Abstract
The multifunctional surface protein CD38 acts as a receptor with ecto-enzymatic activity, hydrolyzing NAD to generate several products known to exhibit Ca2+-mobilizing properties. Although CD38 is a convenient marker of immune cell development, and an indicator of progression for several diseases, it is not restricted to the immune compartment. To determine the potentially multilayered involvement of CD38 in allergen-induced airway inflammation and hyperreactivity, we dissected the potential role of CD38 as a regulator of immunity, but also pulmonary function. CD38-deficient and wild-type (WT) mice were sensitized and airway challenged with ovalbumin, and subsequently analyzed regarding their level of airway hyperresponsiveness (AHR) in response to methacholine. Parameters of lung inflammation were also analyzed. Similar sets of measurements were obtained from reciprocal bone marrow swapping experiments between CD38−/− and WT mice. Mice lacking CD38 exhibit strongly reduced AHR, which is accompanied by a decrease in typical hallmarks of pulmonary inflammation, including eosinophilia and lymphocytic lung infiltrates, as well as Th2-cytokine levels (IL-4, -5, and -13). Antigen-specific immunoglobulin (Ig)E and IgG1 antibody titers are substantially reduced, consistent with CD38 being crucial for mounting a primary humoral systemic immune response. Reconstitution of lethally irradiated, lung-shielded, CD38-deficient mice with WT bone marrow does not restore WT levels of airway hyperreactivity, nor mucus secretion. The opposite experiment, transferring CD38−/− bone marrow into WT mice, also shows reduced AHR levels. These studies demonstrate that CD38 not only acts as a key modulator of the immune response, but also plays an equally important role as an intrinsic pulmonary component.
Keywords: airway hyperreactivity, pulmonary inflammation, CD38 knockout mouse, bone marrow chimera
CLINICAL RELEVANCE
This study demonstrates that CD38, a surface marker with NAD-hydrolysis activity generating several Ca2+-mobilizing agents, plays a dual role in a mouse model of allergen-induced airway hyperresponsiveness.
A complex disease, asthma is increasingly described as a syndrome with multiple causes and evolving symptoms, resulting from progressive changes of the airway tissues as a consequence of the inflammatory environment (remodeling), culminating in a persistent airway hyperresponsiveness (AHR) (1, 2). Although this is a simplified model, the immunologic processes involved in airway inflammation of asthma are thought to be characterized by an imbalance in T helper type 1 (Th1)–Th2 immune regulation, resulting in increased Th2 cytokines, IL-4, IL-5, IL-9, and IL-13, as well as augmented immunoglobulin (Ig)E titers, lung eosinophilia, and mast cell degranulation.
Beyond the role of the immune system in the etiology of the disease, one main feature of asthma, which leads to its clinical manifestations, is the inappropriate contraction of the airway smooth muscle (3). Our knowledge about the molecular mechanisms underlying this dysfunction remains fragmented, in particular in the light of more recent findings documenting that airway smooth muscle cells not only mediate bronchoconstriction, but also participate in the immune response in the airways (4).
By virtue of its role as a universal cellular second messenger, Ca2+ is involved in numerous events underlying the allergic response and asthmatic disease. Ca2+ is crucial to immune cell activation and the production of cytokines (5), and essential for the contractility of airway smooth muscles (6, 7). Secretory processes such as the exocytotic release of mucus from lung goblet cells are also Ca2+-dependent (8). The variety of regulatory mechanisms and entry pathways affecting cytosolic levels of Ca2+ is a direct reflection of the multifaceted roles of Ca2+ in biology.
CD38 represents a novel type of Ca2+-response modulator. A Type II glycosylated surface molecule, CD38 was for decades primarily known as a convenient lymphocytic developmental marker. It has also been used extensively as an indicator of disease progression for HIV/AIDS (9), and B-cell chronic lymphocytic leukemia (B-CLL) (10). More recent studies have revealed that CD38's distribution and function appear to go far beyond the immune context (11). The extracellular portion of CD38 was recognized as an enzymatic region with NAD-glycohydrolase activity. Since this unexpected finding, numerous studies have investigated the enzymology and various functions of CD38 in immunity, as a receptor and signaling molecule (12, 13). CD38 and its relatives converts NAD into cADP-ribose and ADP-ribose (14). Interestingly, both products have calcium (Ca2+)-mobilizing activity (15). The former is known to modulate Ca2+ release from the endoplasmic reticulum by direct or indirect activation of the Ryanodine receptors (RyR) (16, 17); the latter gates the Ca2+-permeable channel TRPM2 (18). Thus, CD38 is thought to play an important role in Ca2+ signaling in various cell types. In particular, CD38 has been shown to be the main source of cADP-ribose in the lungs, where it could therefore contribute to bronchoconstriction, a highly Ca2+-dependent process (19). Naïve as well as IL-13– or TNF-α–challenged CD38−/− mice display diminished AHR after inhalation of increasing doses of methacholine, a nonallergic stimulus (20–22).
The effect of CD38 deficiency in an antigen-induced allergic mouse model of asthma has not yet been analyzed. Here, we show that CD38−/− mice, compared with wild-type C57BL/6 (WT) mice, after ovalbumin (OVA) sensitization and challenge, do not develop inflammation or eosinophilia in the lungs, and have reduced AHR. To dissect the potential role of CD38 as a regulator of immunity and pulmonary function, we analyzed bone marrow chimeric mice. The results of these experiments further support the notion that CD38 not only acts as a key modulator of the immune response, but also plays an equally important role as an intrinsic component of the lungs.
MATERIALS AND METHODS
Animals
C57BL/6 and CD38−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in our institutional animal facility. All experimental females used in this study were under a protocol approved by the Institutional Animal Care and Use Committee of the National Jewish Medical and Research Center.
Sensitization and Airway Challenge
Female mice 10 to 12 weeks of age were sensitized intraperitoneally by injection of 20 μg OVA (Grade V; Sigma Chemical Co., St. Louis, MO) emulsified in 2.25 mg aluminum hydroxide (AlumInject; Pierce Chemical, Rockford, IL) in a total volume of 100 μl on Days 1 and 14. Mice then received three inhalational challenges of 20 minutes each (Omron nebulizer, Kyoto, Japan) with 1% OVA in saline on Days 28, 29, and 30. Forty-eight hours after the last challenge, AHR was assessed and tissues were obtained for further analysis. Challenged-only mice did not receive any sensitization.
