Abstract
Members of the Sprouty family encode novel proteins that are thought to function primarily as intracellular antagonists of the Ras-signaling pathway. Increased Ras signaling is a critical characteristic of human lung adenocarcinoma, the most common type of non–small cell lung cancer. Sprouty 2 is expressed in the lung epithelium, the tissue layer from which lung cancers arise. We hypothesized that overexpression of Sprouty 2 in the distal lung epithelium would inhibit lung tumorigenesis. To test the hypothesis, the consequences of overexpressing Sprouty 2 in the distal lung epithelium on urethane-induced mouse lung tumorigenesis were determined. Urethane is a chemical carcinogen found in tobacco smoke that causes activating mutations in Kras and induces lung tumors in mice. Sprouty 2–overexpressor mice developed significantly fewer lung tumors compared with their littermate controls (13.2 ± 1.1 versus 18.1 ± 1.3, P = 0.006). Tumor diameter was also significantly smaller in Sprouty 2 overexpressors (0.85 mm ± 0.03 versus 0.95 mm ± 0.02, P = 0.005). Sprouty 2 overexpression did not alter Kras mutational frequencies in urethane-induced tumors, suggesting that the tumor-suppressing effect of Sprouty 2 overexpression acts at a stage after Kras mutation, perhaps by interfering with receptor tyrosine kinase–induced signaling. These results demonstrate that Sprouty 2 overexpression inhibited both tumor initiation and subsequent tumor growth.
Keywords: Sprouty, lung cancer, mouse, chemoprevention, Ras
CLINICAL RELEVANCE
Sprouty 2 is an inhibitor of selected receptor tyrosine kinases and Ras signaling. This research suggests that Sprouty mimetics may have utility in the prevention and treatment of lung cancer.
Lung cancer is the leading cause of cancer death in men and women in the United States. Smoking accounts for roughly 90% of cases in men and 70% in women. Non–small cell lung cancer (NSCLC) accounts for approximately 80% of lung cancers. NSCLC is subdivided into squamous cell carcinoma, large cell carcinoma, and adenocarcinoma, with the latter being most common (∼ 40% of all lung cancers) (1, 2).
Fifteen to twenty percent of human NSCLCs, particularly adenocarcinomas, have point mutations in the KRAS proto-oncogene, in most cases at codon 12. These mutations prevent hydrolysis of KRAS-GTP (3). As a result, KRAS signaling becomes constitutive, leading to a strong proliferative stimulus (1). While a minority of human NSCLCs contain gain-of-function mutations in KRAS, increased Ras signaling, via several different mechanisms, appears to occur in most NSCLCs. For example, the receptor tyrosine kinase (RTK) class of growth factor receptors classically signal through Ras. Growth factors and their receptors appear to affect human lung cancer development. Members of the epidermal growth factor (EGF) and EGF receptor (EGFR) families appear to function as autocrine loops in many NSCLCs. In the vast majority of NSCLC, EGFR and/or one of its ligands is over expressed. Another member of the EGFR family, HER2/neu, is expressed in approximately 35% of NSCLCs. Increased expression of these ligands and/or their receptors is thought to result in increased intracellular signaling through the Ras/Raf/MAP kinase pathway, leading to proliferation. Blockade of these receptors with various reagents in human lung cancer cell lines inhibits proliferation (1). Basic fibroblast growth factor (bFGF; aka FGF 2) binds to members of the FGFR family. Forty-nine to seventy percent of adenocarcinomas of the lung express bFGF. FGFRs, like members of the EGFR family, are RTKs, and they classically signal via Ras (1). It remains unclear whether this enhanced Ras signaling plays early, late, multiple, or continuous roles in the many stages leading to overt human lung cancer.
The mouse as a model system for the study of lung cancer has many strengths (4). Inbred strains of mice have different genetic susceptibilities for developing spontaneous lung tumors, most of which resemble adenomas. In mice, susceptibility to spontaneous lung tumor development generally correlates with susceptibility to chemically induced lung tumors. Like spontaneous lung tumors, the induced tumors usually are adenomas.
Similar to some human adenocarcinomas, many spontaneous and induced mouse lung tumors have an activating mutation in murine Kras, usually codon 12 or 61. For example, urethane induces mutations at codon 61, and induction of a Kras mutation appears to be an early (if not the earliest) event in urethane-induced tumorigenesis (4). Recent studies have shown that an activating mutation in Kras is sufficient to induce mouse lung tumors (5, 6). In addition, persistent Kras signaling appears to function as a survival signal in this model, since tumors disappeared if such signaling was abolished (6).
Thus, much has been learned about gain-of-function mutations that lead to increased Kras signaling in human and mouse lung tumors. In contrast, little is known about the potential role antagonists of the Kras signaling pathway may play in lung carcinogenesis. Loss-of-function mutations in these genes would also be predicted to increase Kras signaling, and therefore may play a role in lung tumorigenesis.
One example of a negative regulator is the Sprouty (Spry) family, whose members encode proteins that function as intracellular antagonists of the Ras signaling pathway (7, 8). There are four vertebrate members of the family. Sproutys have been shown to antagonize EGFR (8), FGFR (7, 9), VEGF-R (10), and c-MET (aka hepatocyte growth factor receptor [HGF]) mediated Ras signaling (11). However, Sproutys do not appear to antagonize signaling by all RTKs. The mechanism(s) that generates this selective antagonism of specific RTK-mediated Ras signaling is not known. In addition, it remains unclear if specific Sproutys antagonize specific RTKs. However, Spry has been overexpressed in Drosophila, frogs, chicks, zebrafish, and mice. In each instance, Sprouty appeared to antagonize RTK-induced Ras signaling (12–14). When Spry2 was specifically overexpressed in embryonic mouse lung epithelium, defects in lung development were observed (15). These defects were consistent with inhibition of EGFR and/or FGFR-mediated Ras signaling. However, recent in vitro studies have suggested that overexpression of Spry2 can enhance EGFR signaling (12–14).
