res 2006;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franciosi LG, Page CP, Celli BR, Cazzola M, Walker MJ, Danhof M, Rabe KF, Della Pasqua OE. Markers of disease severity in chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2006;19:189–199. [DOI] [PubMed] [Google Scholar]