Determination of Airway Responsiveness
Female mice were anesthetized (pentobarbital sodium 70–100 mg/kg by intraperitoneal injection), tracheotomized, and connected to a computer-controlled small animal mechanical ventilator (flexiVent; SCIREQ, Montreal, PQ, Canada) for mechanical ventilation at 200 breaths/minute and a tidal volume of 0.2 ml against a positive end-expiratory pressure of 3 to 4 cm H2O. Mice were paralyzed with an intraperitoneal injection of pancuronium bromide (0.08 mg/kg), and then exposed to an aerosol of increasing doses of methacholine (0, 6.25, 12.5, 25, 50, and100 mg/ml of saline) for 10 seconds by ultrasonic nebulizer while the mice were ventilated at 30 breaths/minute with a tidal volume of 0.4 ml. At the end of the 10-second challenge, mechanical ventilation was resumed at 200 breaths/minute with a tidal volume of 0.2 ml. Every 10 seconds for the following 5 minutes, ventilation was interrupted to allow a 1-second passive expiration followed by the application of a 2-second broadband (1–20.5 Hz) volume perturbation to the lungs while the pressure required to generate the perturbation was measured. The pressure and flow data obtained during application of each volume perturbation were used to calculate a complex input impedance (Z) of the respiratory system, and Z was fit to a constant phase model of the lung (23). Plots show the changes in Rn (Newtonian resistance) that represents the resistance of the central airways, and G (tissue damping) that is closely related to tissue resistance.
Determination of Cell Numbers in Bronchoalveolar Lavage Fluids
After methacholine challenge, lungs were lavaged via the tracheal cannula with 1 ml HBSS. Total cell numbers were determined by counting cells with a Coulter Counter (Beckman-Coulter, Hialeah, FL). Differential cell counts were made from cytospin preparations (Cytospin 2; Shandon, Runcorn, Cheshire, UK), stained with Leukostat (Fisher Diagnostics, Pittsburgh, PA). Cells were identified as macrophages, lymphocytes, eosinophils, and neutrophils by standard hematologic procedures, and at least 200 cells were counted.
Lung and Peribronchial Lymph Node Leukocyte Isolation and Culture
Lungs and peribronchial lymph nodes (PBLNs) were harvested from either challenged-only or sensitized and challenged animals. After lavage, lungs were perfused and treated with collagenase types II and IV (Sigma-Aldrich) and dispase II (Roche, Basel, Switzerland) in HBSS containing fetal bovine serum (FBS) for 75 minutes at 37°C on a shaker, and leukocytes were obtained after filtration and centrifugation on a percoll gradient. Cells were resuspended in HBSS, counted with a hemocytometer, and cytospin slides were made. Slides were stained with Leukostat and cell differentiation was determined counting at least 200 cells using a light microscope. Single-cell suspension was obtained from the PBLNs by passing the tissue through stainless steel mesh and resuspended at 2 × 106 cells/ml in RPMI 1640 medium (GIBCO, Grand Island, NY) containing 10% FBS, penicillin (100U/ml), streptomycin (100 g/ml), and 5 mM glutamine. Cells were plated in 96-well round-bottom plates and cultured for 4 days in the presence of 100μg/ml OVA at 37°C. Cell-free supernatants were harvested and stored at −20°C for cytokine determination.
Cytokine Production Analyses
The cytokines secreted into the BAL fluid samples and in the supernatant of cultured lymph nodes were determined by enzyme-linked immunosorbent assay (ELISA) using the Pierce SearchLight multiplex assay (ThermoFisher Scientific, Rockford, IL). IL-4, IL-5, IL-10, IL-13, IFN-γ and TNF-α were measured following the manufacturer's instructions.
Measurement of Serum OVA-Specific IgG1 and IgE
OVA-specific IgG1 and IgE levels in the serum were measured by ELISA. Briefly, the plates were coated with 5μg/ml OVA overnight at 4°C and then blocked with 1% bovine serum albumin in PBS for 1 hour at 37°C. The samples were placed in the wells, and the plates were incubated for 1 hour at room temperature. Sample blank wells did not receive sera but were otherwise treated similarly. The bound Ig were detected with polyclonal goat anti-IgE or anti-IgG1 Alkaline Phosphatase–conjugated antibodies (Southern Biotech, Birmingham, AL). The plates were developed by addition of phosphatase substrate and read in an ELISA plate reader at 405 nm after 30 to 45 minutes of incubation at room temperature.
Histology of Lung Sections
Lungs were harvested, inflated, and immersed in 10% phosphate-buffered formalin. Lung tissues were embedded in paraffin, cut at 5 μm thickness, and stained with hematoxylin and eosin for evaluation of cell infiltrates and with periodic acid-Schiff (PAS) for evaluation of mucus-secreting cells.
Lung Shielding and Bone Marrow Transfer
Before irradiation (900 Rads, cesium source), mice were anesthetized through intrapritoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) in saline, and placed, or not, under a 1-cm-thick lead shield to protect the lung during the irradiation.
During their recovery time, bone marrow cell suspension from naïve mice was prepared by smashing the bone in a potter. Cells were transferred via intravenous injection of 1 × 107 cells in 200 μl of sterile HBSS. Six weeks after the transfer, recipient mice were immunized with OVA, and the presence of donor cells was assessed in peripheral blood 1 week later. Recipient mice were sensitized again with OVA on Day 14, and were airway challenged on Days 28, 29, and 30, as described above. On Day 32, airway function was assessed and tissue isolated for further analysis.
Statistical Analyses
Values of all measurements are expressed as the mean ± SEM. Significance between individual groups was determined by an unpaired, two-tailed Student's t test. Significance levels were set at a P value of 0.05.
RESULTS
CD38−/− Mice Are Resistant to Antigen-Induced Inflammation and AHR
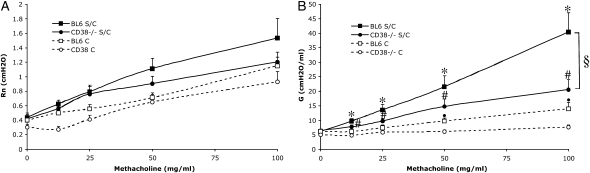
To investigate the potential involvement of CD38 in antigen-mediated lung inflammation, we analyzed CD38−/− mice using the well-established OVA protocol as described in Materials and Methods. We examined the change in central airway resistance (Rn) and tissue resistance (G) of CD38−/− mice in comparison to wild-type C57BL/6 (WT) in response to increasing doses of inhaled methacholine after OVA sensitization and challenge, or challenge only (Figures 1A and 1B). We found no significant difference in Rn (Figure 1A), but a slight decrease in lung function in response to methacholine when comparing the lung tissue resistance G values from challenged-only CD38−/− animals with those from similarly treated WT mice (Figure 1B), although not as pronounced as described in a previous study (20). There was no significant difference between the WT and mutant strains if we expressed the results as percentage change from baseline (data not shown). The difference between the study by Deshpande and coworkers and our own study could originate from the applied methodology, since this previous study used the single-compartment model (plethysmograph chamber from Buxco Electronics Inc., Sharon, CT), whereas we used the constant phase model (Flexivent; Scireq), making it difficult to directly compare the obtained results. Also, whereas we consistently used female animals, this was not specified in the other study, possibly explaining the apparent higher response seen there in WT animals (220% versus in our measurements 150% maximum increase at 100 mg/ml methacholine, as compared with saline challenge).