Spry2 is expressed in adult lung epithelium. Recently, Spry2 expression has been reported in a number of human NSCLC tumors and cell lines (16). While considerable variation occurs between tumors and cell lines, Spry2 protein and mRNA expression is generally repressed. Ectopic expression of Spry2 decreased cell proliferation and migration and blocked tumor formation in immunodeficient mice. In a latent Kras murine model of adenocarcinoma, conditional null Spry2 mice developed increased numbers of lung tumors, supporting a tumor-suppressive effect (17). Whether Spry2 overexpression would suppress tumorigenesis has not been tested.
We hypothesized that overexpression of this putative Ras signaling antagonist in the lung epithelium would inhibit lung tumor development. To test this hypothesis, lung tumor formation was characterized in adult mice that overexpressed Spry2 in lung epithelium and their littermate controls after they were exposed to the chemical carcinogen urethane, which causes lung tumors in mice and induces gain-of-function mutations in Kras (4).
MATERIALS AND METHODS
Generation of Mice with Lung Epithelium–Specific Overexpression of Spry2
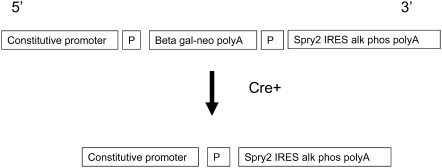
The generation of lung epithelial–specific Spry2 overexpressor mice was based on that used by Lobe and coworkers to construct the Z/AP Cre recombinase reporter mouse (18). A modified version of this vector was obtained (kind gift of C. Lobe, Sunnybrook and Women's Health Sciences Center, Toronto, ON, Canada). Several unique cloning sites replaced the human placental alkaline phosphatase (AP) cDNA. An internal ribosomal entry site (IRES) AP cDNA was inserted downstream of the cloning sites. The coding region of Spry2 was PCR amplified, sequenced, and cloned downstream of the 3′ loxP site and upstream of the IRES AP (see Figure 1). Importantly, the promoter in this construct is ubiquitous and constitutively active. Thus, in the unrecombined configuration, the β-galactosidase–neomycin fusion cDNA, which is flanked on either side by loxP sequences, is constitutively expressed in all cells, but the downstream Spry2 IRES AP cDNAs are not expressed. Cre recombinase recognizes loxP sequences, and irreversibly deletes intervening DNA. Consequently, in the presence of tetracycline-inducible Cre, the β-galactosidase–neomycin fusion cDNA is removed, and Spry2 and IRES AP are now constitutively expressed even when doxycycline is no longer present. Thus, AP is a reporter for Cre-mediated recombination and marks cells that overexpress Spry2.
Figure 1.
Schematic representation of Spry2 conditional gain-of-function transgene. The unrecombined allele constitutively expresses the β-galactosidase–neomycin fusion cDNA. In the presence of the Cre recombinase, the β-galactosidase–neomycin fusion cDNA is deleted, and the Spry2 IRES HPLAP cDNA is constitutively expressed. “Constitutive promoter” = CMV enhancer element fused to the Chick β actin promoter; P = loxP; IRES = internal ribosomal entry site.
Spc-rtTA and Tet-o-Cre transgenic mice were kindly provided by Dr. Jeffrey Whitsett (Cincinatti Children's Hospital Medical Center). The development and characterization of these transgenic mouse lines have been described previously (19). The Spry2GOF line was backcrossed to FVBN mice for three to four generations. The Spc-rtTA and Tet-o-Cre transgenic mice were backcrossed to FVBN for five to six generations. These mice were then bred to generate triple transgenic mice and littermate controls.
A PCR-based strategy was used to identify presence or absence of each transgene from genomic DNA extracted from tails. Previously published SPC and Cre primer sequences and conditions were used (19, 20). The following primers and conditions were used to detect the unrecombined Spry2GOF transgene: forward, 5′-gcttgggtggagaggctattc-3′; reverse, 5′-caaggtgagatgacaggagatc-3′; annealing temperature was 54°C for 30 seconds; 72°C extension time was 45 seconds; 35 cycles.
To induce constitutive overexpression of Spry2, all 30- to 35-day-old mice received doxycycline (0.5 mg/ml final concentration; Sigma, St. Louis, MO) in their drinking water for 1 week (19). Fresh doxycycline drinking water was provided every 48 hours. After 1 week, mice drank regular water for the duration of the protocol.
Western Blot
Five- to seven-week-old triple transgenic mice and littermate controls received doxycline for 1 week followed by 1 week of regular water. After mice were killed, the lungs were dissected free of the body and homogenized in 1.5 ml of ice-cold lysis buffer (50 mM Tris-HCL, pH 7.4; 150 mM NaCl; 0.25% sodium deoxycholate; 1% IGEPAL; 0.1% SDS) containing 50 μl of protease inhibitor cocktail (Sigma). The lung homogenate was transferred to a microfuge tube and spun in a microfuge at 16,000 × g for 10 minutes at 4C. The supernatant was collected, aliquotted, and stored at −80°C until further analysis.
