Abstract
Rationale: Transthoracic Doppler echocardiography is recommended for screening for the presence of pulmonary hypertension (PH). However, some recent studies have suggested that Doppler echocardiographic pulmonary artery pressure estimates may frequently be inaccurate.
Objectives: Evaluate the accuracy of Doppler echocardiography for estimating pulmonary artery pressure and cardiac output.
Methods: We conducted a prospective study on patients with various forms of PH who underwent comprehensive Doppler echocardiography within 1 hour of a clinically indicated right-heart catheterization to compare noninvasive hemodynamic estimates with invasively measured values.
Measurements and Main Results: A total of 65 patients completed the study protocol. Using Bland-Altman analytic methods, the bias for the echocardiographic estimates of the pulmonary artery systolic pressure was −0.6 mm Hg with 95% limits of agreement ranging from +38.8 to −40.0 mm Hg. Doppler echocardiography was inaccurate (defined as being greater than ±10 mm Hg of the invasive measurement) in 48% of cases. Overestimation and underestimation of pulmonary artery systolic pressure by Doppler echocardiography occurred with a similar frequency (16 vs. 15 instances, respectively). The magnitude of pressure underestimation was greater than overestimation (−30 ± 16 vs. +19 ± 11 mm Hg; P = 0.03); underestimates by Doppler also led more often to misclassification of the severity of the PH. For cardiac output measurement, the bias was −0.1 L/min with 95% limits of agreement ranging from +2.2 to −2.4 L/min.
Conclusions: Doppler echocardiography may frequently be inaccurate in estimating pulmonary artery pressure and cardiac output in patients being evaluated for PH.
Keywords: echocardiography, pulmonary hypertension, pulmonary systolic pressure, cardiac output, accuracy
Scientific Knowledge on the Subject
Although Doppler echocardiography (DE) is recommended as a screening tool for the diagnosis of pulmonary hypertension (PH), its accuracy in estimating pulmonary artery systolic pressure in PH patients has been questioned. The value of DE for estimating cardiac output (CO) in these patients has not been assessed.
What This Study Adds to the Field
This prospective study demonstrates that DE can frequently overestimate and underestimate pulmonary artery pressure in PH patients. This error is in part explained by inaccuracies of right atrial pressure estimation and poor Doppler imaging of the transtricuspid regurgitant jet. The estimation of CO by DE does not appear to be reliable.
Pulmonary hypertension (PH), a syndrome characterized by increased pulmonary vascular resistance and remodeling, is associated with significant morbidity and mortality, which are directly related to cardiac function (1). Although the definitive diagnosis of PH is currently established through right-heart catheterization, accurate noninvasive assessment of pulmonary arterial pressure and cardiac output (CO) is desirable both for diagnostic purposes and to assess response to therapy.
Transthoracic Doppler echocardiography (DE) is recommended as the initial noninvasive modality in the screening and evaluation of PH (2). Echocardiography can be used to evaluate right-sided chamber size and function and the presence of pericardial effusion, which are known to impact survival (3–5). Frequently, DE is used to estimate the right ventricular systolic pressure by estimating the pressure gradient between the right ventricle and the right atrium using the modified Bernoulli equation, 4v2, where v equals the velocity of the tricuspid regurgitant jet. An estimated right atrial pressure is added to this number to approximate the right ventricular systolic pressure, which equals the pulmonary artery systolic pressure in the absence of pulmonic stenosis. Using this method, several investigators have demonstrated an adequate correlation between the Doppler estimates and direct measurements with right-heart catheterization (6, 7), although the accuracy of DE has been called in to question in certain clinical settings (8, 9).
Other than measuring pulmonary pressures, DE has the potential to provide additional information important in the management of patients with PH, such as assessment of CO, an important prognostic indicator of survival in these patients (1). However, little is known about the accuracy of Doppler-estimated CO in patients with pulmonary hypertension.
The purpose of this study was to prospectively evaluate the accuracy of DE in estimating pulmonary artery systolic pressure and CO in consecutive patients referred to a single center for evaluation or treatment of PH and in whom DE was performed within 1 hour of hemodynamic assessment by right-heart catheterization. Some of the results of the current study have been previously reported in abstract form (10).
METHODS
This study was conducted by the PH Program at Johns Hopkins University. The Institutional Review Board approved the conduct of this study, and all patients gave informed consent before enrollment. Consecutive patients referred for right-heart catheterization for the diagnosis or management of PH were asked to participate in the study. Study patients underwent a transthoracic DE within 1 hour of completing the right-heart catheterization. A subset of patients from this cohort was reported previously (3, 11).