Figure 1.
Decreased hyperresponsiveness in CD38−/− mice after ovalbumin (OVA) sensitization and challenge. (A) Central airway resistance (Rn) and (B) tissue resistance (G) of WT-BL6 and CD38−/− mice (raw data) in response to increasing concentrations of aerosolized methacholine after OVA sensitization and challenge (S/C), or challenge only (C). Symbols represent the geometric mean and error bars show SEM. BL6 C, n = 8; CD38−/− C, n = 8; BL6 S/C, n = 14; CD38−/− S/C, n = 11. *P < 0.05 for BL6 S/C compared with BL6 C; #P < 0.05 for CD38−/− S/C compared with CD38−/− C; §P < 0.05 for BL6 S/C compared with CD38−/− S/C; ·P < 0.05 for BL6 C compared with CD38−/− C.
WT mice sensitized and challenged (S/C) with OVA displayed the expected alterations in lung function, characterized by significant increases in Rn (Figure 1A) and G (Figure 1B) in response to methacholine. In contrast, CD38−/− S/C mice did not develop any significant increase in Rn in response to aerosolized methacholine (Figure 1A). Furthermore, changes in tissue resistance (G) exhibited by sensitized and challenged CD38−/− mice, although higher than in challenged-only mice for the two highest methacholine doses, clearly did not reach the level of WT mice (Figure 1B). We therefore concluded that CD38 is crucially involved in mounting an allergen-induced pulmonary response.
Inflammatory Cell Infiltrates Are Reduced in the Lungs of CD38−/− Mice
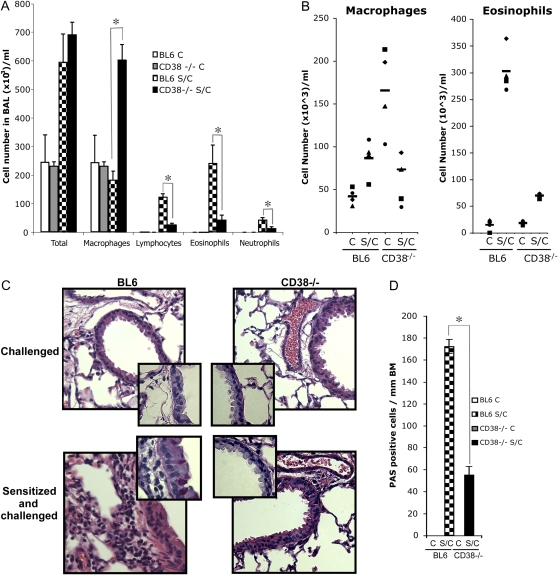
Inflammatory pulmonary cell infiltrates are a common correlative of diminished lung function. We therefore asked whether the absence of AHR response in CD38−/− mice was accompanied by alterations in the inflammatory response in the lungs. We compared total and differential number of cells in the bronchoalveolar lavage (BAL) fluid obtained from challenged-only and from OVA-sensitized and -challenged WT and CD38−/− mice (Figure 2A). As anticipated, we found a substantial increase in the number of cells in the BAL of WT mice after allergen sensitization and challenge, mostly reflecting the recruitment of eosinophils, lymphocytes, and neutrophils. Although the total number of cells found in the BAL of S/C CD38-deficient mice is comparable to that in S/C WT mice, this augmentation is largely attributable to a significant increase in macrophages, an intriguing phenomenon that we did not anticipate. Interestingly, we did not find a decreased number of macrophages in digested lung tissues, but observed higher numbers of macrophages in the lung tissue of CD38−/− mice that were only challenged (Figure 2B). Importantly, eosinophils, lymphocytes, and neutrophils, the immunocytes characteristics of an allergic inflammatory situation, were not significantly elevated in the S/C CD38−/− animals, suggesting a major disturbance of the allergen-induced pulmonary immune response in these mice. To determine whether the phenotype described above correlates with histologic changes, we analyzed the pulmonary tissues of S/C WT and CD38−/− mice. Even though lung sections of naïve BL6 and CD38−/− mice appear similar, after OVA sensitization and challenge we observed a reduced increase in inflammatory cell infiltrate in CD38−/− mice as compared with WT BL6 mice (Figure 2C). Furthermore, staining of these lung samples with PAS to detect mucus secretion revealed that the large airways from CD38−/− sensitized and challenged mice contained 3-fold less PAS-positive cells per millimeter of basal membrane compared with WT (Figure 2D). Overall, these results demonstrate that in the absence of CD38, mice develop only very attenuated characteristic features of antigen-induced allergic asthma.
Figure 2.
Reduced airway inflammation and mucus secretion in CD38−/− mice after OVA sensitization and challenge. (A) Leukocyte numbers in the bronchoalveolar lavage (BAL) of challenged (C) or sensitized and challenged (S/C) mice. *P < 0.05 for BL6 S/C compared with CD38−/− S/C. (B) Composition of cells extracted from the lungs, as described in Materials and Methods, after performing BAL and lung perfusion. (C) Hematoxylin and eosin (H&E)-stained lung sections from challenged or sensitized and challenged WT-BL6 and CD38−/− mice. Magnification of insets: ×100 of periodic acid Schiff (PAS)-stained sections. Bars = 50 μm. (D) Number of PAS-positive cells/mm of basal membrane (BM) identified from lung sections obtained from challenged (C) or sensitized and challenged (S/C) BL6 and CD38−/− mice. *P < 0.05 for BL6 S/C compared with CD38−/− S/C.
The Humoral Systemic OVA Response Is Strongly Decreased, but Not Abrogated, in CD38−/− Mice
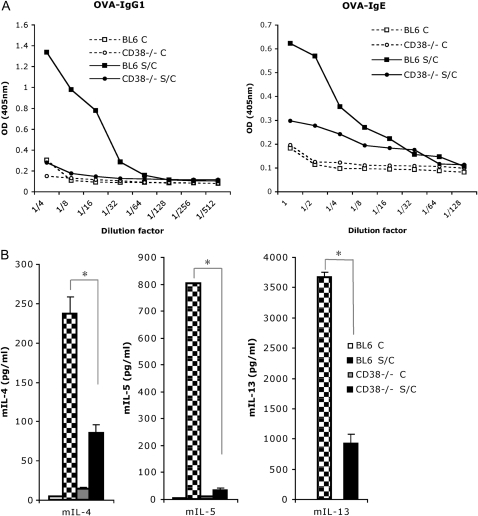
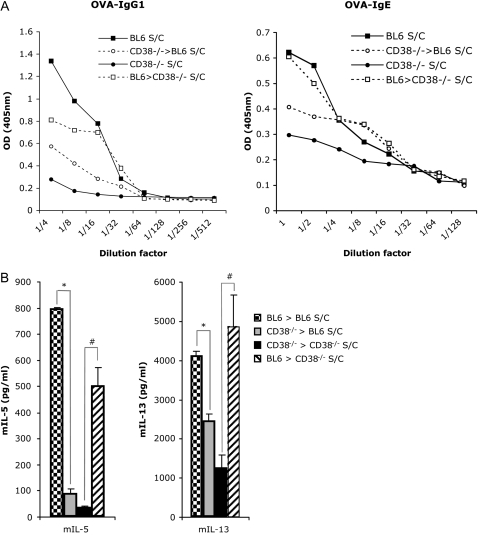
Antigen-specific IgE production is characteristic of a Th2-dominated allergic lung response (24, 25). We assessed the level of OVA-specific IgE and IgG1 production in the serum of mice that were OVA challenged only, versus sensitized and challenged. We found that titers of OVA-specific IgE and IgG1 were strongly decreased in CD38−/− mice after immunization as compared with WT mice (Figure 3A). Therefore, it appears that CD38-deficient mice are capable of mounting only a weak primary humoral systemic OVA response. This confirms a previous study documenting the inefficient (but not absent) priming of T cells by dendritic cells (DCs) lacking CD38 (26). To further delineate the role of CD38 in the immune allergen-induced asthma, we next aimed at determining the cytokine production levels in WT and mutant mice.