Total protein concentration in the lung homogenates was determined using the BCA kit (Pierce, Rockford, IL) following the manufacturer's directions. Ten micrograms of protein were loaded in to each well of a 15% SDS-PAGE Criterion gel (Bio-Rad, Hercules, CA). Proteins were separated and then transferred to an Immobilon membrane (Millipore, Bedford, MA) using the Criterion system (Bio-Rad).
The membrane was blocked with 5% nonfat dried milk powder in PBS + 0.1% Tween (PBST). Rabbit polyclonal anti-Spry2 antibody (Upstate, Charlottesville, VA) was used at 1:2,500 dilution, and the blot incubated overnight at 4°C. The blot was washed with PBST, and then incubated with a horseradish peroxidase–tagged goat anti-rabbit secondary antibody at a 1:5,000 dilution (Santa Cruz Biotechnology, Santa Cruz, CA). After washing with PBST, bands were visualized using the ECL plus Western Blotting kit (Amersham, Piscataway, NJ).
Detection of Human Placental Alkaline Phosphatase Activity
Immunologic reagents for the detection of Spry2 protein by immunohistochemistry are not readily available; therefore, we used expression of the co-expressed IRES AP as a marker of Spry2 expression. Triple transgenic mice and littermate controls were killed after receiving doxycyline for 1 week. The trachea was cannulated with an 18-gauge angiocatheter (BD, Franklin Lakes, NJ) and the lungs were inflation-fixed with 10% buffered formalin (Fisher, Pittsburgh, PA) under 25 cm of water pressure for 15 minutes. The lungs and trachea were then removed and immersed in 10% formalin overnight. Lungs were washed extensively in phosphate-buffered saline (PBS). Fixed lungs in PBS were then immersed in a 70°C water bath for 30 minutes to inactivate endogenous alkaline phosphatases. After a brief rinse in distilled water, lungs were incubated in BM purple (Roche, Indianapolis, IN) for 24 hours with gentle agitation, and then washed in PBS with 5 mM EDTA to stop the reaction. Lungs were placed in 30% sucrose overnight at 4°C, and then embedded in Tissue Tek OCT (Miles, Elkhart, IN). Ten-micrometer frozen sections were cut and collected on Superfrost Plus slides (Fisher, Pittsburgh, PA). Slides were washed in PBS, coverslipped, and images captured on a Leica upright microscope (Leica, Wetzlar, Germany) fitted with a SPOT RT digital camera (Diagnostic Instruments, Sterling Heights, MI).
Carcinogenesis Protocol
Five- to seven-week-old Spry2GOF mice and their littermate controls were given doxycycline in their drinking water for 1 week. The mice then drank regular water for the duration of the protocol. Six- to eight-week-old mice were weighed the day before injection. Mice were given a single intraperitoneal injection of urethane (1 mg urethane dissolved in normal saline/gram of body weight; Sigma); total volume injected was 300 microliters. Sixteen weeks after injection, mice were anesthetized by an intraperitoneal injection of 70 mg/kg of ketamine and 14 mg/kg xylazine, and then killed by transection of the abdominal aorta and the vena cava. The lungs were removed and placed in ice-cold PBS. The experimental protocol was approved by the Case Western Reserve University IACUC.
Enumeration and Measurement of Lung Tumors
Lungs were examined, and tumors were identified and dissected out of the lung parenchyma under a dissecting microscope. Tumor diameter was measured using a digital micrometer. Tumors were fixed in 10% buffered formalin (Fisher, Pittsburgh, PA) and then submitted to a core histology lab for paraffin embedding and sectioning. For RNA extraction, freshly dissected tumors were stored in RNALater (Ambion, Austin, TX) at −80°C.
Immunohistochemistry and TUNEL
Five-micrometer-thick sections of paraffin-embedded tumors were collected. TUNEL assay was performed according to the manufacturer's directions (Roche, Indianapolis, IN). Anti–Ki-67 (1:100 dilution; Abcam, Cambridge, MA), anti-phosphohistone (1:50; Upstate), and anti-activated caspase 3 (1:50; R&D, Minneapolis, MN) antibodies were purchased. Biotinylated secondary antibodies were purchased (Vector, Burlingame, CA), and the ABC detection kit was used according to the manufacturer's directions. Sections were counterstained with hematoxylin (Vector). For each antibody, immunohistochemistry was performed with or without antigen retrieval. For antigen retrieval, slides were immersed in 10 mM sodium citrate pH 6.0 (Sigma), heated under pressure in a pressure cooker for 3 to 5 minutes, pressure relieved, and the slides allowed to cool for 20 minutes. For Ki-67, pressure cooker time was increased to 15 minutes.
TUNEL-positive cells and cells positive for Ki-67, phosphohistone, and activated caspase 3 expression from representative fields were manually counted. At least six fields were counted for each assay. Tumors from three control and three Spry2GOF mice were examined.
RNA Extraction, RT-PCR, and Sequencing
Tumors were homogenized and RNA extracted using the RNA Easy kit (Qiagen, Valencia, CA) following the manufacturer's directions. RT-PCR was performed using the 1-step RT-PCR kit (Qiagen). Kras-specific primer pair (forward primer, 5′-gcctgctgaaaatgactgagta-3′; reverse primer, 5′-tgtcttgtctttgctgaggtct-3′) was used to amplify a product, including codons 12 and 61. The PCR product was sequenced in a core sequencing facility.
Statistics
Statistical analysis was performed using Prism4 software (GraphPad, San Diego, CA); t test was used to compare groups. A P value less than 0.05 was considered significant. Bar graphs show mean values ± SEM.
