Abstract
Invariant NKT cells are a hybrid cell type of Natural Killer cells and T cells, whose development is dependent on thymic positive selection mediated by double positive thymocytes through their recognition of natural ligands presented by CD1d, a non-polymorphic, non-MHC, MHC-like antigen presenting molecule. Genetic evidence suggested that β-glucosylceramide derived glycosphingolipids (GSLs) are natural ligands for NKT cells. N-butyldeoxygalactonojirimycin (NB-DGJ), a drug that specifically inhibits the glucosylceramide synthase, inhibits the endogenous ligands for NKT cells. Furthermore, we and others have found a β-linked glycosphingolipid, isoglobotriaosylceramide (iGb3), is a stimulatory NKT ligand. The iGb3 synthase knockout mice have a normal NKT development and function, indicating that other ligands exist and remain to be identified. In this study, we have performed a glycosphingolipidomics study of mouse thymus, and studied mice mutants which are deficient in β-hexosaminidase b or α-galactosidase A, two glycosidases that are up- and down-stream agents of iGb3 turnover, respectively. Our mass spectrometry methods generated a first database for glycosphingolipids expressed by mouse thymus, which are specifically regulated by rate-limiting glycosidases. Among the identified thymic glycosphingolipids, only iGb3 is a stimulatory ligand for NKT cells, suggesting that large scale fractionation, enrichment and characterization of minor species of glycosphingolipids, be necessary for identifying additional ligands for NKT cells. Our results also provide early insights into cellular lipidomics studies, with a specific focus on the important immunological functions of glycosphingolipids.
Keywords: Natural Killer T (NKT) cells, CD1d, T cell receptor, glycomics, lipidomics, glycosphingolipids, isoglobotriaosylceramide (iGb3), iGb3 synthase, linear ion trap mass spectrometry
Introduction
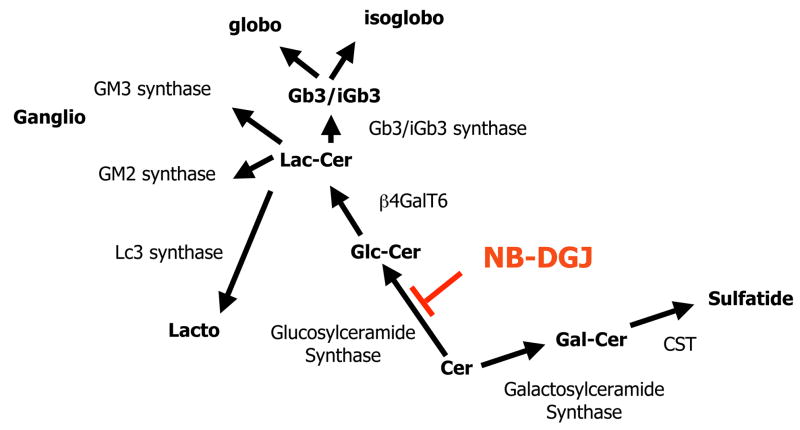
Glycosphingolipids (GSLs) are a class of biomacromolecules assembled through step wise action of glycosyltransferases. Composed of a hydrophobic ceramide part and a hydrophilic complex carbohydrate part, GSLs are synthesized in the endoplasmic reticulum and Golgi complex, where they traffic to the outside layer of plasma membrane. The cell surface GSLs are constantly recycled to the lysosome where they are degraded by glycosidases with the assistance of sphingolipid activator proteins (saposins, 1–5). In mouse and human tissues, GSLs of the globo-, isoglobo, lacto/neolacto-, ganglio-, gala-series have been identified (1–5). The globo, isoglobo, lacto/neolacto-, and ganglio-series of GSLs are assembled from glucosylceramide, while gala-series of GSLs and sulfatides are assembled from galactosylceramide (6, Figure 1).
Figure 1. Glycosphingolipid synthesis in mouse.
All mouse GSLs stem from β-glucosylceramide or β-galactosylceramide, and are assembled by key glycosyltransferaes to become lacto-, ganglio-, globo, isoglobo, sulfo- series of complex glycosphingolpids. N-butyldeoxygalactonojirimycin (NB-DGJ) specifically inhibits the synthesis of glucosylceramide derived glycosphingolipids, through inhibiting the glucosylceramide synthase. β4GalT: β1,4 galactosyltransferase 6 (lactosylceramide synthase); CST: cerebroside sulfotransferase.
The recognition of self GLSs by T lymphocytes was first reported by De Libero’s group (7–9), who found a series of T cell clones from multiple sclerosis patients recognize sulfatides and gangliosides. These glycosphingolpid antigens are presented by CD1 molecules, a family of non-MHC, non-polymorphic, MHC-like antigen presenting molecules. GSLs also serve as candidate antigens which mediate the development of a subset of innate T lymphocytes which express Natural Killer cell markers, named as NKT cells. In contrast to conventional T cells, NKT cells are self reactive to mouse thymocytes (10–13), through recognition of endogenous ligands present in mouse thymus. Invariant NKT cells (Type I) are the major NKT cells, which express invariant, evolutionally conserved T cell receptor (Vα14 in mouse and Vα24 in human), and are defined by their recognition of α-galactosylceramide, a marine sponge derived GSL (14). However, all mammalian glycolipids stem from β-linked monohexosylceramides, β-glucosylceramide or β-galactosylceramide (1–6, Figure 1). α-linked monohexosylceramide structures have not been found in mammals, although they have been found in pathogenic bacteria which are related to human autoimmune disease primary biliary cirrhosis (Novosphingobium aromaticivorans, 15–17). α-linked monohexosyldiacylglycerol has been found in Borrelia burgdorferi, which causes Lyme disease (18), while presence of such structure in mammals has not been reported.
The GSLs derived from β-linked monohexosylceramides were first proposed as candidates for NKT ligands by Stanic et al (19). A mutant cell line which had suppressed expression of β-glucosylceramide synthase, showed a significant defect in stimulating NKT cells. In addition, the groups of Platt and Cerundolo have reported that N-butyldeoxygalactonojirimycin (NB-DGJ), a pharmaceutical which specifically inhibits the β-glucosylceramide synthase (20), inhibits the human dendritic cells’ ability to stimulate NKT cells (21). Zhou, Teyton and Bendelac first reported (22) that in vivo development of invariant NKT cells is related to GSL metabolism; they showed that saposins, a family of lipid transfer proteins which “grab” GSLs and present them for glycosidase digestion (1), are required for NKT cell development. In vitro lipid transfer assays indicated that saposins are specific for loading of GSLs to CD1d, while the loading of the other species of membrane lipids, phospholipids, does not require the assistance of saposins (22). The results of the lipid transfer assays are consistent with the fact that the development of type II NKT cells, which are also CD1d dependent but recognize different lipids, is not impaired in saposin knockout mice. We and others also reported that the in vivo development of NKT cells may be impaired by defects in GSL trafficking to the lysosome, and general lysosomal storage of GSLs may compete with the loading of NKT ligands (23–26). NPC1 mice, with a defect in glycolipid trafficking to late endosome, showed severe defect in NKT cell development (23–25). GM1 gangliosidosis β-galactosidase KO mice showed a defect in NKT cell development with a mechanism hypothesized to be competition of NKT ligand loading by accumulated lysosomal gangliosides (24–25). Finally, we and others found that a GSL, isoglobotrihexosylceramide (Galα3Galβ4Glcβ1Cer, iGb3), is recognized both by mouse Vα14 and human Vα24 natural killer T (NKT) cells (27–32).
The effects of iGb3 have been found by several groups to be substantially different from those of α-galactosylceramide. These stimulatory properties of iGb3 are unique for the extensively studied TCR repertoire (Vβ8, Vβ7, and Vβ2) of endogenous ligands (29, 31, 33), leading to the suggestion that iGb3 might account for the well known autoreactivity of NKT cells, and might also carry out important roles in NKT cell development and/or function, particularly in the numerous non-infectious disease conditions in which they have been implicated. However, by studying iGb3 synthase knockout mice, Porubsky et al (34) have challenged the physiological relevance of iGb3, since no defect in NKT development was found in these mice.
The identities of natural ligands for invariant NKT cells remain the most critical unanswered questions in the NKT field. A clear and comprehensive understanding of GSL and non-GSL metabolism and distribution patterns in mouse thymus is critical for studies on NKT biology. Since we and others have already found that a GSL, iGb3, is a natural ligand for the majority of invariant NKT cell population, we have chosen first to study the problem using sensitive and specific mass spectrometry (MS) based glycosphingolipidomics.
It is generally accepted that MS is an indispensable method for structural glycomics studies, especially for identifying and characterizing low abundance ligands (35–38). We recently combined all of the potential advantages of electrospray ionization linear ion trap mass spectrometry (ESI-LIT-MS) methodology, including the detection of isomeric structures using signature diagnostic ions, observable only in MS4 and MS5 spectra, for the highly sensitive identification and quantitation of GSLs present in the form of multiple isobaric mixtures (39–40). Here we have applied MALDI-TOF-MS and ESI-LIT-MSn techniques to analyze neutral and acidic GSLs purified from mouse thymus. In addition, we have studied the β-hexosamidase b KO and Fabry mice, the two mouse models of glycolipid storage diseases that were used in our previous studies on NKT biology (22, 28). We expect that glycosphingolipidomic analysis of these 2 strains of mutant mice will provide a basis for clarifying the discrepancies in results reported by three different laboratories (22, 25, 28, 41).