Figure 3.
Measurements of serum OVA-specific immunoglobulin (Ig)E and IgG1 levels in CD38−/− mice after OVA sensitization and challenge, and of the Th2 cytokines IL-4, -5, and -13 from peribronchial lymph nodes harvested from CD38−/− and BL6 mice. (A) Levels of serum OVA-specific IgE and IgG1 in challenged (C) or sensitized and challenged (S/C) mice. (B) Levels of IL-4, IL-5, and IL-13 in the supernatants of cell suspensions from peribronchial lymph node that were obtained from challenged (C) or sensitized and challenged (S/C) BL6 and CD38−/− mice, and cultured for 4 days in the presence of 100 μg/ml OVA at 37°C. *P < 0.05 for BL6 S/C compared with CD38−/− S/C.
Th2 Cytokine Production from Cultured Lung and Peribronchal Lymph Nodes Is Decreased in CD38−/− Mice
Because the reliable detection of cytokines in the BAL fluid is particularly challenging in the C57BL/6 genetic background, we opted to analyze the levels of secreted cytokines from cultured lymph nodes using a multiplex infrared ELISA assay (Searchlight, Pierce). To this aim, leukocyte suspensions obtained from peribronchial draining lymph nodes from WT and CD38−/− mice challenged only, or sensitized and challenged, were generated 48 hours after the final challenge, and cultured in the presence of OVA for restimulation. We found very consistently the levels of the Th2-cytokines IL-4, IL-13, and IL-5 to be lower in CD38−/− samples (Figure 3B). Interestingly the levels of IL-5 were most drastically reduced, in accordance with the lack of eosinophilia observed in the lungs of CD38−/− mice. The increase in IL-4 and IL-13 upon sensitization and challenge were severely diminished, but not eliminated (3-fold increase over challenge-only samples), possibly explaining why the primary systemic immune response is lessened, but not abrogated (Figure 3A).
Beyond Th2-cytokines, we looked at IFN-γ, a cytokine characteristic of Th1-mediated immune responses, and could not detect it in any of our samples (detection threshold in the assay was 10 pg/ml).
Reciprocal Bone Marrow Chimeras between CD38−/− and WT Mice Show Levels of Pulmonary Inflammation Similar to Those of the Donor Strain
Because CD38 is not only expressed in the immune context, but is also known to be present on various other cell types, including airway smooth muscle cells (27), we aimed at further dissecting the participation of CD38 in OVA-induced airway inflammation and hyperreactivity. To accomplish this, we performed bone marrow transfer experiments by replacing the hematopoietic compartment of WT mice with CD38-deficient bone marrow and vice versa. Control BM swaps transferring WT-BL6 BM into irradiated WT-BL6 mice (BL6 > BL6), and CD38−/− into CD38−/− mice (CD38−/− > CD38−/−) were also performed.
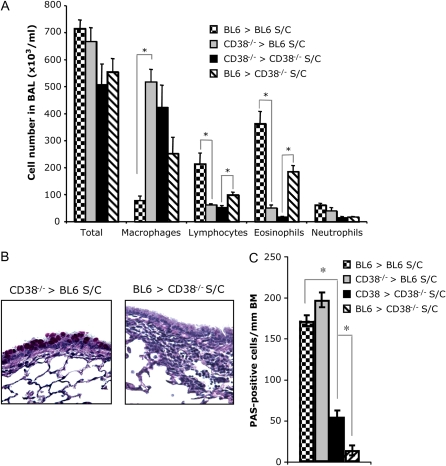
As anticipated, when we examined total and differential number of cells in the BAL fluid obtained from CD38−/− mice reconstituted with WT-BL6 bone marrow (BL6 > CD38−/−), we found the total cell numbers to reach the same level as in S/C WT samples. The influx of eosinophils and lymphocytes to the lungs is also restored in these mice after OVA sensitization and challenge, albeit to a lesser extent (Figure 4A).
Figure 4.
Reciprocal bone marrow swaps between WT-BL6 and CD38−/− mice result in the restoration of the inflammatory phenotype to the BM-donor phenotype, but not of the mucus secretion: Reconstitution of lethally irradiated CD38−/− mice with BL6 bone marrow (BL6 > CD38) restores airway inflammation after OVA sensitization and challenge, whereas reconstitution of BL6 mice with CD38−/− bone marrow suppresses it. (A) Leukocyte numbers in the BAL of irradiated BL6 and CD38−/− mice, reconstituted either with parental or with reciprocal bone marrow, after ovalbumin sensitization and challenge (S/C). (B) H&E-stained lung sections from naïve sensitized and challenged (S/C) BL6 and CD38−/− mice, either intact or reconstituted with reciprocal bone marrow swaps. (C) Number of PAS-positive cells/mm of basal membrane (BM) identified from lung sections obtained from sensitized and challenged (S/C) BL6 and CD38−/− mice, either intact or reconstituted with reciprocal bone marrow. *P < 0.05.
The reciprocal experiment, reconstituting irradiated WT animals with CD38−/− bone marrow (CD38−/− > BL6), results in a phenotype resembling the observations made in the CD38−/− mice (and in CD38−/− recipient mice reconstituted with CD38−/− BM), since these chimeras show no significant eosinophilia or lymphocytic presence in their BAL after OVA sensitization and challenge. We also reproduced our finding that macrophage numbers in the BAL of mice with a CD38-deficient immune system are elevated when compared with WT (Figure 4A).
To assess the inflammatory response in the lung tissues, we examined histologic changes in lung sections. In accordance with the results obtained in the BAL, the cell infiltration was severely reduced to absent in BL6 mice reconstituted with CD38−/− bone marrow when compared with WT-BL6 mice, and greatly enhanced in CD38−/− mice reconstituted with BL6 bone marrow compared with CD38−/− mice (Figure 4B).