RESULTS
Lung Epithelium–Specific Overexpression of Spry2
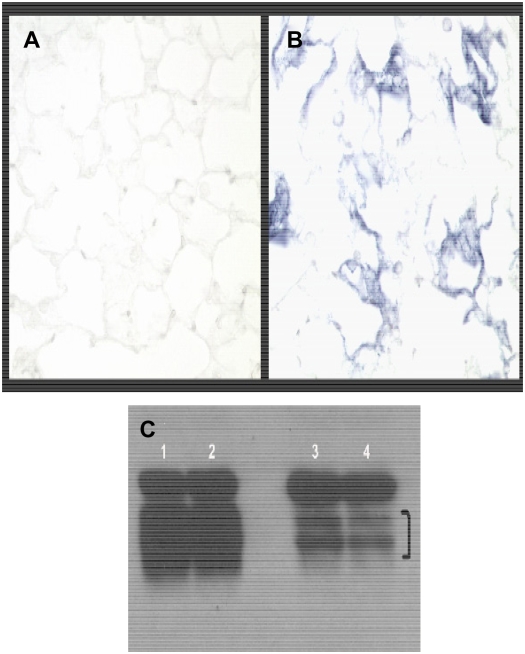
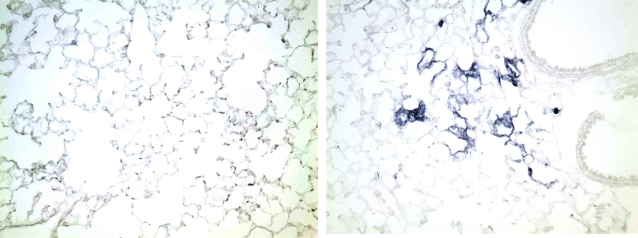
To determine if the Spry2GOF transgene could be recombined specifically in the lung epithelium, which would result in expression of Spry2 and the IRES AP, previously characterized SP-C rtTA and Tet-O-Cre transgenic mouse lines were obtained. Mice that inherit the SP-C rtTA and Tet-O-Cre transgenes have been shown to express Cre specifically in the lung epithelium in the presence of doxycycline (19). These mice were bred to the Spry2GOF transgenic line to generate triple transgenic offspring that could constitutively overexpress Spry2 specifically in the lung epithelium. Administration of doxycycline to the drinking water of pregnant females beginning at about Embryonic Day (E)6.5 resulted in transgenic Spry2 being overexpressed throughout the developing lung epithelium of triple transgenic embryos. Littermate controls only expressed endogenous Spry2 in normal domains (data not shown). In the absence of doxycycline, lungs of triple transgenic embryos only expressed endogenous Spry2 in normal domains through about E16.5 to E17. As described previously, the SP-C rtTA transgene becomes leaky during late gestation and the perinatal period, presumably due to the same factors that led to the pronounced up-regulation of endogenous SP-C during this same time period (19). In our triple transgenic mice, this leakiness results in patchy but widespread expression of Cre in distal lung epithelium. Where expressed, Cre deletes the β-galactosidase–neomycin fusion cDNA, and Spry2 and IRES AP become constitutively expressed even if doxycycline is subsequently removed because Cre-mediated deletion is irreversible and the promoter is constitutive (data not shown). Subsequent postnatal administration of doxycycline results in more uniform and widespread expression of Cre, leading to more uniform and widespread constitutive overexpression of Spry2 and the IRES AP in the distal lung epithelium as shown by AP histochemical staining in Figures 2A and 2B. However, low-power views of the lung reveal considerable variation in the level of expression of IRES AP, a surrogate for Spry2 (Figure 3), with some areas showing intense AP histochemical activity and others below the threshold of detection.
Figure 2.
Overexpression of the recombined Spry2 IRES AP transgene in the lung. SPC-rtTA, Tet-o-Cre, SpryGOF triple transgenic adult mouse and a littermate control drank water supplemented with doxycycline for 1 week. Mice were then killed, and lungs were processed for alkaline phosphatase histochemical staining and then cryosectioned. (A) Littermate control lungs show no alkaline phosphatase staining. (B) In contrast, triple transgenic lungs show alkaline phosphatase staining in alveoli due to Cre-mediated recombination of the Spry2GOF transgene, which results in expression of both Spry2 and the AP reporter cDNAs. Magnification = ×200. (C) Western blot demonstrating doublet of Spry2 protein in lung homogenates from two Spry2 overexpressors (lanes 1 and 2) and littermate controls (lanes 3 and 4). Bracket marks doublet.
Figure 3.
Low-power view of alkaline phosphatase histochemical stained lung from littermate control (left panel) and triple transgenic (right panel) mice from Figure 2, demonstrating significant patchiness in staining for alkaline phosphatase, a surrogate for Spry2 expression.
The lungs of these mice are histologically indistinguishable from littermate controls 1 year after constitutive overexpression was induced in the lung. In addition, lung overexpressors are fertile, and are behaviorally indistinguishable from littermate controls at 16 months of age.
Western blot analysis of littermate control and Spry2 overexpressor lung homogenates showed significantly more Spry2 protein in overexpressor lung homogenates compared with littermate controls (Figure 2C). The two bands detected likely represent a baseline and a post-translationally modified form of the protein, as Sproutys can undergo modifications such as phosphorylation (13). The Western blot also demonstrates the constitutive nature of the promoter driving Spry2GOF transgene expression, since the Spry2 overexpressor mice were killed 1 week after their last exposure to doxycyline-supplemented water, but their lung homogenates continued to express significantly more Spry2 protein than did those of littermate controls. In addition, 1 month after exposure to doxycycline, levels of Sprouty 2 protein in lung homogenates are not different from those seen from mice killed at the end of 1 week of doxycycline administration (data not shown).