Experimental
Mice
Hexb KO mouse strain was from Dr. Richard L Proia at NIDDK, NIH (42). Fabry mice was from Dr. Ashok B. Kulkarni, NIDCR, NIH (43). Gb3 synthase KO mice was generated and maintained as described (44). All mice were backcrossed to C57B6 background for more than 10 generations. Mice aged between 6 to 8 weeks were used for experiments. Thymuses were collected and pooled for biochemical analysis.
Fetal thymus organ culture
Fetal thymuses from day 14 embryo of C57B6 mice were cultured as described (22). For drug treatment, NB-DGJ (Toronto Research Chemicals, Canada) was added since beginning of the 14 day culture period, at the concentration of 10 μg/ml and 100 μg/ml.
Bone marrow derived dendritic cells
Mouse bone marrow derived dendritic cells were generated by culturing bone marrow progenitor cells in presence of GM-CSF and IL4. The total culturing period was 14 days. Both immature and mature dendritic cells were studied. To mature the dendritic cells, CpG ODN (ODN 1826) was added to culture medium in the last day of culture at the concentration of 2 μg/ml.
Extraction of glycosphingolipids
GSLs were extracted as described (39–40). Mouse thymus or mouse bone marrow derived dendritic cells were stored in 16×100 mm glass tubes under −80°C. Lipids were extracted by extensive sonication for four times with mixed polarity solvents. The first and last solvent used was chloroform-methanol 1:1 (v/v). The second and third solvent used was isopropanol-hexane-water 55:25:20 (v/v/v, upper phase removed by aspiration before use). Sonication was followed by centrifugation to pellet the insoluble material. The supernatants were pooled and dried under nitrogen stream at 40°C, and subjected to preliminary analysis by high performance thin layer chromatography (HPTLC).
Separation of neutral and acidic lipids
Neutral and acidic lipids were fractionated by anion exchange chromatography on a small column of DEAE Sephadex A-25. The acidic lipid fraction was eluted with 0.8M sodium acetate in methanol. Both neutral and acidic fractions were dried, desalted by dialysis, dried, and analyzed by HPTLC.
Florisil fractionation of neutral GSLs
The DEAE Sephadex A-25 pass-through fraction was dried under vacuum in the presence of P2O5 for 3 hours and peracetylated with 1 ml pyridine and 0.5 ml acetic anhydride in the dark at room temperature overnight. The peracetylated material was dried, with the addition of 2 ml toluene 3x to ensure complete evaporation. A Florisil (Sigma-Aldrich, St. Louis, MO) column (30–60 mesh, 10×80 mm) was equilibrated in 1,2-dichloroethane-hexane 4:1 (v/v). The peracetylated sample was applied in this solvent, and the column was then washed with 20 ml of the same solvent, followed by 20 ml 1,2-dichloroethane. Neutral peracetylated GSLs were eluted with 40 ml 1,2-dichloroethane-acetone 1:1 (v/v). The fractions were dried and deacetylated with 1 ml 0.5 M sodium methoxide in 2 ml methanol for 3 hours at room temperature. The mixture was neutralized with methanolic acetic acid, dried, and then desalted by dialysis. The GSL contents of all three fractions were compared by HPTLC.
Per-N,O-methylation of GSLs
GSLs (1–20 μg) were introduced into a conical-glass vial, and dimethyl sulfoxide (150 μl) was added without using special drying conditions or inert gas atmosphere. Powdered sodium hydroxide (40–60 mg) was then added to the sample solution, and was stirred at room temperature until completely dissolved. Iodomethane (80 μL) was added with a syringe, and the mixture was shaked at room temperature for 1 hour. The methylation reaction was quenched with water (2 ml). The permethylated products were extracted 3 times by addition of dichloromethane (2 ml). The combined dichloromethane extracts were then washed 3 times with water (2 mL each). Following the final wash, the samples were transferred to a new tube, and dried under nitrogen stream at 35–40°C.
MALDI-TOF mass spectrometry analysis
MALDI-TOF-MS and MALDI-TOF/TOF-MS/MS experiments were performed on a MALDI TOF-TOF Mass Spectrometer (Applied Biosystems 4700, Foster City, CA). The matrix was prepared by mixing 50% volume of 10 mg/ml dihydroxybenzoic acid (DHB) in acetonitrile and 50% volume of 0.1% trifluoroacetic acid (TFA) in water. The DHB matrix was spotted on the MALDI target (1 μL), followed by a 1 μL (containing 100 ng GSLs) spot of sample dissolved in methanol, and allowed to dry before analysis. MS experiments were acquired using the reflectron settings in the positive mode. MS/MS data were obtained using the 1 kV mode with argon or air as collision gas (CID cell gas pressure 3.5 × 10–6 to 9 × 10-7 torr). MS spectra were summed from 1000 to 10000 laser shots, for MS/MS 5000 to 50000 shots were summed.
Electrospray-Ionization Ion-Trap mass spectrometry (ES-LIT-MSn) of permethylated neutral GSLs
Mass spectrometry (MS and MSn) was carried out in positive ion mode on a linear ion trap mass spectrometer (LTQ, ThermoFinnigan, San Jose, CA), using a nanoelectrospray source for direct infusion of samples (dissolved in methanol) by static nanospray, at capillary temperature 230°C, with injection time as 100.00 ms, activation time as 30 ms, activation Q-value as 0.250, isolation width as m/z 1.5, and acquisition time as 3 min to 10 min. Electrospray voltage was 1 kV. Static nanoelectrospray needles were from Proxeon Biosciences (Denmark). Normalized collision energies were set to leave a minimal residual abundance of precursor ion; in this case 30% was used for all product ion scans. All ions were detected as sodium adducts. To obtain MS5 spectra specifically from the characteristic internal HO-3Gal3/4Gal-1-ene disaccharides, each iGb4/Gb4 molecular species (or lipoform, observed at, e.g., m/z X), was subjected to multistep fragmentation via the MS5 pathway as described (39). To obtain MS4 spectra specifically from the characteristic nonreducing Gal3/4Gal-1-ene disaccharides, each iGb3/Gb3 molecular species (or lipoform, observed at, e.g., m/z X), was subjected to multistep fragmentation via the MS4 pathway as described (40).
Artificial iGb3 and asiao-GM1 as quantitation standard
A chemically synthesized, “short-tail” iGb3 (C8), was added as internal standard before permethylation experiments, as published previously (40). Asialo-GM1 (Matreya Cat. No. 1064) was used as internal standard for analyzing charged GSLs.
Other GSL standards for Ion-Trap MS fragmentation assays
Lactosylceramide (Matreya Cat. No. 1500), Gb3 (Matreya Cat. No. 1067), Gb4 (Matreya Cat. No. 1068), asialo-GM1 (Matreya Cat. No. 1064), GM1 (Matreya Cat. No. 1061), asialo-GM2 (Matreya Cat. No. 1512), GM2 (Matreya Cat. No. 1502), GM3 (Matreya Cat. No. 1503), GM4 (Matreya Cat. No. 1535), GD1a (Matreya Cat. No. 1062), GD1b (Matreya Cat. No. 1501), GD3 (Matreya Cat. No. 1504), GT1b (Matreya Cat. No. 1063), GQ1b (Matreya Cat. No. 1516), and sulfatide (Matreya Cat. No. 1049) were from Matreya, Pleasant Gap, PA. Synthetic sugar part of Gb4, GM1, asialo-GM1, iGb3, and Gb3 were provided by Drs. Ola Blixt and James Paulson, Consortium of Functional Glycomics. iGb4 and digalactosylceramide (Galα1,4Gal Cer ), purified from cat intestine, were from Dr. Sussan Tenenberg, Gorteberg University.
Stimulation toward NKT cell hybridomas
GSLs were stored in DMSO at 2 mg/ml. GSLs were pulsed to 50,000 mouse BMDCs at 100 ng/ml for overnight, and mixed with 50,000 NKT cell hybridomas for overnight. IL2 released by NKT hybridomas were measured by CTLL assays. NKT hybridomas were DN32.D3 (22) and 23.8A (provided by Dr. Laurent Gapin, 29).
Flow cytometry analysis of NKT cells
NKT cells were stained by PBS57 (αGalCer analog) loaded mouse CD1d tetramer (APC color, provided by NIAID tetramer facility at Emory University, GA), in combination with anti-CD3 and anti-CD44 monoclonal antibodies (eBiosciences, CA).