Surprising is the observation that after OVA sensitization and challenge, BL6 mice reconstituted with CD38−/− bone marrow contained close to 10-fold more PAS-positive cells per millimeter of basal membrane than CD38−/− mice reconstituted with BL6 bone marrow (Figure 4C). This suggests that the decrease in mucus production observed in the CD38-deficient mice (Figure 2C) mostly originates from a defect extrinsic to the immune system, and that CD38 is required for allergen-induced goblet cell hyperplasia, since WT levels of mucus production were only reached when CD38 was present in the lung epithelium.
Levels of OVA-Specific IgE and IgG1 in the Reciprocal BM Chimeras between CD38−/− and WT Mice Resemble Those Observed in the BM Donor Strains
We confirmed our previous finding that the primary systemic immune response is diminished in the absence of CD38 in the immune compartment, since the CD38−/− into BL6-WT chimeras show titers of OVA-specific IgE and IgG1 close to these observed in CD38−/− mice, which are substantially lower than in WT animals. Conversely, we found that the BL6 into CD38−/− BM chimeric mice exhibit levels of OVA IgE and IgG1 levels comparable to those of S/C BL6 WT animals (Figure 5A).
Figure 5.
Measurements of serum OVA-specific IgE and IgG1 levels in bone marrow chimeric mice after OVA sensitization and challenge, and of the Th2 cytokines IL-5 and -13 from peribronchial lymph nodes harvested from these mice. (A) Levels of serum OVA-specific IgE and IgG1 in lethally irradiated BL6 or CD38−/− mice, reconstituted either with parental or with reciprocal bone marrow, after OVA sensitization and challenge (S/C). (B) Levels of IL-5 and IL-13 in supernatants from peribronchial lymph node cultured for 4 days in the presence of 100 μg/ml OVA that were obtained from lethally irradiated BL6 or CD38−/− mice, reconstituted either with parental or with reciprocal bone marrow, after OVA sensitization and challenge (S/C). *P < 0.05 for BL6 S/C compared with CD38−/− > BL6 S/C. #P < 0.05 for CD38−/− S/C compared with BL6 > CD38−/− S/C.
Th2-Cytokine Production from Cultured Peribronchal Lymph Nodes Is Restored in BL6 > CD38−/− Chimeric Mice
As described above, we measured amounts of cytokines in the supernatant of cultured lymph node cell suspensions. The two control chimeras BL6 > BL6 and CD38−/− > CD38−/− consolidated our results that IL-5 and IL-13 production are strongly decreased upon deletion of CD38 when compared with a WT response. Large increase in both these cytokines to levels close to the BL6 > BL6 positive control were documented in the BL6 > CD38−/− chimeras, but not in the reverse BM transfer experiment (Figure 5B). The measured levels of IL-4 in these experiments were statistically not relevant and therefore unfortunately inconclusive.
Overall, these results demonstrate that reintroduction of CD38 into the immune compartment of otherwise CD38-deficient animals is sufficient to recover Th2 cytokine production in the OVA model.
Lead Shielding of the Lungs during Irradiation Helps Prevent Pulmonary Damage
Since we wished to include lung function parameters in our assessments of the CD38−/−< > BL6 bone marrow chimeras, we were faced with the hurdle of lung damage after the irradiation of the recipient animals. We therefore established a lung-shielding procedure by covering the thoracic area of anesthesized mice with a 1-cm-thick stripe of lead during the irradiation procedure (see Figure E1A in the online supplement). Considering the 1-cm thickness of the shield (d), and that our radiation source is cesium (0.7 MeV), resulting in an absorption coefficient of 1.5 (μ), radiation under the shielded area is reduced by 80% (I(d) = I0.e−μd = 100.e−1.5 = 22.3% of the original radiation is reaching the tissues).
We found that in this particular experiment, at 10 weeks after reconstitution, the cell transfer efficiency did not appear to be majorly affected by the shielding protocol, as documented by the percentage of GFP-positive cells found in the BAL and spleen of a non-GFP mouse reconstituted with bone marrow from a GFP-donor (Figure E1B). Our assessment of lung function after OVA sensitization and challenge in these mice shows that the lead shielding restored the baseline values for Rn and G as well as the elastance to levels comparable with those of nonirradiated mice (Figures E2A and E2B), and prevented the increase in responsiveness in central airway resistance Rn (Figure E2A). On the other hand, the significant decrease in lung tissue resistance in the smaller airways (G) observed in the irradiated mice compared with the nonirradiated controls was not ameliorated by the shielding (Figure E2B). The cellular composition of the BAL after OVA sensitization and challenge was not significantly affected by the irradiation nor the shielding at the analyzed time point of 2.5 months after irradiation (Figure E2C). Noticeably, in the absence of shielding, we observed hemorrhage in the lungs of mice after lung function measurements, over 10 weeks after the irradiation took place. This suggests that the endothelial integrity has experienced long-lasting damages through the irradiation procedure, resulting in the rupture of the vessels through mechanic stress. This phenomenon was prevented by the lead-shielding of the lungs (Figure E2D).
These results document the impact of the irradiation protocol on some of the pulmonary parameters that we are planning to monitor in the bone marrow chimera experiments, and the protective effect of the applied lung shielding protocol. However, there are also disadvantages to the shielding procedure, since it results in the protection of some draining lymph nodes, as well as of the local bone marrow, and of the resident lung macrophages. Consequently, the immune system of the recipient animals would be expected to be partially preserved, making it potentially more complex to evaluate the contribution of the donor cells to the observed phenotype. Still, we concluded that it is worthwhile to use the lead shielding protocol to protect the lungs during the irradiation to avoid artifacts originating from pulmonary tissue damage.
Reciprocal Bone Marrow Chimeras between CD38−/− and WT Mice Both Display Reduced Antigen-Induced AHR
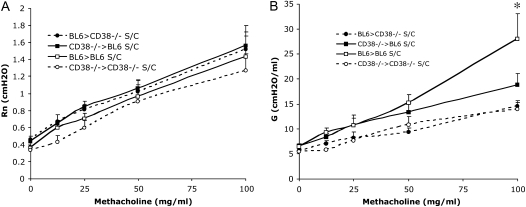
As previously, we examined the change in central airway resistance (Rn) and tissue resistance (G), this time of WT-BL6 mice reconstituted with CD38−/− bone marrow, and of CD38−/− mice reconstituted with WT-BL6 bone marrow, in response to increasing doses of inhaled methacholine, after OVA sensitization and challenge (Figures 6A and 6B). After the bone marrow transfer procedures with lung shielding, we found that 7 weeks after reconstitution, 80–85%% of the B220+ cells in the blood were exhibiting the CD38 phenotype of the donor (Figure E3). As previously, control BM swaps BL6 > BL6 and CD38−/− > CD38−/− were also performed. In all groups of mice, the baseline values (before methacholine challenge) for both Rn and G were comparable. The control bone marrow transfers confirmed our previous observations that CD38−/−-deficient animals show reduced AHR as documented by the significant drop in the lung tissue resistance G values when comparing lung function parameters of S/C BL6 > BL6 with those of S/C CD38−/− > CD38−/− animals. Further, we found that sensitized and challenged WT-BL6 mice reconstituted with CD38−/− bone marrow developed only very weak AHR, as the increase in G response to aerosolized methacholine is comparable to the responses obtained in the CD38−/− > CD38−/− BM chimeras (Figure 6B). This is true even when taking into account our previous finding that irradiation alone leads to a decrease in the change in G in response to methacholine (Figure E2B). Indeed, when compared with the control chimeras where WT mice were reconstituted with WT bone marrow, AHR still appears significantly reduced in the CD38−/− > BL6 chimeras, demonstrating that beyond procedural effects, the deficiency in CD38 is causal to the further reduction in G increase (Figure 6B). Thus, it appears that CD38 deficiency in the immune context is sufficient to inhibit the development of AHR.