Fewer Tumors in Mice Overexpressing Spry2 in the Lung Epithelium
The Spry2GOF lines were established on a mixed genetic background. Since genetic background can have a profound effect on susceptibility to urethane-induced lung tumors (4), the Spry2GOF line was backcrossed three to four generations to the FVBN inbred strain. The SPC-rtTA and tet-O-Cre transgenic lines were backcrossed to FVBN at least six generations each before they were crossed to the Spry2GOF line to produce the triple transgenic and littermate control mice. Thus, the experimental mice should be in a greater than 93% FVBN genetic background.
Five- to six-week-old experimental mice were given doxycycline in their drinking water beginning 1 week before injection of urethane. In triple transgenic mice, widespread lung epithelium overepression of Spry2 should be induced after approximately 72 hours of doxycycline (19). After 1 week of doxycycline water, all mice received a single intraperitoneal injection of urethane. Sixteen weeks later, the mice were killed and examined.
All mice developed tumors. Thus, tumor incidence was 100% in each group (n = 21 for Spry2GOF group; n = 26 for littermate control group).
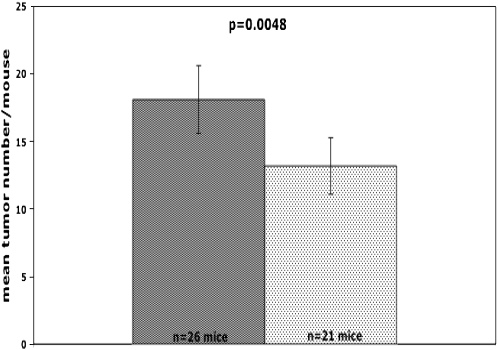
A standard measure of tumor burden is to count the number of tumors visible from inspection of the pleural surface with the aid of a dissecting scope (4). As shown in Figure 4, mean number of lung tumors per mouse was significantly lower than for mice that overexpressed Spry2 in the lung epithelium compared with their littermate controls (13.2 ± 1.1 versus 18.1 ± 1.3, mean ± SEM; P = 0.006). This 27% reduction in lung tumors suggests that overexpression of Spry2 in the lung epithelium inhibited tumor initiation.
Figure 4.
Mice that overexpress Spry2 (lightly shaded bars) in the lung epithelium developed significantly fewer urethane-induced lung tumors than littermate controls (darkly shaded bars) 16 weeks after injection of urethane (13.2 ± 1.1 versus 18.1 ± 1.3, mean ± SEM; P = 0.006).
In our system, Cre-mediated recombination results in overexpression of both Spry2 and human placental AP. To address the possibility that the anti-tumor effects were due to overexpression of human placental AP, the SP-C rtTA and Tet-O-Cre transgenic mice were bred to the Z/AP transgenic mouse line, which contains the same promoter and human placental AP as found in the Spry2GOF transgene. Triple transgenic offspring and littermate controls underwent the same doxycycline and urethane protocols. Urethane-induced lung tumor number was not significantly different between mice that only overexpressed human placental AP in the lung epithelium compared with their littermate controls (data not shown). This result indicates that it is overexpression of Spry2 and not AP that is responsible for the effects on urethane-induced tumorigenesis.
Effect of Overexpressing Spry2 in the Lung Epithelium on Urethane-Induced Kras Mutations
Urethane-induced tumors typically have a gain-of-function point mutation at codon 61 of Kras. A codon 61 mutation that results in glutamine becoming leucine is associated with adenoma formation, while a glutamine becoming arginine is associated with a more malignant phenotype (21). Since adenomas are benign, the associated Kras mutation is thought to signal less efficiently than the mutation associated with the more malignant phenotype (4). We hypothesized that fewer tumors were found in mice overexpressing Spry2 because this Ras-signaling antagonist was able to antagonize the less efficient mutant Kras signal and therefore able to inhibit adenoma development. This hypothesis predicted that significantly fewer tumors in the Spry2GOF group would express the glutamine to leucine mutation.
To begin to test this hypothesis, RNA was extracted from 30 randomly selected tumors from the Spry2GOF mice and 33 from littermate controls. RNA was reverse transcribed and PCR was performed using Kras-specific primers. The PCR product was sequenced. At codon 61, 24/30 (80%) Spry2GOF tumors and 28/33 (85%) control tumors had a CAA to CGA mutation. This mutation results in glutamine becoming arginine, and is associated with adenocarcinomas. The other tumors had a CAA to CTA mutation at codon 61, which leads to a leucine, and is associated with adenomas. The difference in mutation rates was not statistically significant and is not likely to represent a biologically important phenomenon.