Results
NB-DGJ treatment abolishes the development of invariant NKT cells
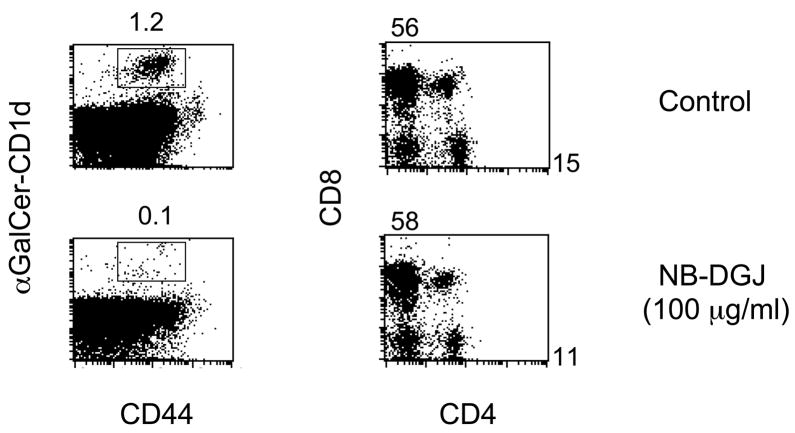
NB-DGJ treatment of cultured fetal thymuses completely blocked the development of NKT cells (Figure 2). In contrast, the development of conventional CD4 and CD8 T cells remained unchanged. This was observed at both 100 μg/ml and 10 μg/ml concentration of drug treatment.
Figure 2. NB-DGJ completely abolishes the NKT cell development.
Fetal thymus organ culture was performed as previously described (22). Thymuses from fetal mice (Day 14 after pregnancy) were cultured in 6 well transwell plates (Corning, NY) in EHAA/RPMI medium with 10% Fetal Calf Serum. NB-DGJ (10 μg/ml and 100 μg/ml) was added at the beginning of culture. The thymuses were cultured to allow the development of NKT cells. After 14 days, the thymocytes were harvested and analyzed by flow cytometry after staining by αGalCer/CD1d tetramer and anti-mouse CD44 monoclonal antibody (Pharmingen, CA). To exclude the possibility of toxicity to thymocytes, the development of conventional CD4 and CD8 T cells were used as controls. No reduction of CD4 or CD8 numbers was found in either percentage or total cell numbers.
Mass spectrometry assays generate first profiles of neutral and acidic glycosphingolipids in mouse thymus
To gain a clear and precise view of the GSL structures in mouse thymus, we used both MALDI-TOF-MS and ESI-LIT-MSn techniques. Table 1 lists the GSLs that were identified from 6–8 week mouse thymi. Representative MALDI-TOF-MS profiles for neutral and acidic GSLs are presented in Supplementary Figure 1. The identities of GSLs were verified by MALDI-TOF/TOF-MS/MS and ESI-LIT-MSn analysis, with the fragmentation patterns compared to those of authentic GSL standards (Supplementary Figure 2).
Table 1.
Glycosphingolipids identified in mouse thymus.
| Neutral GSLs | |
|---|---|
| Monosaccharide-ceramidea (Hex-Cer) | Glc-Cer (Glc β1 Cer) |
| Disaccharide-ceramideb (Hex-Hex-Cer) | Lac-Cer (Gal β4 Glc β1Cer) Digalactosylceramide (Gal α4 Gal β1Cer) |
| Trisaccharide-ceramide (Hex-Hex-Hex-Cer) | iGb3 (Gal α3 Gal β4 Glc Cer) Gb3 (Gal α4 Gal β4 Glc Cer) |
| Trisaccharide-ceramidec (HexNAc-Hex-Hex-Cer) | Lc3 (GlcNAc β3 Gal β4 Glc Cer) Asialo-GM2 (GalNAc β4 Gal β4 Glc Cer) |
| Tetrasaccharide-ceramide (HexNAc-Hex-Hex-Hex-Cer) | iGb4 (GalNAc β3 Gal α3 Gal β4 Glc Cer) Gb4 (GalNAc β3 Gal α4 Gal β4 Glc Cer) |
|
| |
|
Acidic GSLs | |
| Monosialyl-gangliosides | GM3 (NeuAc α3 Gal β4 Glc Cer) GM2 (GalNAc β4 <NeuAc α3> Gal β4 Glc Cer) GM1 (Gal β3 GalNAc β4 <NeuAc α3> Gal β4 Glc Cer) GM1b (NeuAc α3 Gal β3 GalNAc β4 Gal β4 Glc Cer) |
| Disialyl-gangliosides | GD1a (<NeuAc α3> Gal β3 GalNAc β4 <NeuAc α3> Gal β4 Glc Cer) GD1b (Gal β3 GalNAc β4 (<NeuAc α8 NeuAc α3> Gal β4 Glc Cer) |
| Trisialy-gangliosides | GT1a (<NeuAc 8 NeuAc α3> Gal β3 GalNAc β4 <NeuAc α3> Gal β4 Glc Cer) GT1b (<NeuAc α3> Gal β3 GalNAc β4 < NeuAc 8 NeuAc α3> Gal β4 Glc Cer) |
| Tetrasialyl-gangliosides | GQ1b (<NeuAc 8 NeuAc α3> Gal β3 GalNAc β4 < NeuAc 8 NeuAc α3> Gal β4 Glc Cer) |
: The presence of Glc-Cer in the thymus of C57BL6 strain of mice was previous reported (52). We have found Glc β1 Cer is the major structure by NMR analysis (data not shown).
: The disaccharide-ceramide structure in mouse thymus is a mixture of Lac-Cer and Digalactosylceramide. This was verified by comparing the MS3 ion patterns, analyzed by ion trap mass spectrometry to standard Lac-Cer and Digalactosylceramide (Supplementary online Figure 2).
: The trisaccharide-ceramide structures of mouse thymus remain to be studied. Preliminary analysis by ion trap mass spectrometry suggested the structures of Lc3 and asialo-GM2 (data not shown).
Glycosidases specifically regulate glycosphingolipid profiles in glycosidase mutant mice
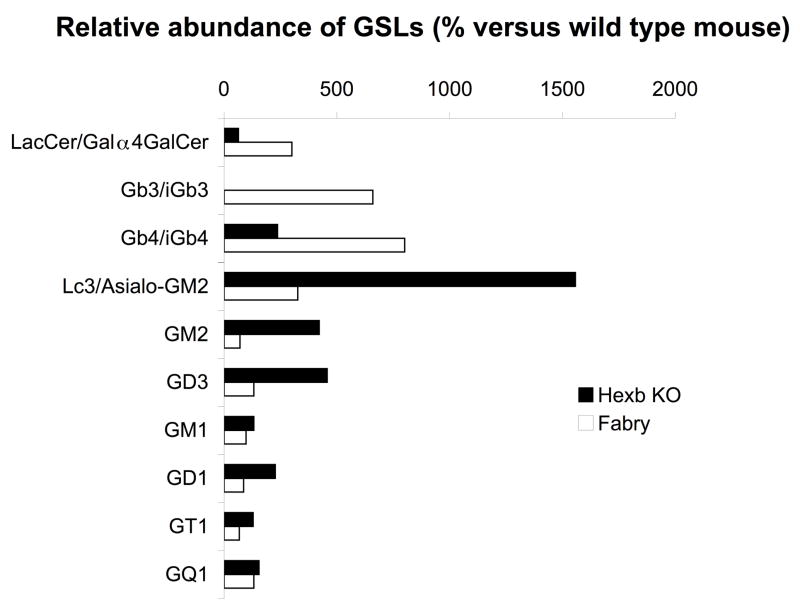
α-galactosidase and β-hexosaminidase are two enzymes involved in GSL degradation. NKT cell development has been studied in mutant mice, and contradictory findings have been reported by different groups. To understand the effects of disruption of the enzyme activities on the relevant components at the biochemical level, we have performed glycosphingolipidomic analysis of these mutant mice strains. To compare the relative abundance of GSLs detected in Hexb KO mice, Fabry mice, and wild type mice, we have added an internal standard (artificial iGb3 for neutral GSLs, and asialo-GM1 (Gg4) for acidic GSLs, respectively). Figure 3 shows the ratio of each GSL in Hexb KO mice and Fabry mice, with respect to the same component in wild type mice, based on comparison of relative ion abundances of each detected GSL to the artificial internal standards (Supplementary Table 1). We found total Gb3+iGb3 triglycosylceramide (6.5 fold ratio to wild type), and total Gb4+iGb4 tetraglycosylceramide (8 fold ratio to wild type) were accumulated in Fabry mice, along with a diglycosylceramide fraction and an additional triglycosylceramide fraction containing a terminal HexNAc residue, whose exact structure remains to be studied. In Hexb mice, we found an accumulation of Gb4/iGb4 (2.4 fold), Lc3+ asialo-GM2 (15.5 fold), GM2 (4.2 fold), GD3 (4.5 fold), and GD1 (2.2 fold). On the other hand, a severe reduction (>95%) was found for iGb3+Gb3 GSLs.
Figure 3. Specific alterations of glycosphingolipids in Hexb KO mice and Fabry mice.
Thymic GSLs from Hexb KO, Fabry, and wild type mice were extracted, permethylated, and analyzed by MALDI-MS. The relative ion abundance of MS1 ions of Hex-Hex-Cer (Gal β1,4 GlcCer and Gal α1,4 GalCer), Hex-Hex-Hex-Cer (Gb3, Gal α1,4 Gal β1,4 GlcCer and iGb3, Gal α1,3 Gal β1,4 GlcCer), HexNAc-Hex-Hex-Hex-Cer (Gb4, Gal β1,4 Gal α1,4 Gal β1,4 GlcCer, and iGb4, Gal β1,4 Gal α1,3 Gal β1,4 GlcCer), HexNAc-Hex-Hex-Cer (Lc3, GlcNAc β1,3 Gal β1,4 GlcCer, and asialo-GM2, GalNAc β1,4 Gal β1,4 GlcCer), GM2, GD3, GM1, GD1, GT1, and GQ1 were compared to internal standards (“short tail” iGb3 for neutral GSLs, and asialo-GM1 for acidic GSLs). The ratio of above GSLs in Hexb mice and Fabry mice versus wild type mice were calculated according to Supplementary Table 1. Gal-terminated GSLs were accumulated in Fabry mice. HexNAc terminated GSLs were accumulated in Hexb KO mice. Gb3 and iGb3 were absent in Hexb KO mice.