Right Heart Catheterization
Right heart catheterization was performed at rest without sedation by members of the research team (M.F. and H.C.). Pressure measurements were taken from the right atrium, right ventricle, and pulmonary artery at the end of expiration. CO was determined using the thermodilution method using an average of a minimum of three measurements. Left-to-right shunting was ruled out by oximetry.
Transthoracic Doppler Echocardiography
All patients had a comprehensive two-dimensional DE protocol within 1 hour of right-heart catheterization, as described previously (3). Echocardiograms were performed by one technician (E.C.) using a Philips Sonos 5500 with a 3.2 MHz transducer (Philips Medical Systems, Andover, MA). Images were recorded in multiple views thus obtaining optimal imaging for the primary analysis. Right atrial pressure (RAP) was estimated by evaluating the inferior vena cava (IVC) size and change with respiration (12). Briefly, RAP was estimated to be 5 mm Hg when the IVC diameter was less than 20 mm and the collapsibility greater than 50%; 10 mm Hg when IVC diameter was less than 20 mm and collapsibility less than 50%; 15 mm Hg when IVC diameter was greater than 20 mm and collapsibility greater than 50%; and 20 mm Hg when IVC diameter was greater than 20 mm and collapsibility less than 50%. Continuous wave Doppler was used to measure the peak velocity of the tricuspid regurgitant (TR) jet at end-expiration. The interpreting cardiologist (P.F.) also graded the quality of the continuous wave Doppler envelope: excellent (high-intensity Doppler signal with full “spade shaped” Doppler envelope with clearly visualized peak), good (moderate- or high-intensity Doppler envelope with mild signal dropout at the peak velocity), fair (moderate-to-low intensity Doppler envelope with moderate-to-high signal dropout at the peak velocity), and poor (low intensity Doppler envelope with poorly visualized peak velocity). The degree of TR was assessed semi-quantitatively (graded 0–3), as previously reported (3). Doppler estimated CO was derived from the Doppler-estimated stroke volume using the velocity time integral (VTI) of flow through the left ventricular outflow tract (LVOT), the diameter of the LVOT, and heart rate recorded during the imaging study, using the following formula: CODoppler = [VTILVOT × (Diameter of LVOT)2 × (0.785)] × heart rate (13). Echocardiograms were interpreted by a single cardiologist (P.F.) who was blinded to the patients' medical history and diagnosis and to the results of the right-heart catheterization.
Statistical Analysis
Descriptive statistics were used to describe the study population using Stata 8.0 (College Park, Texas). Pressures determined by right-heart catheterization and DE were compared using analysis described by Bland and Altman (14). Accuracy was predefined as 95% limits of agreement within ±10 mm Hg for pulmonary artery pressure estimates and ±1 L/min for cardiac output measurements.
RESULTS
Seventy-five consecutive right-heart catheterizations were performed between March and October 2004 on patients referred to the PH program. Three patients refused to participate in the study; two patients could not have echocardiograms performed because of scheduling conflicts; one patient underwent an exercise right-heart catheterization; and one patient was excluded because a left heart catheterization was required at the time of the right-heart catheterization. For two patients who had a repeat right-heart catheterization during the study period; only the first study pairing of DE and catheterization was used in the analysis. The echocardiogram on one other patient could not be adequately interpreted for this study. Therefore, data from a total of 65 patients were available for the final analysis.
Patient Characteristics
The baseline characteristics of the study population are presented in Table 1. The patients tended to be white females with an average age of 54.0 years. The majority of the patients had pulmonary arterial hypertension (WHO Group I) as defined by the Venice classification system (15). Other forms of PH were related to interstitial lung disease (5 patients), pulmonary venous hypertension (4 patients), and obstructive sleep apnea (2 patients). Vasodilator challenges were performed when appropriate with inhaled nitric oxide. Only one patient had a positive vasodilator response as previously defined (16), and the pulmonary artery pressures returned to pretesting levels immediately after cessation of the agent. A minority of patients (32%) were undergoing follow-up catheterization and were already on specific therapy for PH. No changes in therapy were made during the brief time between the catheterization and the echocardiogram.
TABLE 1.
PATIENT DEMOGRAPHICS
| Age, yrs (SD) | 54.0 (14.7) |
| Caucasian, n (%) | 49 (75.4%) |
| Female, n (%) | 55 (84.6%) |
| PH Present, n (%) | 58 (89.2%) |
| Diagnosis | |
| Idiopathic PAH | 14 |
| PAH-CTD | 23 |
| Other*, n | 21 |
Definition of abbreviations: CTD = connective tissue disease; PAH = pulmonary arterial hypertension; PH = pulmonary hypertension.