Smaller Tumors in Mice Overexpressing Spry2 in the Lung Epithelium
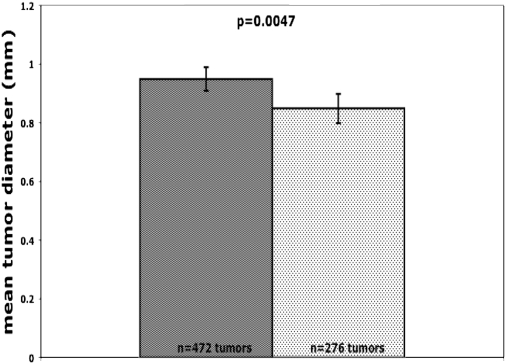
Transgenic expression of Kras with a gain-of-function mutation was sufficient to induce lung tumors (5, 6). This model also demonstrated that continued expression of Kras with a gain-of-function mutation was required to sustain tumor growth. In its absence, tumor cells underwent apoptosis (6). Since Sprouty 2 appears to function as an antagonist of Ras signaling, we hypothesized that urethane-induced lung tumors would be smaller in Spry2GOF mice because of inhibition of this survival signal. All tumors were dissected out of the lung and measured with a digital micrometer. Four hundred seventy-two tumors were found in littermate control mice, and 276 in Spry2GOF mice. As shown in Figure 5, mean tumor diameter in control mice was significantly larger than in Spry2GOF mice (0.95 mm ± 0.02, and 0.85 mm ± 0.03, mean ± SEM; P = 0.005). Assuming approximately spherical shape of the tumors, this difference translates to an approximately 40% smaller average tumor volume in the Spry2 overexpressor mice.
Figure 5.
Mice that overexpress Spry2 (lightly shaded bars) in the lung epithelium developed significantly smaller urethane-induced lung tumors than littermate controls (darkly shaded bars) 16 weeks after injection of urethane (0.85 mm ± 0.03 versus 0.95 mm ± 0.02, mean ± SEM; P = 0.005).
Apoptosis Rates in Lung Tumors
Having found that mean urethane-induced tumor size was smaller in SpryGOF mice, we investigated if tumors in these mice were smaller because they had a higher rate of apoptosis. To determine cell death rates, TUNEL assays were performed on representative sections from tumors 16 weeks after urethane injection. The apoptosis rate within tumors from control mice (7.77/10x field ± 2.0) was not significantly different from Spry2GOF mice (7.4/10x field ± 2, P = 0.800). Similar results were obtained using an anti-activated Caspase 3 antibody (data not shown).
Proliferation Rates in Lung Tumors
Increased Ras signaling induces proliferation of many cell types. Transgenic expression of Kras with a gain-of-function mutation was sufficient to induce proliferation of lung epithelial cells, which went on to form lung tumors (5, 6). Overexpression of Spry2, a Ras signaling antagonist, would be predicted to inhibit proliferation. The difference in mean urethane-induced tumor size could be due to a slower proliferation rate in the Spry2GOF tumors.
To address this possibility, proliferation rate was determined by anti–Ki-67 immunohistochemistry on representative paraffin sections of 16-week-old tumors. The proliferation rate within tumors from control mice (5.77% ± 1.16) was not significantly different from the proliferation rate found in the Spry2GOF tumors (5.48% ± 1.07, P = 0.870). Similar results were obtained using an anti-phosphohistone antibody (data not shown).
DISCUSSION
This study demonstrates that lung epithelium specific constitutive overexpression of Spry2, a putative intracellular antagonist of Ras signaling, resulted in a 27% reduction in multiplicity of urethane-induced lung tumors. Moreover, the tumors that developed were on average smaller than those found in littermate controls. Both the reduction in tumor multiplicity and size were statistically significant.
The finding of fewer tumors suggests that overexpression of Spry2 inhibited urethane-induced tumor initiation. We hypothesized that the reduction in tumor development was due to suppression of tumors arising with a mild Kras mutation at codon 61 (i.e., associated with adenoma formation) (21). Sequencing the Kras mRNA extracted from tumors did not reveal a significant difference in mutation frequencies at codon 61 between Spry2GOF mice and their littermate controls. Thus a skewed mutational spectrum does not explain the 27% reduction in tumor number observed in the Spry2GOF mice. The mechanism by which overexpression of Spry2 in the lung epithelium leads to fewer urethane-induced lung tumors remains unclear, but may occur at stages of carcinogenesis subsequent to Kras mutation. Further investigation will be necessary to understand the mechanism by which Spry2 antagonizes tumorigenesis.
The significantly smaller mean diameter in the Spry2-overexpressing mice suggested that overexpression of Spry2 affected not only tumor initiation, but also subsequent tumor growth. Smaller tumors could arise because of decreased proliferation and/or increased apoptosis. We determined proliferation rates in tumors from Spry2-overexpressing mice and their littermate controls with two different markers. We found no significant difference in proliferation rates using anti–Ki-67 or anti-phosphohistone antibody staining. Our results are in accord with previous studies which demonstrated that urethane-induced lung tumors grow continuously, but at a continuously decreasing rate. Doubling time of tumor volume was approximately 3.5 times faster 4 to 6 weeks after urethane injection compared with 7 to 12 weeks after injection (22, 23). As our analysis was performed 16 weeks after urethane injection, it remains possible that Spry2 overexpression exerted a significant antiproliferative effect at an earlier time point.
Similarly, cell death rates as determined by TUNEL and anti-activated caspase 3 antibody staining were very low and not significantly different between the two groups. Earlier studies showed that cell death is almost undetectable in established urethane-induced lung tumors (22). However, it is clear that low rates of apoptosis can have profound effects on tissue size over a relatively small number of cell cycles (23, 24). In addition, as with tumor cell proliferation, it remains possible that significantly different detectable differences in cell death rates occur earlier. Thus, we cannot exclude the possibility that smaller tumor size in Spry2-overexpresssing mice is due to effects on cell death rates.