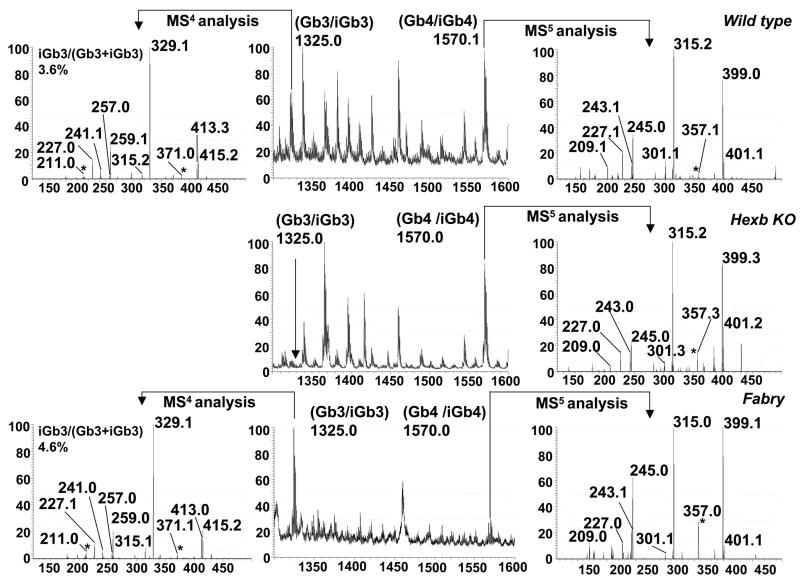
Low abundance of thymic iGb3 is only detectable by ESI-LIT-MSn technology
To address the controversy that iGb3 is not expressed by mouse thymus (34, 45), we analyzed its expression by a more sensitive mass spectrometry method using a linear ion-trap (ESI-LIT-MSn). Trihexosylceramide molecular species observed in MS1 represent both regioisomers (iGb3+Gb3), but we have found that the expression of iGb3 can be detected and measured from characteristic ions produced in MS4 analysis of selected molecular ions (40, Figure 4). We observed multiple molecular ion species of iGb3+Gb3 regioisomers in MS1, due to the variation in their fatty acyl chain lengths; each was subjected to MS4 analysis as described. The expression of iGb3 was detected as higher than 1% in molecular ions of m/z 1277 (1.5%) and m/z 1325 (3.5%) observed in MS1(Supplementary Table 2). In most other trihexosylceramide ions observed in MS1, iGb3 was found to be less than 1%, or undetectable compared to its regioisomer Gb3. The total iGb3 to Gb3 ratio was estimated as 0.4%.
Figure 4. Expression of iGb3 and iGb4 in mouse thymus.
Neutral GSLs from wild type, Hexb KO, and Fabry mice were extracted, permethylated, and analyzed by ion trap mass spectrometry. MS1 ions representing regioisomers of iGb3/Gb3 were selected for MS4 analysis according to published method (40). MS1 ions representing regioisomers of iGb4/Gb4 were selected for MS5 analysis (39). Percentage of iGb3 in iGb3/Gb3 mixtures were calculated according to standard curve as described (40).
The expression of iGb3 is undetectable in Hexb KO mice, due to more than 95% overall reduction of trihexosylceramide levels (iGb3+Gb3). The expression of iGb3 was increased in α-galactosidase A KO mice, easily detected due to the 6.5 fold overall increase of trihexosylceramides (iGb3+Gb3). Noteworthy, the iGb3/Gb3 ratio was only slightly increased in Fabry mice, suggesting that the iGb3 and Gb3 are digested by α-galactosidase with similar efficiency.
We further measured relative levels of iGb4, the lysosomal precursor of iGb3, by MS5 analysis of molecular ions representing regioisomers Gb4 and iGb4 (39). We found a similarly low expression of iGb4 in thymus of the wild type mice (Figure 4). iGb4+Gb4 was increased 2.4 fold in Hexb KO mice, and 7 fold in Fabry KO mice (Figure 4).
Mass spectrometry assays reveal identities of neutral and acidic glycosphingolipids in mouse bone marrow derived dendritic cells
Bone marrow derived dendritic cells (BMDCs) have been widely used to study the biology of NKT cells. It is generally accepted that BMDCs express natural ligands for NKT cells. To have a clear and precise view of the GSL structures expressed by BMDCs, we used both MALDI-TOF/TOF-MS/MS and ESI-LIT-MSn techniques. The GSLs identified are listed in Table 2. Representative MALDI-TOF-MS profiles for neutral GSLs and acidic GSLs are presented in Supplementary Figure 3.
Table 2.
Glycosphingolipids identified in mouse dendritic cells.
| Neutral GSLs | |
|---|---|
| Disaccharide-ceramide (Hex-Hex-Cer) | Lac-Cer (Gal β4 Glc β1Cer) Digalactosylceramide (Gal α4 Gal β1Cer) |
| Trisaccharide-ceramide (Hex-Hex-Hex-Cer) | iGb3 (Gal α3 Gal β4 Glc Cer) Gb3 (Gal α4 Gal β4 Glc Cer) |
| Trisaccharide-ceramide (HexNAc-Hex-Hex-Cer) | Lc3 (GlcNAc β3 Gal β4 Glc Cer) Asialo-GM2 (GalNAc β4 Gal β4 Glc Cer) |
| Tetrasaccharide-ceramide (HexNAc-Hex-Hex-Hex-Cer) | iGb4 (GalNAc β3 Gal α3 Gal β4 Glc Cer) Gb4 (GalNAc β3 Gal α4 Gal β4 Glc Cer) |
| Tetrasaccharide-ceramide (Hex-HexNAc-Hex-Hex-Cer) | Asialo-GM1 (Gal β3 GalNAc β4 Gal β4 Glc Cer) |
| Fossman antigen (HexNAc)3-(Hex)2-Cer | GalNAc α3 GalNAc β3 Gal α4 Gal β4 Glc Cer |
|
| |
|
Acidic GSLs | |
| Monosialyl-gangliosides | GM1 (Gal β3 GalNAc β4 <NeuAc α3> Gal β4 Glc Cer) GM1b (NeuAc α3 Gal β3 GalNAc β4 Gal β4 Glc Cer) |
| Disialyl-gangliosides | GD1a (<NeuAc α3> Gal β3 GalNAc β4 <NeuAc α3> Gal β4 Glc Cer) GD1b (Gal β3 GalNAc β4 (<NeuAc α8 NeuAc α3> Gal β4 Glc Cer) GD3 (NeuAc α8 NeuAc α3 Gal β4 Glc Cer) |
| Trisialyl-gangliosides | GT1a (<NeuAc 8 NeuAc α3> Gal β3 GalNAc β4 <NeuAc α3> Gal β4 Glc Cer) GT1b (<NeuAc α3> Gal β3 GalNAc β4 < NeuAc 8 NeuAc α3> Gal β4 Glc Cer) |
CpG specifically regulate GSL profiles in dendritic cells
To study whether the maturation process of dendritic cells correlates with alteration of GSL expression, we compared the ratio of GSLs detected in mature DCs to those in immature DCs. Figure 5 shows increased levels of Gb3+iGb3 (2.4 fold) and Gb4+iGb4 (2.3 fold) expression, based on comparison of relative ion abundances of each detected GSL to artificial internal standards (Supplementary Table 3). The other significant increase was in the level of Forssman antigen (4.3 fold). No significant changes were found for any acidic GSLs in CpG matured DCs.
Figure 5. Alteration of GSL pattern in mature dendritic cells.
GSLs from immature and CpG-ODN-activated mature bone marrow derived dendritic cells were extracted, permethylated, and analyzed by MALDI-MS. The relative ion abundance of MS1 ions of Hex-Hex-Cer (Gal β1,4 GlcCer and Gal α1,4 GalCer), Hex-Hex-Hex-Cer (Gb3, Gal α1,4 Gal β1,4 GlcCer and iGb3, Gal α1,3 Gal β1,4 GlcCer), HexNAc-Hex-Hex-Hex-Cer (Gb4, Gal β1,4 Gal α1,4 Gal β1,4 GlcCer, and iGb4, Gal β1,4 Gal α1,3 Gal β1,4 GlcCer), HexNAc-Hex-Hex-Cer (Lc3, GlcNAc β1,3 Gal β1,4 GlcCer, and asialo-GM2, GalNAc β1,4 Gal β1,4 GlcCer), Forssman antigen, asialo-GM1, GD1, and GT1 were compared to internal standards (artificial iGb3 and GM1). The ratio of above GSLs in CpG-ODN matured dendritic cells versus immature dendritic cells were calculated according to Supplementary Table 3.