Other patient characteristics include: chronic thromboembolic pulmonary hypertension, 1; interstitial lung disease, 5; obstructive sleep apnea, 2; HIV-related PAH, 1; sickle cell disease, 1; diastolic dysfunction, 4; sarcoidosis, 1; exercise induced, 1; unspecified, 1; portopulmary hypertension, 2; congenital heart disease, 1; chronic obstructive pulmonary disease, 1.
Summary statistics for hemodynamic measurements obtained by right-heart catheterization and DE are presented in Table 2. These values during right-heart catheterization revealed a wide range in right atrial and pulmonary artery pressures, from 2 to 24 mm Hg and 20 to 120 mm Hg, respectively. CO ranged from 1.6 to 11.2 L/min. Results of DE measurements revealed a similar wide range of pressures and outputs. Six patients were found not to have any appreciable tricuspid regurgitation. However, four of these patients had evidence of pulmonary hypertension by catheterization including one with moderate PH (defined as a mean pulmonary artery pressure greater than 35 mm Hg). No patients had evidence of a left-to-right shunt by oximetry.
TABLE 2.
HEMODYNAMIC VALUES OBTAINED BY RIGHT-HEART CATHETERIZATION AND DOPPLER ECHOCARDIOGRAPHY
| Right-Heart Catheterization | n | Mean | SD | |||
|---|---|---|---|---|---|---|
| RAP, mm Hg | 65 | 9.4 | 5.0 | |||
| PASP, mm Hg | 65 | 68.5 | 23.9 | |||
| mPAP, mm Hg | 65 | 41.4 | 14.6 | |||
| CO, L/min | 65 | 4.4 | 1.7 | |||
| Echocardiogram | ||||||
| RAP, mm Hg | 65 | 12.4 | 4.7 | |||
| RVSP, mm Hg | 59 | 70.2 | 25.1 | |||
| CO, L/min | 64 | 4.3 | 1.4 | |||
Definition of abbreviations: CO = cardiac output; mPAP = mean pulmonary artery pressure; PASP = pulmonary artery systolic pressure; RAP = right atrial pressure; RVSP = right ventricular systolic pressure.
Accuracy of Echocardiographic Estimates
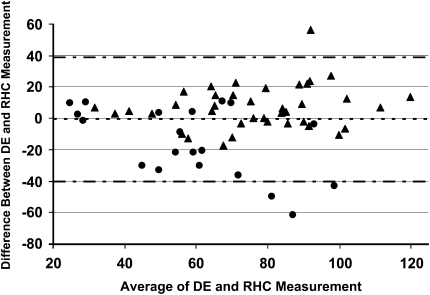
Fifty-nine patients had DE estimates of pulmonary artery pressure. Using Bland-Altman analysis, the bias for the echocardiographic estimates of the pulmonary artery pressures was −0.6 mm Hg with 95% limits of agreement ranging from +38.8 to −40.0 mm Hg (Figure 1). The correlation coefficient for the DE and invasive measurement of pulmonary artery systolic pressure was 0.66 (P < 0.001). Using a definition of an accurate pulmonary artery pressure estimate of within 10 mm Hg of the value from catheterization, 48% of the echocardiographic estimates were accurate. Six of 16 patients (38%) pressure overestimates were greater than 20 mm Hg, whereas 12 of 15 (80%) pressure underestimates were greater than 20 mm Hg. In keeping, the magnitude of underestimation was greater than the overestimation (−30 ± 16 vs. +19 ± 11 mm Hg; P = 0.03). Of note, 10 of 12 (83%) underestimates greater than 20 mm Hg were associated with a fair or poor quality Doppler jet across the tricuspid valve, suggesting inadequate or incomplete resolution of the maximal jet velocity as the cause of underestimation. The TR grade was comparatively less for subjects with “poor” or “fair” versus “good” or “excellent” quality transtricuspid Doppler velocity signals (1.3 ± 0.1 vs. 2.2 ± 0.1; P < 0.001).
Figure 1.
Bland-Altman plot of Doppler echocardiographic estimates of pulmonary artery pressure and right-heart catheterization measurements. The bias was −0.6 mm Hg and the 95% limits of agreement were +38.8 and −40.0 mm Hg. Triangles represent excellent- and good-quality Doppler signal; circles = fair- and poor-quality Doppler signal; dotted line = bias; dash/dotted line = upper and lower limits of agreement. Abbreviations: DE = Doppler echocardiography; PASP = pulmonary artery systolic pressure; RHC = right-heart catheterization.