One limitation of this study is that we did not formally assess the level of Spry2 expression in the tumors arising in the triple transgenic and control mice. It is possible that a fraction of alveolar type II cells not expressing the transgene gave rise to most of the tumors. The variability of AP histochemical activity, a surrogate for Spry2, on low-power views (Figure 3) of the lungs of triple transgenics given doxycycline supports this idea. We do not know the quantitative relationship between AP histochemical staining and Spry2 expression, so it is possible that the AP-negative regions actually do express biologically active levels of Spry2. The likely variability in Spry2 expression does not affect the basic conclusion that Spry2 overexpression inhibits lung tumorigenesis and may suggest that our results are actually an underestimation of this effect. The inhibitory effect of Spry2 overexpression on urethane-induced lung tumorigenesis was modest, but comparable to many other potential lung cancer–chemopreventive agents, including green tea (25), lipoxygenase and leukotriene pathway inhibitors (26), and nonsteroidal anti-inflammatory drugs such as indomethacin (27), ibuprofen, and piroxicam (28). Sprouty 2 mimetics alone may not be effective chemopreventive agents, but may be one component of a multifaceted chemopreventive regimen. In addition, it remains possible that even higher levels of Spry2 overexpression may have greater inhibitory activity against lung tumorigenesis. Unfortunately, inheriting two copies of the Spry2GOF transgene was not compatible with postnatal life. Thus, we were not able to determine if there may be a dose response effect.
Our findings also provide insight into the normal function of Sprouty 2. Most of the published data indicate that Sprouty 2 functions as an antagonist of selected growth factor signaling. Both biochemical and genetic data indicate that Sprouty interacts with components of the Ras signaling pathway that are both upstream and downstream of Ras. Moreover, these studies have shown interactions with components known to promote or inhibit Ras signaling (13, 14). A model reconciling these findings is one in which Sprouty functions as part of a macromolecular complex whose net effect is inhibition of Ras signaling. However, recent in vitro data has shown that overexpression of vertebrate Sprouty 2 can promote EGFR-initiated Ras signaling by interacting with Cbl, and thereby preventing Cbl from interacting with EGFR and targeting the latter for degradation (12). Many pathologic insults to the lung induce EGFR expression, and increased EGFR signaling is found in most human lung cancers. Thus, if Sprouty 2 promotes EGFR-mediated signaling, urethane would be predicted to induce more and/or larger tumors in the Spry2GOF mice. We observed the opposite. Thus, our in vivo findings are consistent with Sprouty 2 functioning as an antagonist of Ras signaling. However, our findings do not exclude the possibility that Sprouty 2 may promote RTK signaling in other contexts. For example, Sprouty 2 may have different functions in different cell types. The in vitro studies were not performed with lung epithelial cell lines.
In the past, Sproutys have been suggested to play a role in breast and prostate cancer (29, 30). Interestingly, loss of rather than enhanced Spry expression was associated with human cancer. It has been suggested that Sproutys may function as tumor suppressors (11, 30). More recently, low Spry2 expression has been reported in human NSCLC; ectopic expression antagonized cell migration (16). Spry2-null mice have increased numbers of lung tumors in a conditional KRAS tumorigenesis model, further supporting a tumor suppressor effect in lung cancer. We now demonstrate that overexpression of Spry2 decreases lung tumor numbers and size in the urethane model, in which KRAS mutation is an early and critical event. This suggests that Spry2 mimetic strategies might be of utility in lung cancer chemoprevention.
In summary, we have shown that overexpression of the Ras signaling antagonist Spry2 in the lung epithelium inhibits, but does not prevent, urethane-induced lung tumor initiation. In addition, such overexpression also inhibits subsequent growth of these tumors. The mechanisms by which overexpression of Spry2 inhibits tumor initiation and tumor growth remain unclear. Moreover, our findings raise the possibility that Sprouty 2 mimetics may have a role in preventing and/or treating lung cancer.
Acknowledgments
The first author developed the conditional Spry2GOF transgenic line as a postdoctoral fellow in Gail Martin's laboratory at the University of California, San Francisco. The authors thank her for her support and encouragement. The authors thank the histopathology core facility in the Skin Diseases Research Center for processing, sectioning, and staining of tissue, and the sequencing core facility in the orthopedics department for sequencing. An early version of this work was presented at the 2003 Thomas L. Petty Aspen Lung Conference and reported in abstract form (31).
This work was supported by the Department of Medicine and the Ireland Cancer Center at Case Western Reserve University, University Hospitals of Cleveland, and IRG-91–022–09 from the American Cancer Society (G.M.); and NCI SPORE in Lung Cancer P50 CA58187 and a Merit Review Grant from the Department of Veterans Affairs (Y.E.M.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0147OC on July 17, 2008
Conflict of Interest Statement: G.M does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.E.M. is a co-inventor on a pending patent for the use of prostacyclin and analogs to prevent cancer.