The expression of iGb3 in mouse bone marrow derived dendritic cells was also investigated. The results show that iGb3 is detectable in certain trihexosylceramide molecular ions at levels of 1 to 2.5% (Supplementary Figure 4), while in many other trihexosylceramide molecular ions, iGb3 is below 1% or undetectable (Supplementary Table 4). The total iGb3 to Gb3 ratio was estimated as 0.8% in both immature and mature DCs.
iGb3 versus Gb3 ratio increases more than 10 fold in Gb3 synthase KO mice
Since the detection of iGb3 is hampered by the overwhelming abundance of its regioisomer Gb3, we hypothesized that we should be able to measure higher ratios of iGb3 in trihexosylceramide molecular species if we examine Gb3 synthase knockout mice. This will also address the possibility that Gb3 could give rise to artifactual signals resembling those from iGb3 in ESI-LIT-MS4 spectra, i.e., through rearrangement or some other process, fragmentation of the terminal disaccharide MS3 product of Gb3 (Galα1-4Gal-1-ene) in MS4 could give rise to background levels of product ion signals putatively specific for iGb3 (Galα1-3Gal-1-ene). These background signals were observed to be below 0.2% for the m/z 211 product, and below 0.4% for the m/z 371 product, in multiple experiments performed in three different mass spectrometry core facilities (40). If we have measured artifactual iGb3 ion signals in MS4 which are solely background Gb3 fragmentation products, we should not detect an increase of the iGb3/Gb3 ratio in Gb3 synthase KO mice.
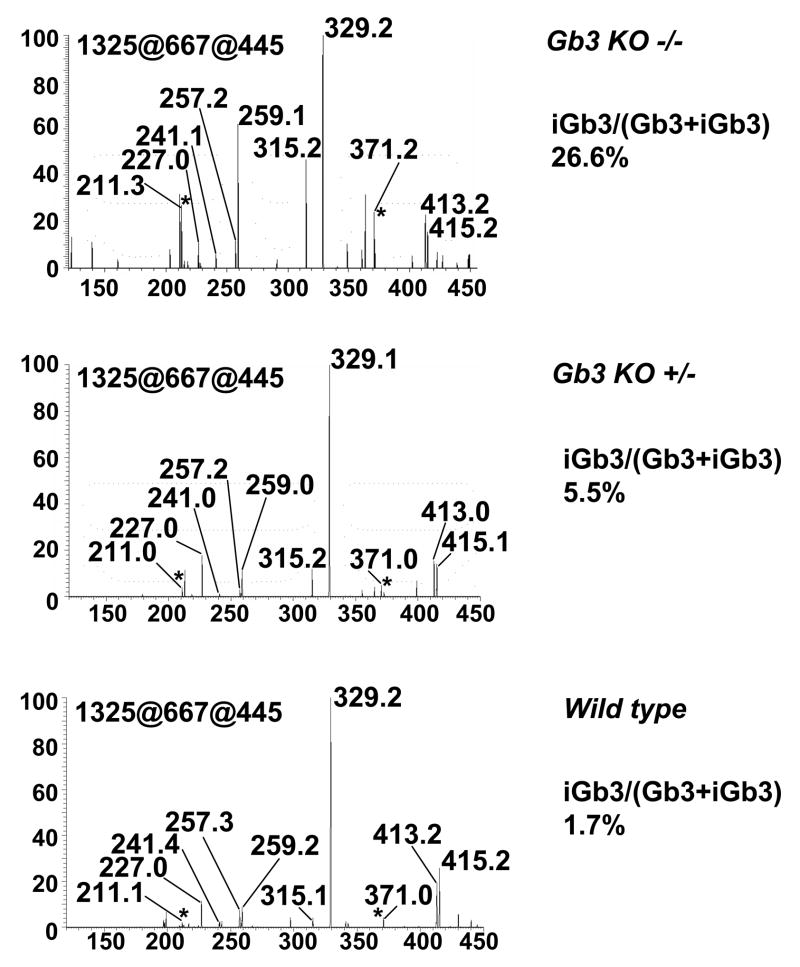
In Figure 6, we compare the iGb3/Gb3 ratio in Gb3 synthase−/−, Gb3 synthase+/−, and wild type mice. A more than 10 fold increase of the iGb3/Gb3 ratio, from 1.7 % to 26.5%, was found in Gb3 synthase KO mice (Supplementary Table 5), when selecting the molecular ion m/z 1325. The total iGb3 to Gb3 ratio in Gb3 synthase KO mice was estimated as 7.5%. Noteworthy, we still found a presence of Gb3 (5% versus wild type control mice) in Gb3 synthase KO mice. A slight increase of iGb3/Gb3 ratio was found in Gb3 synthase+/− mice as well (2–3 fold), which is consistent with our previous finding that Gb3 synthase activity was decreased 50% in heterozygous mutant mice (44).
Figure 6. Increased ratio of iGb3 in Gb3 synthase KO mice.
Neutral GSLs from wild type, Gb3 synthase+/− and Gb3 synthase−/− mice were extracted, permethylated, and analyzed by ion trap mass spectrometry. MS1 ions representing regioisomers of iGb3 & Gb3 were selected for MS4 analysis. Percentage of iGb3 in iGb3 & Gb3 mixtures were calculated according to standard curve as described (40).
We further studied the NKT cells in the thymus of Gb3 synthase KO mice, and found normal NKT cell numbers in thymus, spleen, and bone marrow (Supplementary Figure 5). This finding is consistent with our previous report that Gb3 is not a stimulatory ligand for NKT cells (28), since Gb3 synthase KO mice have much reduced Gb3 expression not measurable by anti-Gb3 antibody staining (44).
Major species of glycosphingolipids do not stimulate NKT cells
To identify new candidate GSL antigens for NKT cells, we examined the stimulatory activities of all of them toward the DN32.D3 and 24.8A hybridomas. We found no detectable stimulation by any of these GSLs except iGb3 (Supplementary Figure 6).
Discussion
Profiles of neutral and acidic glycosphingolipids in mouse thymus
In this study, we report a mass spectrometry-based glycosphingolipidomic analysis, utilizing state-of-the-art MALDI-TOF/TOF-MS/MS and ESI-LIT-MSn instrumental techniques, of the major GSL species in mouse thymus and bone marrow derived dendritic cells. Compared to previous studies using HPLC and fast atom bombardment mass spectrometry (FAB-MS), our results demonstrated a number of significant advantages:
we clearly observed presence of GSL structures not previously reported by others (21, 34,45, 46), including iGb3 and iGb4;
our results were generated with very small amounts of sample (107 mouse bone marrow derived dendritic cells);
tedious HPLC or TLC purification of individual GSLs is avoided;
the method is generally applicable, and results have been reproduced in more than one mass spectrometry core facility.
A significant part of this study required permethylation of the GSL samples, which increases the sensitivity and specificity of carbohydrate structure detection by mass spectrometry, but may destroy or leave undetected sulfated GSL structures. Detection of sulfated GSLs was carried out on native GSLs using ESI-MSn with a precursor ion scanning technique (47), which enables specific searching for GSLs containing a sulfo group. Using this technique, we identified sulfatide molecular species in mouse thymus (Supplementory Figure 7).
In comparison to HPLC-based quantitation methods, and to the traditional use of MS for qualitative glycan profiling and structure analysis, quantitation of glycoconjugates by MS remains a relatively less traveled field. In this study, we have compared the relative ion abundance of neutral GSLs to that of an artificial iGb3, and the acidic GSLs to asialo-GM1. This comparison allowed us to calculate the relative ratios of GSLs in Hexb KO mice and Fabry mice to wild type mice. However, it is difficult to calculate the absolute quantity of each GSL, because significantly different GSLs may have different ionization efficiencies. A GSL standard for each specific GSL, having the same ionization efficiency as the analyte, is required for absolute quantitation. For example, we have shown that the artificial iGb3 has same ionization efficiency as natural iGb3 expressed by mammalian cells (40). In the future, we propose to synthesize additional specific “short-tail” GSL standards for quantitation purpose.
Specificity of glycosidases in regulating glycosphingolipid expression
α-galactosidase KO and Hexb KO mice display specific accumulation of GSLs terminated by sugars that are cleavable by specific glycosidases. Our results indicate that normal GSL degradative processing is only selectively impaired in α-galactosidase KO mice or Hexb KO mice. The finding that Hexb KO mice lack iGb3 expression is consistent with our previous hypothesis that the Hexb enzyme is required for generation of iGb3 (28). We found that Hexb KO cells showed a specific defect in processing and presenting HexNAc terminated glycolipid antigens (iGb4 and GalNAcβ1,3Galα1,1Cer), but not α-Gal terminated complex glycolipids (Galα1,2Galα1,1Cer) and αGalCer antigen (28). Studies from the Cerundolo lab contradicted our findings; Gadola et al (25) reported instead that Hexb KO cells could not process and present Gal α1,2Gal α Cer, nor could they present the surrogate antigen for NKT cells, αGalCer. These discrepancies could be explained by differences in age and genetic background between mouse strains. Indeed, we have already found clear biochemical differences between the Hexb KO mice used by two laboratories. The Hexb KO mice used by the Cerundolo group, as studied by Speak et al (45), showed a similar amount of Gb3 as compared to wild type mice, while we observed more than 95% reduction of Gb3 in thymus from our Hexb KO strain.