The distribution of estimates of the right atrial pressure and the measured pressure is shown in Figure 2. A total of 29 subjects had an echo-estimated RAP of 15 mm Hg or greater, however invasive RAP measurements revealed that 11 of 20 of these patients had an RAP ≤10 mm Hg, and 4 of 20 had an RAP ≤5 mm Hg. In fact, half (8 of 16 subjects) of the cases of PA systolic pressure overestimation were related solely to right atrial pressure overestimation by echocardiography. Comparing the transtricuspid gradient estimate from the echocardiograms and a calculated transtricuspid gradient from the right-heart catheterization yielded a bias of −1.8 mm Hg, with 95% limits of agreement of 33.6 and −37.2 mm Hg.
Figure 2.
Comparison of right atrial pressure as estimated by Doppler echocardiography and right-heart catheterization. RHC = right-heart catheterization.
For clinical perspective, Table 3 compares the pulmonary artery systolic pressure (PASP) obtained by right-heart catheterization with the DE PASP (for under- and overestimate values), along with the corresponding diagnostic category of severity that each subject would fall into for each technique. PH was categorized as severe if the PASP was greater than 65 mm Hg, moderate (55 to 64 mm Hg), and mild (40 to 54 mm Hg). Of note, 47% of patients with pressure underestimation by DE had their PH underestimated by two or more diagnostic categories (major error); 33% of the underestimates were within one diagnostic category (minor error); and 20% of underestimates were within the same diagnostic category. In contrast, only 13% of patients with pressure overestimates by DE had their PH overestimated by two or more diagnostic categories (major error); 31% by one diagnostic category, and 56% of overestimates were within the same diagnostic category.
TABLE 3.
COMPARISON OF PH SEVERITY ACCORDING TO PASP DERIVED FROM RHC VS. PASP ESTIMATED BY DE*
| RHC | DE | Categorycath | CategoryDoppler | Error | ||||
|---|---|---|---|---|---|---|---|---|
| PASP Underestimates
|
||||||||
| 70 | 48 | severe | mild | 2 | ||||
| 76 | 46 | severe | mild | 2 | ||||
| 65 | 43 | severe | mild | 2 | ||||
| 90 | 54 | severe | mild | 2 | ||||
| 72 | 51 | severe | mild | 2 | ||||
| 118 | 56 | severe | moderate | 1 | ||||
| 106 | 56 | severe | moderate | 1 | ||||
| 105 | 94 | severe | severe | 0 | ||||
| 76 | 59 | severe | moderate | 1 | ||||
| 126 | 74 | severe | severe | 0 | ||||
| 119 | 77 | severe | severe | 0 | ||||
| 60 | 30 | moderate | none | 2 | ||||
| 66 | 33 | moderate | none | 2 | ||||
| 64 | 51 | moderate | mild | 1 | ||||
| 61 | 50 | moderate | mild | 1 | ||||
| PASP Overestimates
|
||||||||
| 113 | 127 | severe | severe | 0 | ||||
| 80 | 103 | severe | severe | 0 | ||||
| 96 | 108 | severe | severe | 0 | ||||
| 78 | 100 | severe | severe | 0 | ||||
| 80 | 102 | severe | severe | 0 | ||||
| 70 | 81 | severe | severe | 0 | ||||
| 70 | 89 | severe | severe | 0 | ||||
| 84 | 111 | severe | severe | 0 | ||||
| 65 | 76 | severe | severe | 0 | ||||
| 64 | 120 | moderate | severe | 1 | ||||
| 60 | 82 | moderate | severe | 1 | ||||
| 63 | 78 | moderate | severe | 1 | ||||
| 58 | 73 | moderate | severe | 1 | ||||
| 62 | 73 | moderate | severe | 1 | ||||
| 48 | 65 | mild | severe | 2 | ||||
| 54 | 74 | mild | severe | 2 | ||||
Definition of abbreviations: DE = Doppler echocardiography; PASP = pulmonary artery systolic pressure; PH = pulmonary hypertension; RHC = right-heart catherization.
Classification of PH severity (category) by RHC or DE: severe (≥ 65 mm Hg); moderate (55–64 mm Hg); mild (40–54 mm Hg). The degree of misclassification (error) of the severity of PH by DE is graded from 0–2 (0 = same category; 1 = 1 category of misclassification; 2 = 2 or more categories of misclassification).
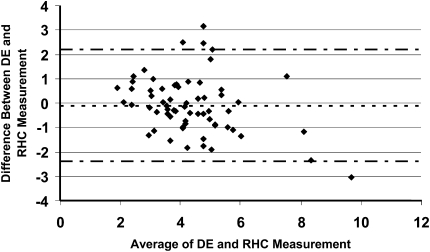
Echocardiographic estimates of cardiac output were compared with thermodilution determinations in Figure 3. The bias of −0.1 L/min with 95% limits of agreement ranging from 2.2 to −2.4 L/min fell outside the predetermined limits of clinical acceptability (see Methods section). The correlation coefficient for the DE and thermodilution cardiac output was 0.74; P < 0.001. We also tested whether the velocity time integral from the right ventricular (RV) outflow tract (VTIRVOT ) could differentiate subjects with a low or relatively preserved cardiac index, the latter being defined as a cardiac index greater than 2.2 L/min/m2. Using receiver operator curve analysis, the area under the curve for the VTIRVOT was 0.82 (95% confidence interval [CI] 0.70–0.94; P < 0.0001); the best VTIRVOT cutpoint for a reduced cardiac index was 12.2, with a sensitivity and specificity of 85.7 and 70.8%, respectively.