References
- 1.Zochbauer-Muller S, Gazdar AF, Minna JD. Molecular pathogenesis of lung cancer. Annu Rev Physiol 2002;64:681–708. [DOI] [PubMed] [Google Scholar]
- 2.Sato M, Shames DS, Gazdar AF, Minna JD. A translational view of the molecular pathogenesis of lung cancer. J horac Oncol. 2007;2:327–343. [DOI] [PubMed] [Google Scholar]
- 3.Khosravi-Far R, Der CJ. The Ras signal transduction pathway. Cancer Metastasis Rev 1994;13:67–89. [DOI] [PubMed] [Google Scholar]
- 4.Malkinson AM. Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer 2001;32:265–279. [DOI] [PubMed] [Google Scholar]
- 5.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 2001;410:1111–1116. [DOI] [PubMed] [Google Scholar]
- 6.Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, Whitsett JA, Koretsky A, Varmus HE. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev 2001;15:3249–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell 1998;92:253–263. [DOI] [PubMed] [Google Scholar]
- 8.Casci T, Vinos J, Freeman M. Sprouty, an intracellular inhibitor of Ras signaling. Cell 1999;96:655–665. [DOI] [PubMed] [Google Scholar]
- 9.Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development 1999;126:4465–4475. [DOI] [PubMed] [Google Scholar]
- 10.Cabrita MA, Christofori G. Sprouty proteins: antagonists of endothelial cell signaling and more. Thromb Haemost 2003;90:586–590. [DOI] [PubMed] [Google Scholar]
- 11.Lee CC, Putnam AJ, Miranti CK, Gustafson M, Wang LM, Vande Woude GF, Gao CF. Overexpression of sprouty 2 inhibits HGF/SF-mediated cell growth, invasion, migration, and cytokinesis. Oncogene 2004;23:5193–5202. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol 2004;5:441–450. [DOI] [PubMed] [Google Scholar]
- 13.Guy GR, Wong ES, Yusoff P, Chandramouli S, Lo TL, Lim J, Fong CW. Sprouty: how does the branch manager work? J Cell Sci 2003;116:3061–3068. [DOI] [PubMed] [Google Scholar]
- 14.Christofori G. Split personalities: the agonistic antagonist Sprouty. Nat Cell Biol 2003;5:377–379. [DOI] [PubMed] [Google Scholar]
- 15.Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, Bellusci S. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech Dev 2001;102:81–94. [DOI] [PubMed] [Google Scholar]
- 16.Sutterluty H, Mayer CE, Setinek U, Attems J, Ovtcharov S, Mikula M, Mikulits W, Micksche M, Berger W. Down-regulation of Sprouty2 in non-small cell lung cancer contributes to tumor malignancy via extracellular signal-regulated kinase pathway-dependent and -independent mechanisms. Mol Cancer Res 2007;5:509–520. [DOI] [PubMed] [Google Scholar]
- 17.Shaw AT, Meissner A, Dowdle JA, Crowley D, Magendantz M, Ouyang C, Parisi T, Rajagopal J, Blank LJ, Bronson RT, et al. Sprouty-2 regulates oncogenic K-ras in lung development and tumorigenesis. Genes Dev 2007;21:694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol 1999;208:281–292. [DOI] [PubMed] [Google Scholar]
- 19.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res 2002;11:21–29. [DOI] [PubMed] [Google Scholar]
- 20.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 2000;275:11858–11864. [DOI] [PubMed] [Google Scholar]
- 21.Nuzum EO, Malkinson AM, Beer DG. Specific Ki-ras codon 61 mutations may determine the development of urethan-induced mouse lung adenomas or adenocarcinomas. Mol Carcinog 1990;3:287–295. [DOI] [PubMed] [Google Scholar]
- 22.Shimkin MB, Polissar MJ. Some quantitative observations on the induction and growth of primary pulmonary tumors in strain A mice receiving urethan. J Natl Cancer Inst 1955;16:75–97. [PubMed] [Google Scholar]
- 23.Dyson P, Heppleston AG. Cell kinetics of urethane induced murine pulmonary adenomata: I. The growth rate. Br J Cancer 1975;31:405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamerton LF, Steel GG. Cell population kinetics in normal and malignant tissues. Prog Biophys Mol Biol 1968;18:245–283. [DOI] [PubMed] [Google Scholar]
- 25.Gunning WT, Kramer PM, Lubet RA, Steele VE, Pereira MA. Chemoprevention of vinyl carbamate-induced lung tumors in strain A mice. Exp Lung Res 2000;26:757–772. [DOI] [PubMed] [Google Scholar]
- 26.Gunning WT, Kramer PM, Steele VE, Pereira MA. Chemoprevention by lipoxygenase and leukotriene pathway inhibitors of vinyl carbamate-induced lung tumors in mice. Cancer Res 2002;62:4199–4201. [PubMed] [Google Scholar]
- 27.Moody TW, Leyton J, Zakowicz H, Hida T, Kang Y, Jakowlew S, You L, Ozbun L, Zia H, Youngberg J, et al. Indomethacin reduces lung adenoma number in A/J mice. Anticancer Res 2001;21:1749–1755. [PubMed] [Google Scholar]
- 28.Jalbert G, Castonguay A. Effects of NSAIDs on NNK-induced pulmonary and gastric tumorigenesis in A/J mice. Cancer Lett 1992;66:21–28. [DOI] [PubMed] [Google Scholar]
- 29.Kwabi-Addo B, Wang J, Erdem H, Vaid A, Castro P, Ayala G, Ittmann M. The expression of Sprouty1, an inhibitor of fibroblast growth factor signal transduction, is decreased in human prostate cancer. Cancer Res 2004;64:4728–4735. [DOI] [PubMed] [Google Scholar]
- 30.Lo TL, Yusoff P, Fong CW, Guo K, McCaw BJ, Phillips WA, Yang H, Wong ES, Leong HF, Zeng Q, et al. The ras/mitogen-activated protein kinase pathway inhibitor and likely tumor suppressor proteins, sprouty 1 and sprouty 2 are deregulated in breast cancer. Cancer Res 2004;64:6127–6136. [DOI] [PubMed] [Google Scholar]
- 31.Minowada G, Miller YE. Sprouty 2 gene in mouse lung tumorigenesis. Chest 2004;125:111S. [DOI] [PubMed] [Google Scholar]