GSL antigen processing and presentation in Fabry mice were first studied by the Kronenberg group (41). In their study, α-galactosidase A deficient cells were completely defective in presentation of Galα1,2Galα1,1Cer, although no defect was found in presenting αGalCer. Prigozy et al (41) found a 50% reduction of NKT cells in the periphery (spleen), but NKT cells were normal in the thymus. When we first discovered that saposins are required for NKT cell development, we compared the presentation of Galα1,2Galα1,1Cer and αGalCer by saposin KO cells to that of α-galactosidase KO cells (22). We found a defect in presenting αGalCer by saposin KO cells, but not α-galactosidase KO cells, while neither saposin KO and α-galactosidase KO cells could present Galα1,2Galα1,1Cer. Similar to Kronenberg’s group, we could not find a defect of NKT cells in the thymus of α-galactosidase KO mice. We have found normal numbers of NKT cells in thymus and spleen of 4 to 8 weeks old Fabry mice (22). The defect (90% reduction in thymus) reported by Gadola et al (25) may be due to a different Fabry mouse strain used in their study. They could find Gb3, but no other GSLs, in the thymus of their Fabry mice (45); in contrast, we observed an 8-fold increase of Gb4 expression in our Fabry mice (supplementary Figure 1). It is also interesting to note that although iGb3 expression is increased (5 fold) in our Fabry mice, we did not find significant increase of stimulation toward NKT cells by thymocytes from Fabry mice (22) compared to wild type mice, and the underlying mechanisms remain to be studied.
We used Hexb KO mice in our discovery of the first natural ligand for NKT cells, through the reasoning that iGb3 is cleaved from iGb4 by β-hexosaminidases. However, we agree with most investigators in the field that iGb3 may not be the only natural ligand. We have pointed this out since our first report of the antigenic activity of iGb3, and in review articles (27, 28). Since we found iGb3 by an indirect approach, testing GSL candidates that could be generated in the lysosome by β-hexosaminidases, we can not exclude the possibility that other natural ligands could also be present. The exact mechanisms responsible for the defect of NKT cell development in Hexb KO mice remain unclear. It may be due to defect in generating natural ligands such as iGb3, or a secondary effect of GSL accumulation (Figure 3, especially gangliosides), or other more severe secondary effects which may even cause defect in loading of α-galactosylceramide, as reported by Gadola et al (25) in a different Hexb KO mouse strain. A complete view of all these biochemical and cell biological parameters is still currently missing.
The current study is the first to describe in detail the GSL profiles of glycosidase mutant immune cells. GSL profiles in Hexb KO and Fabry mice have been previously characterized at the organ level, including brain, kidney, and liver. The Sandhoff group reported the accumulation of HexNAc terminated GSLs in kidney of Hexb KO mice (48). The Shayman group reported that Gb3 was increased in liver and kidney of Fabry mice, while the level of Gb4, present at a similar amount to Gb3, was not significantly changed (49–50). These results suggest that expression of GSLs in different cell types is regulated by complex processes. The regulation may operate at the transcriptional level of glycosyltransferases and glycosidases, through post-translational trafficking of these glyco-enzymes and their activator proteins (such as saposins), and through lysosomal acidification (51).
Low abundance of iGb3 expression by mouse thymus and mouse dendritic cells
We found that the level of iGb3 in mouse immune cells is overwhelmed by expression of the Gb3 regioisomer. In most trihexosylceramide molecular species detectable at the MS1 level, the abundance of iGb3 MS4 product ions is less than 1% or undetectable. However, levels of iGb3 higher than 1% were detected upon MS4 analysis of particular trihexosylceramide molecular species corresponding to certain ceramide compositions (lipoforms). Our data are consistent with findings by the Platt group (45), that overall the level iGb3 is below 1% of total iGb3 & Gb3 mixture. However, because the ESI-LIT-MSn method can measure the iGb3 levels in individual lipoforms (differing in this case by m/z increments corresponding to fatty-N-acyl chain length and unsaturation), the presence of iGb3 can be differentially detected in a few molecular species, even when it is below the limit of detectability in the majority of them. In contrast, the HPLC method employed by the Platt group involves cleavage of the glycan from the ceramides of all iGb3 and Gb3 species prior to analysis. Even if the the resulting mixture of isomeric glycans were well resolved in the subsequent HPLC separation, the relative sensitivity of iGb3 detection in a much larger pool of Gb3 is paradoxically reduced, rather than increased, if its expression was confined to a limited set of lipoforms. The ability to detect iGb3 expression on a single lipoform out of many is a noteworthy advantage of the MSn methodology.
It remains to be studied whether such a low level of iGb3 expression in thymocytes and dendritic cells is sufficient for providing stimulatory signals to NKT cells at an in vivo setting, although it is generally accepted that single peptide-MHC complex expression in an antigen presenting cell is sufficient to trigger TCR signaling. In addition to direct TCR stimulation by natural ligands, costimulatory pathways may contribute to the full activation program of NKT cells. We and others have recently found that B7 family of co-stimulatory molecules are critical for the second wave of NKT cell expansion during their development (52–54).
The physiological relevance of iGb3 has been further challenged by the groups of Platt and Sandrin (45, 55–56), based on their finding that iGb3 is absent in mouse and human immune cells (thymus and dendritic cells). The Platt group employed an HPLC method to assay oligosaccharides cleaved from total thymic GSLs following fluorescence-labeling (45); the method relies purely on retention behavior for identification. The Sandrin group used a monoclonal antibody (15.101) to stain human tissue sections (55–56), although the specificity of their antibody remains questionable (57). Both laboratories have concluded that iGb3 is not expressed in humans, but in contrast to their findings, we have detected iGb3 and its lysosomal precursor iGb4 in human thymus and dendritic cells by an ion trap mass spectrometry method (39–40, and manuscript in preparation), which is considerably more sensitive than HPLC method (40, 45), and more specific than antibody staining (57). Christiansen et al (56) further concluded that human iGb3 synthase is a pseudogene, based on negative finding by RT-PCR experiments, and the negative finding that a hybrid rat-human iGb3 synthase enzyme failed to generate products that react to antibody 15.101. However, the antibody staining method used to measure glycosyltransferase activities by detection of their products has a far lower sensitivity compared to mass spectrometry methods (39–40), or conventional methods based on measuring the transfer of radioisotope labeled sugar donors.
The enzymatic mechanism of iGb3 synthesis in mouse thymus is not clearly understood yet. We first cloned the mouse iGb3 synthase from mouse thymus (28). Subsequently, mRNA blotting analysis by Milland et al. showed that this enzyme is most abundantly expressed by mouse thymus, as compared to other organs (36). Thus it is difficult to understand why iGb3 is expressed at such a low abundance in thymus. We have studied whether other α1,3-glycosyltransferases can synthesize iGb3, and found α1,3-galactosyltransferase 1 (α3GalT1) produced iGb3 when transfected to Chinese Hamster Ovary (CHO) cells (Supplementary Figure 8). Using recombinant bovine α3GalT1 protein, we also synthesized iGb3 in a cell-free system (data not shown). However, the efficiency of α3GalT1 in synthesizing iGb3 is 10 fold lower than iGb3 synthase in CHO cell transfection experiments, suggesting the latter is the major enzyme. The low level of iGb3 in mouse thymus may be due to yet unknown negative regulators on enzyme activity. Alternatively, there may exist enzyme-cofactors required for efficient assembly of iGb3.
Stimulatory activity toward NKT cells by major species of glycosphingolipids
In our preliminary screening for stimulatory activities of the GSLs, we have not found any others that are stimulatory ligands besides iGb3. Further studies are currently under way to measure the stimulatory activities of these ligands using additional NKT cell clones, as well as fresh NKT cells. On the other hand, we reason that minor species of GSLs can only be purified and characterized through large scale fractionation of GSLs from pooled mouse thymuses.
Currently, there are two series of lipid species to consider as candidates for the major natural ligands of NKT cells: phospholipids and glycosphingolipids. Supporting the involvement of phospholipids, Gumperz et al (58) reported that phosphatidylcholine and phosphatidylinosital stimulate a few NKT cell hybridomas in plate-bound assays. Evidence supporting a role for GSLs includes genetic data that glucosylceramide synthase is involved in natural ligand production (19); that NB-DGJ inhibits the antigen presenting cells’ ability to stimulate NKT cells (21, Figure 2); and that charged GSLs from mature dendritic cells stimulate fresh liver NKT cells in an IFN-α dependent manner (59). Although our data (Figure 2) and data from the groups of Joyce (19) and Cerundolo (21) strongly support that GSL metabolism influences the production of natural ligand, it is still not conclusive that the major natural ligands must be GSLs. Specifically, glucosylceramide has been reported as critical for intracellular trafficking of certain membrane proteins. For example, in the melanoma tumor cell line B16 mutant GM95, which does not synthesize glucosylceramide due to inactivation of β-glucosylceramide synthase, a severe defect was found in the trafficking of melanin from the endoplasmic reticulum to the melanosome compartment, leading to a “white” phenotype of the cell line (60), although the trafficking of CD1d was normal in GM95 cells (19). At this time, we can not exclude non-GSLs as the candidates for natural ligands.