Figure 6.
No restoration of the AHR phenotype to the BM-donor phenotype in reciprocal bone marrow swaps between WT-BL6 and CD38−/− mice after OVA sensitization and challenge. (A) Central airway resistance (Rn) and (B) tissue resistance (G) of BL6 and CD38−/− mice whose lungs were lead-shielded to protect them from radiation damage, and reconstituted with either parental or reciprocal bone marrow, in response to increasing concentrations of aerosolized methacholine after sensitization and challenge (S/C). Symbols represent the geometric mean and error bars show SEM. BL6 > BL6 S/C, n = 7; CD38−/− > CD38−/− S/C, n = 8; BL6 > CD38−/− S/C, n = 12; CD38−/− > BL6 S/C, n = 12. *P < 0.05 for BL6 S/C compared with BL6 > CD38−/− and CD38−/− > BL6 S/C.
Importantly, the reciprocal bone marrow swap, CD38−/− mice reconstituted with BL6 bone marrow, did not restore AHR to the wild-type BL6 level either, as assessed by changes in the tissue resistance G (Figure 6B). This result implies that the role of CD38 in allergen-induced asthma is not limited to its participation in the immune response, but extends to its presence in the lung tissues. This argues in favor of a dual model of CD38 function, as an immunologic and intrinsic pulmonary tissue component, both playing a nonnegligible role in AHR.
DISCUSSION
The initiation and exacerbation of asthma involve a complex interplay between the immune system and local pulmonary components that result in the characteristic clinical picture of impaired respiratory function. Increase in airway smooth muscle (ASM) mass and their contractility, decreased elasticity of the lung, epithelial damage and mucus hypersecretion, all lead to airflow obstruction, and are some of the lung-intrinsic parameters that are discussed in conjunction with asthma (28). The studies presented here aimed at investigating the participation in allergic asthma of CD38, a surface marker with ectoenzymatic activity that converts extracellular NAD into several products, including free ADP-ribose and cADPR, both compounds reported to exhibit Ca2+-mobilization properties. Based on its role as a universal cellular messenger, Ca2+ is involved in crucial aspects of all physiologic processes relevant to asthma, from the immune response to ASM and epithelial cell functions.
In three prior studies analyzing the effect of CD38 deficiency on airway function, it was reported that naïve CD38 knockout mice show diminished responsiveness to methacholine (20), as well as after IL-13 and TNF-α challenge (21, 22). Since both models do not require the initiatory immune response, and IL-13 elicited an inflammatory situation in the lungs of the CD38−/− mice comparable with those of WT animals, the authors concluded that CD38's involvement in these cases must be intrinsic to the lung. In the present study, we have tested how allergen-induced asthma is impacted by the absence of CD38 using the mouse OVA model. We found parameters of pulmonary inflammation and AHR to be decreased in CD38−/− animals. Reciprocal bone marrow swap experiments between WT and CD38-deficient mice described here support the notion that CD38 plays a dual role in this model, not only as a modulator of inflammatory events, but also as an intrinsic component of the pulmonary tissues, both in regulating the airway constriction, and possibly mucus secretion from the epithelial cells.
Regarding the immune aspect of CD38 deficiency in the OVA asthma model, the significant decrease in antigen-specific IgG1 and IgE indicates defective systemic adaptive immunity. This finding is consistent with published reports that CD38-deficient dendritic cells fail to migrate to lymph nodes cells (26, 29), ultimately resulting in the lack of efficient priming of Th2, which in turn do not produce sufficient amounts of cytokines such as IL-4/5 and -13 that are the signal required for eosinophil recruitment. Future studies, for example through adoptive transfer of selected immune cell populations, will need to demonstrate that this mechanism underlies the diminished immune response in the OVA model of allergen-induced AHR. Beyond the defect in DC-function, it has also been demonstrated that neutrophils lacking CD38 have intrinsic migratory defects, although not to all chemoattractants, since for example IL-8 responsiveness appears intact. How eosinophilic function might be influenced by CD38 is unclear, although their ability to respond to IL-13 challenge and the resulting eotaxin production, is not affected in vivo (21). One unexpected aspect of our study is the elevation in macrophage numbers observed in the BAL of sensitized and challenged CD38−/− mice, a finding confirmed in chimeric mice whose immune system was reconstituted with CD38−/− BM. There is currently only sparse information in the literature about the potential involvement of CD38 in macrophage homeostasis or function (30, 31). Moreover, the role of alveolar macrophages (AM) in the context of asthma and of the murine OVA model analyzed here remains poorly defined, although several studies suggest that AMs contribute to an attenuation of AHR (32, 33). To address some of these questions, it will therefore be of interest to for example perform in vivo clodronate-depletion of AMs to investigate their potential contribution in reducing AHR in CD38−/− mice.
The absence of the typical hallmarks of inflammation after OVA sensitization and challenge we observed in CD38−/− mice is therefore consistent with a model in which, at an early time point of allergen-induced asthma, CD38 is essential to mount an immune response to the allergen. However, as mentioned above, the resulting inflammatory cascade, once the Th2 chemokines are present, has been shown not to be affected by the lack of CD38. On the other hand, the severity of the pulmonary reaction is strongly decreased in the absence of CD38, independently of its role in the immune context, as shown by the reduced AHR parameters that remain comparable to CD38-deficent mice in BM chimeras where CD38−/− mice were reconstituted with a WT immune compartment. This points at a role of CD38 in the context of the pulmonary tissues themselves.