Figure 3.
Bland-Altman plot of cardiac output estimated by DE and measured by right-heart catheterization. The bias was −0.1 L/min and the 95% limits of agreement were 2.2 and −2.4 L/min. Diamonds represent difference in CO; dotted line = bias; dash/dotted line = upper and lower limits of agreement. Abbreviations: CO = cardiac output; DE = Doppler echocardiography; RHC = right-heart catheterization.
DISCUSSION
This study evaluated the utility of echocardiography for the estimation of pulmonary hemodynamics and CO in a population of patients referred for right-heart catheterization at a single PH center. Both tests were completed sequentially and within a very short timeframe (1 hour). Despite these optimized conditions, echocardiographic estimates for right atrial and pulmonary artery pressures differed significantly from those determined by invasive measurements, as assessed by Bland-Altman analysis, with a tendency to both overestimate and underestimate the pulmonary artery systolic pressure. Underestimations were larger in magnitude and more often significantly misclassified the severity of PH. Finally, measurements of CO by DE, which has been validated in other patient populations, appeared least useful.
Clinical interest in using DE to estimate right ventricular pressure arose after Berger and colleagues and Currie and colleagues demonstrated good correlation between echocardiographic estimates and directly measured pressures (6, 7). However, establishing good correlation does not imply that one test is an accurate substitute for another. More recent studies have focused on the value of echocardiography in assessing RV function by various techniques, including measurement of the tricuspid annular plane systolic excursion (TAPSE), two-dimensional strain, tissue Doppler echocardiography, or the speckle tracking method (3, 17, 18). However, RV systolic pressure (RVSP) remains the most commonly emphasized echo-derived parameter in routine clinical practice, particularly as the diagnosis of PH often hinges upon its initial recognition by Doppler echocardiography. Also, direct comparisons of echocardiographic estimates (e.g., RVSP) and hemodynamic values obtained by right-heart catheterization, with the two procedures being performed within an acceptable time frame, have been relatively lacking in PH studies.
Bland and Altman developed statistical methods to better assess the equivalence or accuracy of one test compared with another (14), which we used for the present study. The Bland-Altman method is arguably a more optimal way to compare two numerical tests. Although conclusions from the present study (related to the accuracy of DE) are at odds with those reached by Berger and colleagues and Currie and colleagues, they are in agreement with studies by Arcasoy and colleagues and Fisher and colleagues (8, 9). The former study assessed the utility of DE in patients with advanced lung disease who had been referred for lung transplantation. Although DE values correlated with hemodynamic measurements (the two studies were performed within 72 hours of each other), DE was found to be frequently inaccurate and to overestimate the degree of PH. The latter study related to patients participating in the cardiovascular substudy of the National Emphysema Treatment Trial (NETT), using a similar Bland-Altman analysis in these patients with end-stage chronic obstructive pulmonary disease (COPD); the authors concluded that DE estimates of pulmonary artery pressures correlated very poorly with hemodynamic measurements obtained by cardiac catheterization. In addition, the DE test characteristics of sensitivity and specificity were also poor. The temporal relationship between the DE and hemodynamic measurements in that study was not defined. In another comparison of DE and right-heart catheterization limited to a population of patients with scleroderma (19), and in which the mean interval between the two techniques was an average of 1.8 months, Denton and colleagues found a good correlation (R = 0.83, P < 0.001), however, with large discrepancies in pulmonary artery systolic pressures (PASP), of over 20 mm Hg in approximately 25% of patients, between the two techniques, particularly with increasing PASP. Nevertheless, the authors felt that DE was a useful technique to detect PH in this particular population. In a more recent study of 42 patients with various forms of PH in whom DE was performed simultaneously (in 22 patients) or nonsimultaneously (60 patients, some with repeated measurements) with cardiac catheterization (with the two procedures separated by up to 48 hours), Selimovic and colleagues found good correlations and small absolute differences for PASP and CO between the two approaches (20). However, large discrepancies in individual values for CO and transpulmonary gradient were found.