In summary, our study provides the first in depth view of GSL expression in mouse immune cells, and should provide a basis for future identification of potential thymic GSL ligands for NKT cells. This study also demonstrated the presence of iGb3, the first natural ligand, in mouse thymus, and highlighted the importance of biochemical studies by sensitive, specific, and generally applicable methodologies.
Conclusions
The search for natural ligands for NKT cells has been ongoing for more than a decade without considerable progress. Our study indicates these ligands contain unknown structures which are not reported as major GSLs. Similarly, previous studies by others (61) showed that major membrane phospholipids with known structures are not stimulatory ligands for the majority population of NKT cells. We propose a “lipidomics” approach, i.e., one that identifies new lipid structures and tests their immunological activity, with a priority on those structures similar to known ligands of NKT cells. The generation of first lipidomics database for antigen presenting cells in the immune system will be a fundamental step, and open up an a new avenue to identify lipid and glycolipid targets for the immune-related disorders which have heretofore been challenging to study.
Supplementary Material
Acknowledgments
This work was supported by a start up fund from M.D. Anderson Cancer Center, and a Developmental Award from NCI Joe Moakley Leukemia SPORE grant to D.Z. and an NIH grant (R21 RR20355) to S.B.L. The authors thank Drs. Ola Blixt and Jim Paulson (Consortium of Functional Glycomics, the Scripps Research Institute) for synthetic carbohydrate standards; Dr. Vernon N. Reinhold for providing the MS facilities of the UNH Center for Structural Biology (NIH/NCRR grant P20 RR16459) for these studies; Drs. Jochen Mattner, Albert Bendelac, and Bhanu Prakash Pappu for discussion.
Abbreviations
- α1
3GT, α-1,3 galactosyltransferase I
- ESI-LIT-MS
electrospray ionization linear ion trap mass spectrometry
- Forssman antigen
GalNAcα1,3 GalNAcβ1,3Galα1,4Galβ1,4Glcβ1,1Cer
- Glc-Cer
glucosylceramide
- Gal-Cer
galactosylceramide
- GM3
NeuAcα2,3Galβ1,4Glcβ1,1Cer
- GM2
GalNAcβ1, 4(NeuAcα2,3)Galβ1,4Glcβ1,1Cer
- GSL
glycosphingolipid
- iGb3
isoglobotrihexosylceramide (Galα1,3Galβ1,4Glcβ1,1Cer)
- Lac-Cer
lactosylceramide
- Lc3
GlcNAcβ1,3Galβ1,4Glcβ1,1Cer
- MALDI-TOF-MS
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- NB-DGJ
N-butyldeoxygalactonojirimycin
- TLC
thin layer chromatography
Footnotes
The glycosphingolipid nomenclature follows the recommendations of the IUPAC-IUB Commission on Biochemical Nomenclature (CBN for Lipids: Eur J Biochem. (1998) 257: 293).
Supporting Information Available: This material is available free at http://pubs.acs.org
References
- 1.Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu Rev Cell Dev Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- 2.Yamakawa T, Suzuki A, Hashimoto Y. Genetic control of glycolipid expression. Chem Phys Lipids. 1986 Dec 15;42(1–3):75–90. doi: 10.1016/0009-3084(86)90044-7. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzmann G, Sandhoff K. Metabolism and intracellular transport of glycosphingolipids. Biochemistry. 1990 Dec 11;29(49):10865–71. doi: 10.1021/bi00501a001. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa S, Hirabayashi Y. Glucosylceramide synthase and glycosphingolipid synthesis. Trends Cell Biol. 1998 May;8(5):198–202. doi: 10.1016/s0962-8924(98)01249-5. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd KO, Furukawa K. Biosynthesis and functions of gangliosides: recent advances. Glycoconj J. 1998 Jul;15(7):627–36. doi: 10.1023/a:1006924128550. Review. No abstract available. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki A. Biosynthetic pathways of glycosphingolipids. In: Taniguchi N, Honke K, Fukuda M, editors. Handbook of glycosyltransferases and related genes. Springer-Verlag; Tokyo: 2002. pp. 636–646. [Google Scholar]
- 7.De Libero G, Mori L. Self glycosphingolipids: new antigens recognized by autoreactive T lymphocytes. News Physiol Sci. 2003 Apr;18:71–6. doi: 10.1152/nips.01418.2002. [DOI] [PubMed] [Google Scholar]
- 8.Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. Eur J Immunol. 1999 May;29(5):1667–75. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 9.Shamshiev A, Donda A, Prigozy TI, Mori L, Chigorno V, Benedict CA, Kappos L, Sonnino S, Kronenberg M, De Libero G. The alphabeta T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 2000 Aug;13(2):255–64. doi: 10.1016/s1074-7613(00)00025-x. [DOI] [PubMed] [Google Scholar]
- 10.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995 Dec 1;182(6):2091–6. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmer MI, Colmone A, Felio K, Xu H, Ma A, Wang CR. A cell-type specific CD1d expression program modulates invariant NKT cell development and function. J Immunol. 2006 Feb 1;176(3):1421–30. doi: 10.4049/jimmunol.176.3.1421. [DOI] [PubMed] [Google Scholar]
- 12.Brossay L, Tangri S, Bix M, Cardell S, Locksley R, Kronenberg M. Mouse CD1-autoreactive T cells have diverse patterns of reactivity to CD1+ targets. J Immunol. 1998 Apr 15;160(8):3681–8. [PubMed] [Google Scholar]
- 13.Park SH, Roark JH, Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J Immunol. 1998;160(7):3128–34. [PubMed] [Google Scholar]
- 14.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997 Nov 28;278(5343):1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 15.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005 Mar 24;434(7032):525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 16.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005 Mar 24;434(7032):520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 17.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005 Jun;35(6):1692–701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 18.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006 Sep;7(9):978–86. doi: 10.1038/ni1380. Epub 2006 Aug 20. [DOI] [PubMed] [Google Scholar]
- 19.Stanic AK, De Silva AD, Park JJ, Sriram V, Ichikawa S, Hirabyashi Y, Hayakawa K, Van Kaer L, Brutkiewicz RR, Joyce S. Defective presentation of the CD1d1-restricted natural Va14Ja18 NKT lymphocyte antigen caused by beta-D-glucosylceramide synthase deficiency. Proc Natl Acad Sci U S A. 2003;100(4):1849–54. doi: 10.1073/pnas.0430327100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson U, Butters TD, Dwek RA, Platt FM. N-butyldeoxygalactonojirimycin: a more selective inhibitor of glycosphingolipid biosynthesis than N-butyldeoxynojirimycin, in vitro and in vivo. Biochem Pharmacol. 2000;59(7):821–9. doi: 10.1016/s0006-2952(99)00384-6. [DOI] [PubMed] [Google Scholar]
- 21.Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, Besra GS, Platt FM, Cerundolo V. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A. 2007 Dec 18;104(51):20490–5. doi: 10.1073/pnas.0710145104. Epub 2007 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou D, Cantu C, 3rd, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, Morales CR, Grabowski GA, Benlagha K, Savage P, Bendelac A, Teyton L. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004 Jan 23;303(5657):523–7. doi: 10.1126/science.1092009. Epub 2003 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagiv Y, Hudspeth K, Mattner J, Schrantz N, Stern RK, Zhou D, Savage PB, Teyton L, Bendelac A. Cutting edge: impaired glycosphingolipid trafficking and NKT cell development in mice lacking Niemann-Pick type C1 protein. J Immunol. 2006 Jul 1;177(1):26–30. doi: 10.4049/jimmunol.177.1.26. [DOI] [PubMed] [Google Scholar]
- 24.Schümann J, Facciotti F, Panza L, Michieletti M, Compostella F, Collmann A, Mori L, De Libero G. Differential alteration of lipid antigen presentation to NKT cells due to imbalances in lipid metabolism. Eur J Immunol. 2007 Jun;37(6):1431–41. doi: 10.1002/eji.200737160. [DOI] [PubMed] [Google Scholar]
- 25.Gadola SD, Silk JD, Jeans A, Illarionov PA, Salio M, Besra GS, Dwek R, Butters TD, Platt FM, Cerundolo V. Impaired selection of invariant natural killer T cells in diverse mouse models of glycosphingolipid lysosomal storage diseases. J Exp Med. 2006 Oct 2;203(10):2293–303. doi: 10.1084/jem.20060921. Epub 2006 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrantz N, Sagiv Y, Liu Y, Savage PB, Bendelac A, Teyton L. The Niemann-Pick type C2 protein loads isoglobotrihexosylceramide onto CD1d molecules and contributes to the thymic selection of NKT cells. J Exp Med. 2007 Apr 16;204(4):841–52. doi: 10.1084/jem.20061562. Epub 2007 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou D. The immunological function of iGb3. Curr Protein Pept Sci. 2006 Aug;7(4):325–33. doi: 10.2174/138920306778018007. Review. [DOI] [PubMed] [Google Scholar]