CD38 is known to be expressed on ASM cells (34, 35), and its enzymatic product cyclic ADP-ribose (cADPR) triggers sarcoplasmic reticulum (SR) Ca2+ release in ASM (27, 36). Furthermore, it was demonstrated that β-NADH and mild hypoxia result in cADPR accumulation in pulmonary smooth muscle homogenates (37). Recent studies have shown that the selective cell-permeant cADPR antagonist 8-bromo-cADPR attenuates the elevation in [Ca2+]i to the agonists Acetylcholine (ACh) and endothelin-1 in ASM, but not to histamine (38), and subsequently, that stimulation with ACh indeed leads to cADPR increase in ASM cells (39). Interestingly, CD38 expression appears to be up-regulated in human ASM after exposure to the inflammatory cytokines TNF-α, IFN-γ, IL-1β, and the Th2 cytokine IL-13 (20, 27, 40, 41).
TNF-α has also been found to increase the expression of two genes whose products are essential for mucus production by epithelial cells: the calcium-activated chloride channel gob-5, and the mucin MUC-5AC (42). Very little is currently known about the potential role of CD38-mediated events in the pulmonary epithelium. A few reports document the presence of CD38 in this context, but it has not been investigated in which capacity (43, 44). Our observation that the mucus hypersecretion associated with asthma is not restored in irradiated CD38−/− mice reconstituted with WT-BL6 bone marrow suggests that CD38 takes part in this process. Although this has been insufficiently studied regarding mucin secretion from goblet cells, the essential role of Ca2+ in triggering exocytotic release is vastly documented in other cell types such as neurons. A very recent study has begun to analyze the Ca2+ response of goblets cells after purinergic stimulation (45). It will be interesting to determine how CD38 might contribute to goblet cell function, and more generally, to the currently discussed involvement of epithelial cells in promoting inflammation and tissue remodeling (46).
The precise molecular mechanism by which CD38 might influence immune and smooth muscle cell function remains speculative at this time, but is thought to involve the aforementioned Ca2+-mobilizing properties of the CD38 NAD-hydrolysis reaction products. Over the past decade, much attention has been given to cyclic ADP-ribose, since it was shown to be a potent intracellular Ca2+ store depletion agent via the activation of the ryanodine receptors (RyR) on the surface of the ER/SR. The question whether cADPR directly interacts with RyR or requires a soluble binding protein is still subject to debate (15). By far the most abundant product of CD38's enzymatic activity is, however, free ADP-ribose, which represents 97% of the obtained products. Whereas free ADP-ribose has been considered for a long time a toxic metabolite with no recognized signaling function, the discovery of the ADP-ribose activated TRPM2 channel, a puzzling chimeric molecule consisting of a TRP ion channel with a Ca2+-permeable pore fused to an enzymatic region with homologies to the NUDIX family of hydrolases (NUcleoside DIphosphate bound to X, their class of substrates), sheds new light on this metabolite (18, 47). TRPM2 is well represented in the immune context (48), particularly in cells of the phagocytic lineage, but although it is detectable in the lung by qPCR (49), its cell type–specific pulmonary expression profile has not been analyzed.
In summary, the present study adds to the growing evidence that CD38 plays a complex role in pulmonary inflammation and asthma. Potential molecular targets for asthma treatment that would simultaneously and independently influence the immune and pulmonary cellular components of the disease are not frequent. Therefore, the multifaceted involvement of CD38 supported by the findings presented here holds the promise for future pharmacologic strategies aiming at manipulating various entities of the CD38 pathway, which beyond CD38 itself, could also include further effector molecules such as the TRPM2 ion channel.
Supplementary Material
Acknowledgments
Dr. Katsuyuki Takeda is acknowledged for his kind help and technical advice, and for reviewing this manuscript. The authors thank the National Jewish Histology and FACS facilities, as well as Drs. Azzeddine Dakhama and Steve Groshong for their help in evaluating the histology results presented here. They are also grateful to Dr. Raul Torres for many helpful discussions and suggestions. The authors further express their gratitude to the Sandler family for their generosity and the asthma research program they brought to life.
This work was supported by the Sandler Program in Asthma Research (A.-L. P.), the postdoctoral NIAID training grant A107045-16 (J.M.H.), and a Fellow to Faculty grant from the American Heart Association (W.J.J.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2007-0392OC on October 17, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lloyd CM, Robinson DS. Allergen-induced airway remodelling. Eur Respir J 2007;29:1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore WC, Peters SP. Update in asthma 2006. Am J Respir Crit Care Med 2007;175:649–654. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Helli PB, Catalli A, Chew A, Janssen LJ. Airway smooth muscle excitation-contraction coupling and airway hyperresponsiveness. Can J Physiol Pharmacol 2005;83:725–732. [DOI] [PubMed] [Google Scholar]
- 4.Lazaar AL, Panettieri RA Jr. Airway smooth muscle as a regulator of immune responses and bronchomotor tone. Clin Chest Med 2006;27:53–69. (vi.). [DOI] [PubMed] [Google Scholar]
- 5.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol 2007;7:690–702. [DOI] [PubMed] [Google Scholar]
- 6.Jude JA, Wylam ME, Walseth TF, Kannan MS. Calcium signaling in airway smooth muscle. Proc Am Thorac Soc 2008;5:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc 2008;5:23–31. [DOI] [PubMed] [Google Scholar]
- 8.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol 2008;70:487–512. [DOI] [PubMed] [Google Scholar]
- 9.Langford SE, Ananworanich J, Cooper DA. Predictors of disease progression in HIV infection: a review. AIDS Res Ther 2007;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deaglio S, Vaisitti T, Aydin S, Ferrero E, Malavasi F. In-tandem insight from basic science combined with clinical research: CD38 as both marker and key component of the pathogenetic network underlying chronic lymphocytic leukemia. Blood 2006;108:1135–1144. [DOI] [PubMed] [Google Scholar]
- 11.Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 2007;446:41–45. [DOI] [PubMed] [Google Scholar]
- 12.Lund FE. Signaling properties of CD38 in the mouse immune system: enzyme-dependent and -independent roles in immunity. Mol Med 2006;12:328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malavasi F, Deaglio S, Ferrero E, Funaro A, Sancho J, Ausiello CM, Ortolan E, Vaisitti T, Zubiaur M, Fedele G, et al. CD38 and CD157 as receptors of the immune system: a bridge between innate and adaptive immunity. Mol Med 2006;12:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 1993;262:1056–1059. [DOI] [PubMed] [Google Scholar]