The present study extends the findings of these previous studies to a more assorted, and clinically relevant, population of patients with pulmonary arterial hypertension (PAH; which constituted 68% of our patient sample) as well as other causes of PH. Moreover, our study addresses important limitations of some of these previous studies in being prospective rather than retrospective and limiting the interval between invasive and noninvasive measurements to 1 hour, thus obviating as much as possible potential daily variations in hemodynamic measurements. Importantly, unlike the study by Selimovic and colleagues, we excluded repeated measurements in the same patients, which has the potential to bias results (e.g., if an individual had particularly good or poor echocardiographic windows) and is not appropriate when performing a Bland-Altman analysis. In the present study, Figure 2 demonstrates that PASP obtained by DE was frequently underestimated, often to a significant extent, particularly when quality of the Doppler envelope was fair or poor. This is expected because the accuracy of the Doppler method is contingent upon obtaining the correct peak velocity from which the peak pressure can be estimated. Likewise, our data show that a lesser degree of TR is more common in patients with underestimated pressure by DE, which stands to reason. This data does not account for the six (excluded) subjects with no appreciable TR, four of whom had PH by right-heart catheterization. Thus, a complete or relative lack of TR, although typically associated with a more compensated right ventricle, should not be interpreted as “reassuring” for ruling out the presence of PH. Of note, 12 of 15 patients in whom PA pressure was underestimated by DE had evidence of RV enlargement and/or dysfunction on their DE exam, supporting the notion that the clinician should integrate evidence of RV size and function, along with pressure estimates in the overall DE interpretation.
The PASP was overestimated even when the envelope quality was adequate, with the absolute degree of pressure overestimation greatest at higher pressures. Interestingly, when we evaluated the validity of echo-estimated RAP, we found a wide spread between values obtained by DE and right-heart catheterization, particularly when RAP pressures were deemed elevated by DE (Figure 3). In fact, an inaccurate RAP estimate accounted for half of the cases of noninvasive pressure overestimation. Therefore, these results indicate that the size of the IVC, and its variation with respiration routinely used to estimate RAP, may not be as useful as commonly believed in patients with chronic pulmonary hypertension.
Our data also demonstrate that from a diagnostic or clinical perspective, pressure underestimation was more likely to lead to gross misclassification of the degree of PH in the individual patient; 47% of subjects whose pressure was underestimated by Doppler were misclassified by two or more diagnostic categories (e.g., severe PH by invasive measurement, estimated to be mild PH by DE exam). In contrast, only 13% of subjects with pressure overestimation by Doppler had gross misclassification of their PH severity by DE exam. Therefore, the pressure underestimations were not only greater in magnitude but also more clinically misleading. These data serve to underscore the importance of pursuing definitive PA pressure assessment by right-heart catheterization in the patient with suspected PH if there is uncertainty about the DE estimated value for whatever reason, may it be a suboptimally visualized Doppler signal across the tricuspid valve or evidence of RV dysfunction.
In addition, our study is the first to evaluate the accuracy of echocardiographically estimated CO in a large group of patients with PH. The wide 95% limits of agreement between the thermodilution CO and the noninvasive estimate suggests that echocardiography has limited utility in this patient population in assessing CO, a finding that was suggested in the smaller study by Selimovic and colleagues (20). However, the discrepancies between the DE assessment and the hemodynamic values were smaller when CO was low (Figure 3). Therefore, it is unlikely that changes involving the left ventricle or diastolic ventricular interaction (more common in severe pulmonary hypertension where CO is expected to be low) would significantly affect the accuracy of DE assessment. Some of the noted discrepancy between the two measurements may be due to inherent and clinically “acceptable” variability in the invasive CO estimation by thermodilution, recognizing that an average of three values is taken, allowing up to 10% variation between each measure. Although some reports have shown consistent CO underestimation by the thermodilution method in subjects with significant TR, others have demonstrated that in patients with severe PH, even in the presence of significant TR, the thermodilution has acceptable agreement with the Fick method (21). In addition, in animal models, the creation of acute tricuspid regurgitation has been shown not to affect thermodilution cardiac output measurements (22). In our study, there did not appear to be a serial underestimation of CO in the subgroup of patients with moderate or greater TR (mean bias −0.04 L/min), making TR-related CO underestimation by thermodilution an unlikely culprit for the discrepancies seen between CO estimates by thermodilution and Doppler here. There was a high, but imperfect correlation between heart rate at the time of the catheterization and the DE (r = 0.88), making heart-rate variability a potential but likely minor contributor to the CO differences between the two methods. Another known limitation, and thus source of variability, inherent to this Doppler method of CO estimation is that the diameter of the LVOT must be squared, thus, error in LVOT diameter estimation is subject to amplification (23). This latter issue can be circumvented by simply using the VTI as a surrogate for stroke volume and thus cardiac index. In our dataset, a VTIRVOT less than 12 was a relatively accurate predictor of a cardiac index 2.2 or less, suggesting that easily derived Doppler echocardiographic data can still be used to provide insight into cardiac output in PH patients. Similarly, we recently showed that a TAPSE less than 1.8 was an accurate predictor of a stroke volume index ≤28 ml/m2; thus, even if we cannot obtain precise CO estimations by Doppler, a more integrated use of easily obtained Doppler and 2D echocardiographic measures can still provide useful information in identifying patients with a low cardiac index (3).