- 28.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 29.Scott-Browne JP, Matsuda JL, Mallevaey T, White J, Borg NA, McCluskey J, Rossjohn J, Kappler J, Marrack P, Gapin L. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007 Oct;8(10):1105–13. doi: 10.1038/ni1510. Epub 2007 Sep 9. [DOI] [PubMed] [Google Scholar]
- 30.Xia C, Yao Q, Schumann J, Rossy E, Chen W, Zhu L, Zhang W, De Libero G, Wang PG. Synthesis and biological evaluation of alpha-galactosylceramide (KRN7000) and isoglobotrihexosylceramide (iGb3) Bioorg Med Chem Lett. 2006 Apr 15;16(8):2195–9. doi: 10.1016/j.bmcl.2006.01.040. Epub 206 Feb 2. [DOI] [PubMed] [Google Scholar]
- 31.Schumann J, Mycko MP, Dellabona P, Casorati G, MacDonald HR. Cutting edge: influence of the TCR Vbeta domain on the selection of semi-invariant NKT cells by endogenous ligands. J Immunol. 2006 Feb 15;176(4):2064–8. doi: 10.4049/jimmunol.176.4.2064. [DOI] [PubMed] [Google Scholar]
- 32.Yu KO, Im JS, Illarionov PA, Ndonye RM, Howell AR, Besra GS, Porcelli SA. Production and characterization of monoclonal antibodies against complexes of the NKT cell ligand alpha-galactosylceramide bound to mouse CD1d. J Immunol Methods. 2007 May 31;323(1):11–23. doi: 10.1016/j.jim.2007.03.006. Epub 2007 Apr . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei DG, Curran SA, Savage PB, Teyton L, Bendelac A. Mechanisms imposing the Vbeta bias of Valpha14 natural killer T cells and consequences for microbial glycolipid recognition. J Exp Med. 2006 May 15;203(5):1197–207. doi: 10.1084/jem.20060418. Epub 2006 May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Gröne HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci U S A. 2007 Apr 3;104(14):5977–82. doi: 10.1073/pnas.0611139104. Epub 2007 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levery SB. Glycosphingolipid structural analysis and glycosphingolipidomics. Methods Enzymol. 2005;405:300–69. doi: 10.1016/S0076-6879(05)05012-3. [DOI] [PubMed] [Google Scholar]
- 36.Haslam SM, Julien S, Burchell JM, Monk CR, Ceroni A, Garden OA, Dell A. Characterizing the glycome of the mammalian immune system. Immunol Cell Biol. 2008 Aug 26; doi: 10.1038/icb.2008.54. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Ashline D, Singh S, Hanneman A, Reinhold V. Congruent strategies for carbohydrate sequencing. 1. Mining structural details by MSn. Anal Chem. 2005 Oct 1;77(19):6250–62. doi: 10.1021/ac050724z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinhold VN, Reinhold BB, Costello CE. Carbohydrate molecular weight profiling, sequence, linkage, and branching data: ES-MS and CID. Anal Chem. 1995 Jun 1;67(11):1772–84. doi: 10.1021/ac00107a005. Review. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Teneberg S, Thapa P, Bendelac A, Levery SB, Zhou D. Sensitive detection of isoglobo and globo series tetraglycosylceramides in human thymus by ion trap mass spectrometry. Glycobiology. 2008 Feb;18(2):158–65. doi: 10.1093/glycob/cwm129. Epub 2007 Dec 3. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Zhou D, Xia C, Wang PG, Levery SB. Sensitive quantitation of isoglobotriaosylceramide in the presence of isobaric components using electrospray ionization-ion trap mass spectrometry. Glycobiology. 2008 Feb;18(2):166–76. doi: 10.1093/glycob/cwm127. Epub 2007 Nov 28. [DOI] [PubMed] [Google Scholar]
- 41.Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001 Jan 26;291(5504):664–7. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 42.Sango K, McDonald MP, Crawley JN, Mack ML, Tifft CJ, Skop E, Starr CM, Hoffmann A, Sandhoff K, Suzuki K, Proia RL. Mice lacking both subunits of lysosomal beta-hexosaminidase display gangliosidosis and mucopolysaccharidosis. Nat Genet. 1996 Nov;14(3):348–52. doi: 10.1038/ng1196-348. [DOI] [PubMed] [Google Scholar]
- 43.Ohshima T, Murray GJ, Swaim WD, Longenecker G, Quirk JM, Cardarelli CO, Sugimoto Y, Pastan I, Gottesman MM, Brady RO, Kulkarni AB. alpha-Galactosidase A deficient mice: a model of Fabry disease. Proc Natl Acad Sci U S A. 1997 Mar 18;94(6):2540–4. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okuda T, Tokuda N, Numata S, Ito M, Ohta M, Kawamura K, Wiels J, Urano T, Tajima O, Furukawa K, Furukawa K. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. J Biol Chem. 2006 Apr 14;281(15):10230–5. doi: 10.1074/jbc.M600057200. Epub 2006 Feb 13. [DOI] [PubMed] [Google Scholar]
- 45.Speak AO, Salio M, Neville DC, Fontaine J, Priestman DA, Platt N, Heare T, Butters TD, Dwek RA, Trottein F, Exley MA, Cerundolo V, Platt FM. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci U S A. 2007 Apr 3;104(14):5971–6. doi: 10.1073/pnas.0607285104. Epub 2007 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarting GA, Gajewski A. Glycolipids of murine lymphocyte subpopulations. Structural characterization of thymus gangliosides. J Biol Chem. 1983 May 10;258(9):5893–8. [PubMed] [Google Scholar]
- 47.Hsu FF, Turk J. Studies on sulfatides by quadrupole ion-trap mass spectrometry with electrospray ionization: structural characterization and the fragmentation processes that include an unusual internal galactose residue loss and the classical charge-remote fragmentation. J Am Soc Mass Spectrom. 2004 Apr;15(4):536–46. doi: 10.1016/j.jasms.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Sandhoff R, Hepbildikler ST, Jennemann R, Geyer R, Gieselmann V, Proia RL, Wiegandt H, Grone HJ. Kidney sulfatides in mouse models of inherited glycosphingolipid disorders: determination by nano-electrospray ionization tandem mass spectrometry. J Biol Chem. 2002 Jun 7;277(23):20386–98. doi: 10.1074/jbc.M110641200. Epub 2002 Mar 27. [DOI] [PubMed] [Google Scholar]
- 49.Abe A, Arend LJ, Lee L, Lingwood C, Brady RO, Shayman JA. Glycosphingolipid depletion in fabry disease lymphoblasts with potent inhibitors of glucosylceramide synthase. Kidney Int. 2000 Feb;57(2):446–54. doi: 10.1046/j.1523-1755.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 50.Abe A, Gregory S, Lee L, Killen PD, Brady RO, Kulkarni A, Shayman JA. Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J Clin Invest. 2000 Jun;105(11):1563–71. doi: 10.1172/JCI9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003 Feb 28;299(5611):1400–3. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 52.Chung Y, Nurieva R, Esashi E, Wang YH, Zhou D, Gapin L, Dong C. A critical role of costimulation during intrathymic development of invariant NK T cells. J Immunol. 2008 Feb 15;180(4):2276–83. doi: 10.4049/jimmunol.180.4.2276. [DOI] [PubMed] [Google Scholar]
- 53.Williams JA, Lumsden JM, Yu X, Feigenbaum L, Zhang J, Steinberg SM, Hodes RJ. Regulation of thymic NKT cell development by the B7-CD28 costimulatory pathway. J Immunol. 2008 Jul 15;181(2):907–17. doi: 10.4049/jimmunol.181.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng X, Zhang H, Yin L, Wang CR, Liu Y, Zheng P. Modulation of NKT cell development by B7-CD28 interaction: an expanding horizon for costimulation. PLoS ONE. 2008 Jul 16;3(7):e2703. doi: 10.1371/journal.pone.0002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milland J, Christiansen D, Lazarus BD, Taylor SG, Xing PX, Sandrin MS. The molecular basis for galalpha(1,3)gal expression in animals with a deletion of the alpha1,3galactosyltransferase gene. J Immunol. 2006 Feb 15;176(4):2448–54. doi: 10.4049/jimmunol.176.4.2448. [DOI] [PubMed] [Google Scholar]
- 56.Christiansen D, Milland J, Mouhtouris E, Vaughan H, Pellicci DG, McConville MJ, Godfrey DI, Sandrin MS. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 2008 Jul 15;6(7):e172. doi: 10.1371/journal.pbio.0060172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou D, Levery SB. Response to Milland et al.: Carbohydrate residues downstream of the terminal Galalpha(1,3)Gal epitope modulate the specificity of xenoreactive antibodies. Immunol Cell Biol. 2008 Sep 9; doi: 10.1038/icb.2008.65. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000 Feb;12(2):211–21. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- 59.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, Platt F, Trottein F. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007 Oct;27(4):597–609. doi: 10.1016/j.immuni.2007.08.017. Epub 2007 Oct 18. [DOI] [PubMed] [Google Scholar]
- 60.Sprong H, Degroote S, Claessens T, van Drunen J, Oorschot V, Westerink BH, Hirabayashi Y, Klumperman J, van der Sluijs P, van Meer G. Glycosphingolipids are required for sorting melanosomal proteins in the Golgi complex. J Cell Biol. 2001 Oct 29;155(3):369–80. doi: 10.1083/jcb.200106104. Epub 2001 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parekh VV, Singh AK, Wilson MT, Olivares-Villagómez D, Bezbradica JS, Inazawa H, Ehara H, Sakai T, Serizawa I, Wu L, Wang CR, Joyce S, Van Kaer L. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct alpha- and beta-anomeric glycolipids. J Immunol. 2004 Sep 15;173(6):3693–706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.