- 15.Fliegert R, Gasser A, Guse AH. Regulation of calcium signalling by adenine-based second messengers. Biochem Soc Trans 2007;35:109–114. [DOI] [PubMed] [Google Scholar]
- 16.Meszaros LG, Bak J, Chu A. Cyclic ADP-ribose as an endogenous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature 1993;364:76–79. [DOI] [PubMed] [Google Scholar]
- 17.Galione A, Lee HC, Busa WB. Ca(2+)-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science 1991;253:1143–1146. [DOI] [PubMed] [Google Scholar]
- 18.Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 2001;411:595–599. [DOI] [PubMed] [Google Scholar]
- 19.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J 2007;30:114–133. [DOI] [PubMed] [Google Scholar]
- 20.Deshpande DA, White TA, Guedes AG, Milla C, Walseth TF, Lund FE, Kannan MS. Altered airway responsiveness in CD38-deficient mice. Am J Respir Cell Mol Biol 2005;32:149–156. [DOI] [PubMed] [Google Scholar]
- 21.Guedes AG, Paulin J, Rivero-Nava L, Kita H, Lund FE, Kannan MS. CD38-deficient mice have reduced airway hyperresponsiveness following IL-13 challenge. Am J Physiol Lung Cell Mol Physiol 2006;291:L1286–L1293. [DOI] [PubMed] [Google Scholar]
- 22.Guedes AG, Jude JA, Paulin J, Kita H, Lund FE, Kannan MS. Role of CD38 in TNF-{alpha}-induced airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol 2008;294:L290–L299. [DOI] [PubMed] [Google Scholar]
- 23.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992;72:168–178. [DOI] [PubMed] [Google Scholar]
- 24.Finkelman FD, Katona IM, Urban JF Jr, Holmes J, Ohara J, Tung AS, Sample JV, Paul WE. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol 1988;141:2335–2341. [PubMed] [Google Scholar]
- 25.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med 1998;187:939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partida-Sanchez S, Goodrich S, Kusser K, Oppenheimer N, Randall TD, Lund FE. Regulation of dendritic cell trafficking by the ADP-ribosyl cyclase CD38: impact on the development of humoral immunity. Immunity 2004;20:279–291. [DOI] [PubMed] [Google Scholar]
- 27.Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose-mediated Ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. FASEB J 2003;17:452–454. [DOI] [PubMed] [Google Scholar]
- 28.Berend N, Salome CM, King GG. Mechanisms of airway hyperresponsiveness in asthma. Respirology 2008;13:624–631. [DOI] [PubMed] [Google Scholar]
- 29.Partida-Sanchez S, Rivero-Nava L, Shi G, Lund FE. CD38: an ecto-enzyme at the crossroads of innate and adaptive immune responses. Adv Exp Med Biol 2007;590:171–183. [DOI] [PubMed] [Google Scholar]
- 30.Iqbal J, Zaidi M. CD38 is required for priming by TNF-alpha: a mechanism for extracellular coordination of cell fate. Am J Physiol Renal Physiol 2007;292:F1283–F1290. [DOI] [PubMed] [Google Scholar]
- 31.Song EK, Lee YR, Yu HN, Kim UH, Rah SY, Park KH, Shim IK, Lee SJ, Park YM, Chung WG, et al. Extracellular NAD is a regulator for FcgammaR-mediated phagocytosis in murine macrophages. Biochem Biophys Res Commun 2008;367:156–161. [DOI] [PubMed] [Google Scholar]
- 32.Peters-Golden M. The alveolar macrophage: the forgotten cell in asthma. Am J Respir Cell Mol Biol 2004;31:3–7. [DOI] [PubMed] [Google Scholar]
- 33.Pouliot P, Spahr A, Careau E, Turmel V, Bissonnette EY. Alveolar macrophages from allergic lungs are not committed to a pro-allergic response and can reduce airway hyperresponsiveness following ex vivo culture. Clin Exp Allergy 2008;38:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White TA, Johnson S, Walseth TF, Lee HC, Graeff RM, Munshi CB, Prakash YS, Sieck GC, Kannan MS. Subcellular localization of cyclic ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities in porcine airway smooth muscle. Biochim Biophys Acta 2000;1498:64–71. [DOI] [PubMed] [Google Scholar]
- 35.Sieck GC, White TA, Thompson MA, Pabelick CM, Wylam ME, Prakash YS. Regulation of store-operated Ca2+ entry by CD38 in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2008;294:L378–L385. [DOI] [PubMed] [Google Scholar]
- 36.Prakash YS, Kannan MS, Walseth TF, Sieck GC. Role of cyclic ADP-ribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. Am J Physiol 1998;274:C1653–C1660. [DOI] [PubMed] [Google Scholar]
- 37.Evans AM, Dipp M. Hypoxic pulmonary vasoconstriction: cyclic adenosine diphosphate-ribose, smooth muscle Ca(2+) stores and the endothelium. Respir Physiol Neurobiol 2002;132:3–15. [DOI] [PubMed] [Google Scholar]
- 38.White TA, Kannan MS, Walseth TF. Intracellular calcium signaling through the cADPR pathway is agonist specific in porcine airway smooth muscle. FASEB J 2003;17:482–484. [DOI] [PubMed] [Google Scholar]
- 39.Kip SN, Smelter M, Iyanoye A, Chini EN, Prakash YS, Pabelick CM, Sieck GC. Agonist-induced cyclic ADP ribose production in airway smooth muscle. Arch Biochem Biophys 2006;452:102–107. [DOI] [PubMed] [Google Scholar]
- 40.Tirumurugaan KG, Jude JA, Kang BN, Panettieri RA, Walseth TF, Kannan MS. TNF-alpha induced CD38 expression in human airway smooth muscle cells: role of MAP kinases and transcription factors NF-kappaB and AP-1. Am J Physiol Lung Cell Mol Physiol 2007;292:L1385–L1395. [DOI] [PubMed] [Google Scholar]
- 41.Tirumurugaan KG, Kang BN, Panettieri RA, Foster DN, Walseth TF, Kannan MS. Regulation of the cd38 promoter in human airway smooth muscle cells by TNF-alpha and dexamethasone. Respir Res 2008;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busse PJ, Zhang TF, Srivastava K, Lin BP, Schofield B, Sealfon SC, Li XM. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol 2005;116:1256–1263. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez JE, Deaglio S, Donati D, Beusan IS, Corno F, Aranega A, Forni M, Falini B, Malavasi F. Analysis of the distribution of human CD38 and of its ligand CD31 in normal tissues. J Biol Regul Homeost Agents 1998;12:81–91. [PubMed] [Google Scholar]
- 44.Khoo KM, Chang CF. Purification and characterization of CD38/ADP-ribosyl cyclase from rat lung. Biochem Mol Biol Int 1998;44:841–850. [DOI] [PubMed] [Google Scholar]
- 45.Rossi AH, Salmon WC, Chua M, Davis CW. Calcium signaling in human airway goblet cells following purinergic activation. Am J Physiol Lung Cell Mol Physiol 2007;292:L92–L98. [DOI] [PubMed] [Google Scholar]
- 46.Holgate ST. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol 2007;28:248–251. [DOI] [PubMed] [Google Scholar]
- 47.Eisfeld J, Luckhoff A. Trpm2. Handb Exp Pharmacol 2007;179:237–252. [DOI] [PubMed] [Google Scholar]
- 48.Perraud AL, Knowles HM, Schmitz C. Novel aspects of signaling and ion-homeostasis regulation in immunocytes. The TRPM ion channels and their potential role in modulating the immune response. Mol Immunol 2004;41:657–673. [DOI] [PubMed] [Google Scholar]
- 49.Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 2006;26:159–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.