A potential limitation of this study is that the two measurement methods were not performed simultaneously, which has been done previously only in a small subset of patients (20). However, we felt that performing DE in the catheterization laboratory in a large group of patients studied prospectively would have interfered with adequate hemodynamic measurements and rendered the study technically difficult. Pulmonary pressures in patients with PAH are known to fluctuate significantly over the course of several hours as demonstrated by Rich and colleagues (24). However, the degree of variation noted in our study is far greater than would be expected in such a short time frame without any interventions or change in position or activity (patients were left idle in the supine position during the time, equal to or less than an hour, separating the two procedures). Additional sources of variability are also inherent to obtaining accurate hemodynamic pressure recordings, and thus, for every patient, appropriate transducer position relative to the heart, zero-referencing, and obtaining pressures at end-expiration, were part of standard procedure to minimize these potential issues. Because the study assessed only one time-point, it does not address the validity of following trends in serial measurements. However, based on significant differences between these two methods at one time-point, addressing serial comparative measurements appears futile.
In conclusion, we believe that DE remains an invaluable screening tool for the evaluation and further management of PH. However, our study underlines the significant limitations of DE when used to evaluate right-sided cardiac pressures and CO in patients with PH. DE does not always accurately reflect pulmonary artery and right atrial pressures and can significantly under- or overestimate these values in individual patients. Particular caution should be exercised in assessing PA pressure by DE when the TR jet quality is low, as serious pressure underestimations can occur, leading to missed or delayed diagnosis of a disease with high morbidity and mortality. DE has also been shown to provide an estimate of PVR, measured as the ratio of the tricuspid regurgitant velocity (TRV) to the VTI of the right ventricular outflow tract (RVOT). TRV/VTIRVOT has been shown to predict mortality and adverse cardiovascular events in patients with stable coronary artery disease (25). However, because the relation of TRV/VTIRVOT to PVR has been established in a population with an average PVR of 2 mm Hg/L/min, its application in patients with very high PVR values (i.e., pulmonary vascular disease) remains to be determined. In addition, acceleration time of the pulsed wave Doppler velocity envelope in the RV outflow tract correlates reasonably well with mean PA pressure and, unlike estimates based on tricuspid regurgitation velocity, is available in virtually all patients (26). Regression equations have been proposed to estimate mean pulmonary artery pressure based on echocardiographic findings (27, 28), including formulas using pulmonary artery acceleration time (PAAT), which are somewhat heart rate–dependent but correlate well with mean pulmonary artery pressure in patients with heart rates between 60 and 100 per minute (29).
In summary, DE remains an indispensable screening tool for the assessment of PH; however, clinicians should not be overly reliant on Doppler pressure estimates alone in the initial approach to the patients with suspected PH. Moreover, it cannot be overemphasized that DE does not replace cardiac catheterization for definitive hemodynamic assessment of known or suspected PH. Furthermore, we feel that DE may not be very useful when used serially in assessing changes in pulmonary artery pressure in response to therapy (although this was not specifically addressed in our study), due to significant individual over and underestimation of pressures, which underscores the importance of taking other echo-derived metrics (i.e., measures of RV size and function) into consideration as well. The validity of specific DE measurements or combination of measurements for that purpose will only be answered in carefully designed prospective studies. Finally, this study suggests that DE may be of limited value for the assessment of CO.
Supported by the Johns Hopkins University General Clinical Research Center and NHLBI P50 HL084946 (P.M.H.).
Originally Published in Press as DOI: 10.1164/rccm.200811-1691OC on January 22, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343–349. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, Gaine S. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004;43:40S–47S. [DOI] [PubMed] [Google Scholar]
- 3.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1034–1041. [DOI] [PubMed] [Google Scholar]
- 4.Hinderliter AL, Willis PW, Long W, Clarke WR, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, et al. Frequency and prognostic significance of pericardial effusion in primary pulmonary hypertension. PPH Study Group. Primary pulmonary hypertension. Am J Cardiol 1999;84:481–484. [DOI] [PubMed] [Google Scholar]
- 5.Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002;39:1214–1219. [DOI] [PubMed] [Google Scholar]
- 6.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol 1985;6:359–365. [DOI] [PubMed] [Google Scholar]
- 7.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol 1985;6:750–756. [DOI] [PubMed] [Google Scholar]
- 8.Fisher MR, Criner GJ, Fishman AP, Hassoun PM, Minai OA, Scharf SM, Fessler AH. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. Eur Respir J 2007;30:914–921. [DOI] [PubMed] [Google Scholar]
- 9.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003;167:735–740. [DOI] [PubMed] [Google Scholar]
- 10.Fisher MR, Forfia PR, Chamera E, Housten T, Champion HC, Resar J, Corretti M, Girgis RE, Hassoun P. Accuracy of Doppler echocardiography in the hemodynamic evaluation of pulmonary hypertension. Chest 2004;126:883S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, Champion HC, Girgis RE, Hassoun PM. Hyponatremia predicts right-heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2008;177:1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan JM, Blair JE, Goonewardena S, Ronan A, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 2007;20:857–861. [DOI] [PubMed] [Google Scholar]
- 13.Ihlen H, Amlie JP, Dale J, Forfang K, Nitter-Hauge S, Otterstad JE, Simonsen S, Myhre E. Determination of cardiac output by Doppler echocardiography. Br Heart J 1984;51:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 15.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004;43:5S–12S. [DOI] [PubMed] [Google Scholar]
- 16.Sitbon O, Humbert M, Jais X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Herve P, Simonneau G. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005;111:3105–3111. [DOI] [PubMed] [Google Scholar]
- 17.Borges AC, Knebel F, Eddicks S, Panda A, Schattke S, Witt C, Baumann G. Right ventricular function assessed by two-dimensional strain and tissue Doppler echocardiography in patients with pulmonary arterial hypertension and effect of vasodilator therapy. Am J Cardiol 2006;98:530–534. [DOI] [PubMed] [Google Scholar]
- 18.Pirat B, McCulloch ML, Zoghbi WA. Evaluation of global and regional right ventricular systolic function in patients with pulmonary hypertension using a novel speckle tracking method. Am J Cardiol 2006;98:699–704. [DOI] [PubMed] [Google Scholar]
- 19.Denton CP, Cailes JB, Phillips GD, Wells AU, Black CM, Bois RM. Comparison of Doppler echocardiography and right-heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br J Rheumatol 1997;36:239–243. [DOI] [PubMed] [Google Scholar]
- 20.Selimovic N, Rundqvist B, Bergh CH, Andersson B, Petersson S, Johansson L, Bech-Hanssen O. Assessment of pulmonary vascular resistance by Doppler echocardiography in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2007;26:927–934. [DOI] [PubMed] [Google Scholar]
- 21.Hoeper MM, Maier R, Tongers J, Niedermeyer J, Hohlfeld JM, Hamm M, Fabel H. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am J Respir Crit Care Med 1999;160:535–541. [DOI] [PubMed] [Google Scholar]
- 22.Buffington CW, Nystrom EU. Neither the accuracy nor the precision of thermal dilution cardiac output measurements is altered by acute tricuspid regurgitation in pigs. Anesth Analg 2004;98:884–890. [Table of contents.] [DOI] [PubMed] [Google Scholar]
- 23.Rowland T, Obert P. Doppler echocardiography for the estimation of cardiac output with exercise. Sports Med 2002;32:973–986. [DOI] [PubMed] [Google Scholar]
- 24.Rich S, D'Alonzo GE, Dantzker DR, Levy PS. Magnitude and implications of spontaneous hemodynamic variability in primary pulmonary hypertension. Am J Cardiol 1985;55:159–163. [DOI] [PubMed] [Google Scholar]
- 25.Farzaneh-Far R, Na B, Whooley MA, Schiller NB. Usefulness of noninvasive estimate of pulmonary vascular resistance to predict mortality, heart failure, and adverse cardiovascular events in patients with stable coronary artery disease (from the Heart and Soul Study). Am J Cardiol 2008;101:762–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitabatake A, Inoue M, Asao M, Masuyama T, Tanouchi J, Morita T, Mishima M, Uematsu M, Shimazu T, Hori M, et al. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation 1983;68:302–309. [DOI] [PubMed] [Google Scholar]
- 27.Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Herve P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 2009;135:760–768. [DOI] [PubMed] [Google Scholar]
- 28.Chemla D, Herve P. Estimation of mean pulmonary artery pressure: simpler than expected. Chest 2008;133:592–593. [DOI] [PubMed] [Google Scholar]
- 29.Dabestani A, Mahan G, Gardin JM, Takenaka K, Burn C, Allfie A, Henry WL. Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol 1987;59:662–668. [DOI] [PubMed] [Google Scholar]